Abstract
Women experience dramatic changes in hormones, mood and cognition through different periods of their reproductive lives, particularly during pregnancy and giving birth. While limited human studies of early pregnancy and motherhood showed alteration of cognitive functions in later life, researches on rodents showed a persistent improvement of learning and memory performance in females with history of giving birth compared to virgin controls. Alzheimer's disease (AD), the most common dementia in elderly, is more prevalent in women than in men. One of the risk factors is related to the sharp reduction of estrogen in aged women. It is unknown whether the history of fertility activity plays any roles in altering risk of AD in females, such as altering cognitive function. Would reproductive experience alter the risk of AD in females? If so, what might be the mechanisms of the change? In this study, we examined the effects of reproductive experience on cognitive function in an AD transgenic mouse model (APP23) and age-matched wild-type non-transgenic control mice (WT). Our data showed an age-dependent effect of reproductive experience on learning and memory activity between breeders (had one or more litters) and non-breeders (virgins). More importantly, our data, for the first time, demonstrated a genotype-dependent effect of parity on cognitive function between APP23 and WT mice. At the age of 12 months, WT breeders outperform non-breeders in spatial working and reference memory while APP23 breeders performed worse in spatial learning and memory than age-matched APP23 non-breeders. These genotype- and age-dependent effects of reproductive activity on cognitions are significantly associated with changes of neuropathology of AD in the APP23 mice, expression of proteins related to synaptic plasticity and cognitive functions in the brain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer's disease (AD) is more prevalent in women than in men, and this difference in prevalence remains even after adjusting for age and level of education [1, 2]. The age of AD onset in women is also influenced by factors such as having children. Previous studies showed that women with past fertility experience had higher risk of AD compared to those who had never given birth to a child [3]. Other studies also reported that having children might be linked to cognitive decline [4] and earlier onset of AD [5], while the number of children made no difference in the risk of AD [6]. While it is uncertain whether the lifelong effect of past fertility on cognitive function is related to changes in estrogen levels in women, studies found that the higher estrogen levels usually present in nulliparous women who had no past fertility history may be sufficient to delay the AD onset. However, there is always a possibility that there are more birth control pills users in the nulliparous women, which might be interfering with the outcome of those human studies. The concerns of having additional estrogen such as birth control pills in nulliparous females can be totally excluded in animal studies. Interestingly, multiple researches conducted on rodents showed a persistent improvement of learning and memory performance in females with past history of giving birth compared to virgin controls [7–9].
AD is the most common type of progressive dementia in the elderly. AD neuropathology is characterized by deposition of β-amyloid peptide (Aβ), which is generated from amyloid precursor protein (APP) by enzymatic digestion involving β-secretase and γ-secretase activity, and the extracellular Aβ plaques formation along with accumulation of intracellular neurofibrillary tangles, and neuronal cell loss [10–13]. There are several AD transgenic animal models that have been used to study AD neuropathology and disease-related cognitive impairment. Age-associated Aβ plaque formation, synaptic impairment, and learning and memory decline have been well documented in those transgenic animal models, including APP23 mice as previously reported [14–16]. Our recent studies showed that early estrogen treatment prevents Aβ plaque formation in APP23 mice at later age, suggesting a potential neuroprotective role of estrogen in female AD pathology [17].
Several cognition-related genes have been identified to be related to the hormone changes in pregnancy and postpartum period by multiple studies. Ca2+/calmodulin-dependent protein kinase II (CaMKII) is known to be involved in synaptic plasticity and memory. A down-regulation of CAMKII is found in the brain of individuals with depression, AD as well as in early postpartum period [18–20]. Furthermore, in animal studies, the level of Thr286 phosphorylation of CaMKII in the hippocampus is associated with faster hippocampal-dependent spatial memory formation [21]. The cAMP-response element-binding (CREB) protein is a crucial transcription factor regulating expression of genes involved in neuronal growth and plasticity and plays a role in neuronal survival. Studies showed that estradiol induces spine plasticity in the hippocampus via rapid membrane effects and slower transcriptional regulation via the CREB pathway [22–24]. Furthermore, the number of cells with positive immunostaining for phospho-CREB in the medial preoptic area of the hypothalamus, a key region for the expression of genes involved in maternal behavior, is increased about 3-fold in female maternal mice who had exposure to their pups [25]. A multifunctional mitochondrial enzyme 17β-hydroxysteroid dehydrogenase type 10 (HSD10), formerly called endoplasmic reticulum-associated Aβ-binding protein (ERAB), is involved in maintaining brain synaptic functions and elevation of HSD10 is associated with AD [26–28]. Studies found that the gene that codes for HSD10 is located on chromosome X which might be associated with gene-related dementia [29]. Moreover, the elevation of HSD10 levels in synaptic mitochondria might eliminate estrogen neuroprotective activity and impair hippocampal-dependent learning and memory [28].
Together, increasing interest in reproductive activities (fertility experience) and ageing related cognitive function in females suggest that early fertility experience in females have a long lasting effect on learning and memory activities later in life. However, most findings from human studies are contradictory with the reports from animal studies. This contrary might be due to most human studies containing insufficient information on birth control pills usage, especially in nulliparous women, which might significantly affect the outcome of the cognitive functional analyses [30, 31]. To examine whether reproductive activity affect age-related cognitive decline in AD, we investigated the spatial learning and memories, neuropathology of AD and synaptic associated proteins expression in the brain of APP23 mice with or without fertility history. Age-matched WT mice with or without fertility experience were included as controls. We identified an age- and genotype-dependent effect of fertility on cognitive function with ageing.
Results
Early Fertility in APP23 Mice with Swedish Mutation Failed to Enhance Spontaneous Cognition
Female behavior changes with pregnancy and motherhood in both human and non-human studies. Ageing is associated with decrease of alternation [32]. Thus, before examining APP gene mutated mice, we first examined whether such reproductive activity altered cognitive function during normal ageing. We tested female WT breeders and non-breeders at age of 6 and 12 months for spontaneous cognition in the Y maze, a nature and hippocampal-dependent behavior as an initial test of memory function. At the age of behavioral tests, all breeders had given birth at least once and were tested at least 1 month after the last delivery and non-breeders were virgins without history of pregnancy or giving birth. As shown in Fig. 1a, at 12 months of age, WT breeders maintain 60 % of alternation rate compared to WT non-breeders who showed great reduction of alternation behavior as part of normal ageing [32]. To examine whether AD's gene, i.e., a popular one, Swedish mutation in APP gene, would affect fertility-enhanced cognition, we used APP23 mice, a mouse model of AD which contains a Swedish mutation [33]. Specifically, we examined the spontaneous alternation in young (6 months old) and aged (12 months old) APP23 transgenic mice. In contrast to WT breeders that showed an improved alternation rate, APP23 breeders showed a tendency of reduction of alternation rate compared to age-matched APP23 non-breeders in both young and aged mice as shown in Fig. 1b. To examine whether reproductive activity alters spatial working memory, we also examined two-trail recognition memory. Our data showed that WT breeders entered more frequently into and spent more time in the novel arm than WT non-breeders, while APP23 breeders with APP Swedish mutation showed the same exploration to the novel arm as age-matched APP23 non-breeders (Fig. 2a and b). Interestingly, APP23 breeders showed a higher number of arm entries than APP23 non-breeders, while WT breeders failed to show a significant difference in total number of arm entries compared to WT non-breeders (Fig. 2c). The result suggests that the deficiency of spontaneous alternation behavioral in APP23 breeder mice is not related to lacking of locomotion.
Effects of fertility experience on spontaneous alternation behavior in WT and APP23 mice. At 6 and 12 months of age, alternation performance was examined in WT (a) and APP23 (b). Breeders had given birth at least once and non-breeders were virgins. Values are given as mean ± SEM. Asterisk indicates a p value of <0.05 compared to age-matched non-breeders
Effects of fertility on recognition memory WT and APP23 mice. Two-trial recognition memory test was performed in the Y maze. Percentage of entries to novel arm (a), time spent in novel arm (b) and total number of entries to all arms (c) were examined and compared between breeders and non-breeders for both WT and APP23 mice at 12 months of age. Values are given as mean ± SEM. Asterisk indicates a p value of <0.05 compared to genotype-matched non-breeders
Further Spatial Memory Tests by Barns Maze Confirm that Fertility Activity Failed to Improve Cognition in APP23 Mice Containing Swedish Mutation
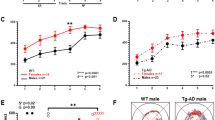
The Barnes maze spatial memory is used as an equivalent to the Morris water maze test without water stress, especially since water stress can modulate phosphorylation of proteins, including tau [34, 35]. Learning abilities were tested using the Barnes maze at 6 or 12 months of age for 4 consecutive days. Figure 3 shows that all mice learned to locate the target hole during the course of the training period (days 1–4), as indicated by a progressive reduction in target latencies and the number of errors. As shown in Fig. 3d, both WT breeders and APP23 breeders showed a better spatial learning curve compared to genotype-matched non-breeders at age of 12 months (using total errors as a measurement), while APP23 breeders showed a longer target latency than APP23 non-breeders at age of 12 months (Fig. 3b). There was a similar learning curve between breeders and non-breeders at 6 months of age for both genotypes (Fig. 3a and c), suggesting an age-associated effect of fertility on learning. To examine the spatial reference memory, animals were examined on day 5 and day 12 for 90 s for short-term and long-term memories, respectively. As shown in Fig. 4a and c, the WT breeders required significantly shorter time to enter the target hole (shorter target latency time) than that of WT non-breeders in both short-term and long-term memory tests at 6 and 12 months of age. No statistical significance was found between WT breeders and WT non-breeders in long-term reference memory test at 6 months of age. Unlike WT breeders, APP23 breeders showed a longer time to enter the target hole compared to age-matched APP23 non-breeders (Fig. 4b and d). Our data suggested that fertility activity does not affect learning until later in life but does affect spatial memory as early as at age of 6 months.
Effects of fertility on spatial learning curves in WT and APP23 mice during the 4-day learning trails in Barns maze test. Target latency generated from mice at 6 months of age (a) and 12 months of age (b) in the Barnes maze reference memory test. Total errors made by mice at 6 months of age (a) and 12 months of age (d) in the Barnes maze reference memory test. Values are given as mean ± SEM. Asterisk indicates a p value of <0.05 compared to genotype-matched non-breeders
Effects of fertility on spatial memory in WT and APP23 mice. Target latency was recorded on day 5 and day 12 probe trails as time to enter the escape hole in WT mice at 6 months (a) and 12 months (c) of age, and APP23 mice at age of 6 months (b) and 12 months (d). Values are given as mean ± SEM. Asterisk indicates a p value of <0.05 compared to genotype-matched non-breeders
Fertility Alters Brain Amyloid Pathology in APP23 Mice
Although human studies showed that fertility experience increases risk of AD in women, our studies of APP23 transgenic mice showed that reproductive activity improves learning performance at 12 months of age (Fig. 3d), but significantly impairs short- or long-term spatial reference memory at 6 and 12 months of age (Fig. 4b and d). To further investigate whether fertility induces pathology alteration in the brain of APP23 mice, we detected amyloid plaques, a landmark of AD pathology, in the brain of APP23 breeders and APP23 non-breeders. As shown in Fig. 5, at the age of 12 months, APP23 breeders developed more plaques (more in numbers and larger in size) in the cortex and hippocampus than APP23 non-breeders. This marks the first report of the evidence that reproductive activity affects AD neuropathology.
Effects of fertility on Aβ plaques formation in APP23 mice. Aβ accumulation in APP23 mice cortex and hippocampus were identified with immunostaining of monoclonal antibody against amino acid residues 1–16 of Aβ (6E10) and the histological ground was counterstained with haematoxylin (a). The 6E10-immunopositive structures were counted and statistical analysis (b). Bar: 100 μm
Fertility Activity Alters Cognitive-Related Protein Expression
One of the questions we are facing is that WT breeders are doing better in spatial memory than WT non-breeders, which is opposite to what we observed with those that contain Swedish mutation, the APP23 breeders. To examine whether synaptic related proteins are also affected by reproductive activities — specifically, to examine whether the influence of fertility on cognition is caused by alteration of synapse or cognition related gene expression, we first examined synaptic proteins such as SYP and SNAP25 as shown in Fig. 6a and b. Our data showed an elevation of SYP expression in the brain of WT breeders compared to that in WT non-breeders only. However, fertility in APP23 mice showed a mild inhibitory effect on SYP protein expression, since APP23 breeders showed lower SYP expression than that in APP23 non-breeders. There is no significant difference in SNAP25 expression levels between breeders and non-breeders. CREB is a transcription factor which has been shown to be integral in the formation of spatial memory and may possess therapeutic potential for AD [36]. We detected CREB protein expression in breeders and non-breeders. As shown in Fig. 6a and b, a reduction in CREB expression level was noticed in the brain of APP23 breeders compared to that in APP23 non-breeders, while no changes were observed between breeders and non-breeders of WT mice.
Effects of fertility on synaptic associated protein expression in WT and APP23 mice. Western blot analysis of CREB, SYN, SYNAP25 (a, b) and ERAB and p-CaMKII (c, d) protein expression in the brain of WT non-breeders (WT-NB), WT breeders (WT-B), APP23 non-breeders (APP-NB) and APP23 breeders (APP-B) at 12 months of age. The bar graphs present normalized density of each target protein with value as mean ± SEM. Asterisk indicates a p value of <0.05 compared to genotype-matched non-breeders
To further understand the effect of reproductive activity on regulation of cognitive-associated proteins, we extended our investigation to ERAB (HSD17B10) and phosphorylated CaMKII. As shown in Fig. 6c and d, there was an increase in ERAB (dimer) level in APP23 breeders compared to that in APP23 non-breeders (p < 0.05), and no effect of fertility on p-CaMKII was found in APP23 and WT mice.
Discussion
To assess the specificity of spatial working memory related to the fertility activity, we chose to test spontaneous alternation and spatial working memory in the Y maze and spatial reference and working memories in Barnes maze to avoid water stress since water stress has been reported to modulate phosphorylation of proteins, including tau [34, 35]. To examine the effect of reproductive activity on AD related cognitive function, we examined learning and memory activities in APP23 transgenic mice with age-matched wild type mice as control group. Our data demonstrated, for the first time, that virgin APP23 mice (non-breeders) outperform APP23 mice with a history of having one or more litters (breeders) in both spontaneous alternation behavior (Fig. 1b) and spatial reference and working memories (Figs. 2 and 4). However, APP23 breeders showed no difference in spatial learning compared to age-matched APP23 non-breeders (Fig. 3), suggesting a functional-specific effect of reproductive activity on cognitive function. In addition, the effect of reproductive activity on cognitive function is genotype-dependent. In contrast to non-breeders outperforming breeders in APP23 mice, WT breeders outperformed WT non-breeders in alternation behavioral (Fig. 2a), recognition working memory (Fig. 2a and b), and spatial reference memory (Fig. 3). Together, our data is in support of several human reports that number of pregnancies is a risk factor for AD [3, 6, 37].
Reproductive experiences in females, pregnancy and giving birth, constitute special conditions in women's lives that affect various physiological and endocrinological systems. The reproductive hormones produced during pregnancy and postpartum period cause changes in the brain, such as increasing of the size of the brain cell bodies, extending the length of dendritic branches, and altering of neurogenesis in several brain regions [38–40]. The reproductive activity associated changes of cognitive function with ageing have also been reported in human studies as well as animal investigations with some controversial findings. For example, in human studies, pregnant women showed impairments in verbal memory [41], word fluency, word list learning [42, 43], and performance on priming tasks and incidental learning tasks [44, 45], when compared to non-pregnant controls. Other human studies with a smaller sample size showed no differences in memory performance between pregnant subjects and non-pregnant controls [46, 47]. However, findings from animal studies showed an improved cognitive performance in pregnant rats as well as in the rats during postpartum period [8, 48–51]. It is noteworthy that human studies of reproductive activity in risk of AD are most weakened by their lack of information about contraception usage in the nulliparous control females who had no past fertility history. Nulliparous females are more likely to take birth control pills as contraceptives and these pills contain mixed sex hormones that can impact verbal and spatial abilities [30, 31]. While it is unclear what causes the discrepancies in findings of cognitive function between human and animal studies, our studies on the effect of fertility on cognitive function in AD transgenic mouse model marks the first demonstration of a direct evidence that reproductive activities impairs both spatial working and reference memories in aged female APP23 mice which is in support of several human reports that number of pregnancies is a risk factor for AD.
In addition, our studies also showed an age-dependent effect of fertility on cognitive function, showing that reproductive activity had greater effects on cognitive function in older mice (12 months old) than in younger mice (6 months). The age-dependent effect of reproductive activity on cognition is also consistent with previous reports which found that reproductive experience induced behavioral changes last longer than the postpartum or mother care periods and may have important impacts later in life [17, 52]. Furthermore, a recent longitudinal clinical study demonstrated an interesting correlation between an early serum estrogen levels and cognitive decline years later in aged women [53], providing more clinical support of a long-lasting impact of endogenous estrogen on cognitive function.
To further understand whether the reproductive activity in APP23 mice alters neuropathological processes in the brain, we examined the Aβ plaques in the brain of APP23 breeders and non-breeders at 12 months of age. In line with our cognition data, APP23 breeders developed greater numbers and larger size of Aβ plaques in both the hippocampus and cortex as shown in Fig. 5. Although it is unclear by what mechanisms fertility affected brain Aβ deposition and plaque formation, our recent studies of APP23 mice have not only demonstrated an objective evidence of the influence of reproductive activity on learning and memory function in AD, but also presented a pathological evidence of fertility-related change of AD pathology in the brain. Our data, for the first time, suggested a potential underlying mechanism of the spatial memory impairment found in the APP23 breeders.
Spontaneous alternation, spatial working and reference memories are hippocampal and cortex dependent cognitive functions. To understand the genotype-dependent effect of fertility activity on brain synaptic plasticity, we examined several synaptic associated proteins by Western blot analysis. As shown in Fig. 6b, the expression of synaptophysin (SYP), the major synaptic vesicle protein, is up-regulated in WT breeders compared to WT non-breeders, while a down-regulation of SYP and CREB expressions was found in APP23 breeders compared to APP23 non-breeders. There is no difference in synaptosomal-associated protein 25 (SNAP25) between breeders and non-breeders, suggesting that fertility did not affect the trans-SNARE complex, which is important in recruiting synaptic vesicle and plasma membranes together to form a tight complex. However, the up-regulation of SYP in WT breeders suggests a possible mechanism of enhanced cognitive function observed in behavioral studies. In contrast, a down-regulation of SYP in APP23 breeders might contribute to the cognitive impairment identified in the Y maze and Barnes maze studies. The CREB protein is a crucial transcription factor regulating the expression of genes involved in neuronal growth and plasticity and associates with spatial memory. CREB in neurons is necessary for the late stage of long-term potentiation [54]. Impairment of CREB phosporylation may be a pathological component in AD, and the pharmacological induction of CREB phosphorylation has been proposed as a therapeutic approach for AD [55]. Whether the reduction of CREB expression levels found in APP23 breeders was induced by the down-regulation of phosphorylation of CREB requires further investigation. Moreover, we also examined phophorylated Ca2+/calmodulin-dependent protein kinase II (p-CaMKII) protein expression in aged APP23 and WT mice, and observed an up-regulation of p-CaMKII in APP23 breeders compared to APP23 non-breeders as shown in Fig. 6d. Because the activity of CaMKII is essential in learning and memory and the expression of p-CaMKII is critical for synaptic plasticity [56–58], an up-regulation of p-CaKMII in APP23 breeders is an unexpected surprise. Recent studies reported that the levels of p-CaMKII protein were decreased in hippocampus the CA1 and CA3 brain regions of AD and vascular dementia models [59], and the p-CaMKII expression level in the hippocampus is associated with noise-induced stress [60], suggesting brain region-specific effects of p-CaMKII in the brains. Brain region-specific expression of p-CaMKII was also reported in other animal models [61]. Whether the overall increase in p-CaMKII protein level found in the brains of APP23 breeders represents any brain region-specific level changes of p-CaMKII needs to be further examined. It is possible that a down-regulation of p-CaMKII expression in the hippocampus caused a complementary up-regulation of p-CaMKII in other brain regions.
In summary, our studies show that cognitive function in females is influenced by past fertility activities in an age- and genotype-dependent manner. The effect of fertility on cognitive function lasts longer than the postpartum period and becomes even more affective later in life. Our studies of fertility's influence on cognitive function in APP23 mice suggested, for the first time, that reproductive activity causes cognitive impairment with no interference of any extra estrogen uptake, such as birth control pills in human studies, and this fertility-induced cognitive impairment is associated with promoting AD pathology and down-regulation of synaptic associated protein expression in the brain. In contrast, WT mice with a history of giving birth outperformed WT non-breeders, showing better cognitive performance and up-regulation of synaptic proteins. It is known that the changes of sex hormones during pregnancy and postpartum periods promote neurogenesis and improve neuronal network [38, 48, 50]. It is unknown if and how AD-related pathogenesis, such as overproduction of Aβ by gene mutation, prevent and even reverse the fertility-induced up-regulation of synaptic proteins in later of life. While the molecular mechanisms of the fertility-related changes in cognitive function remain unknown and the APP23 mouse line is one of transgenic models of AD amyloid pathogenesis which might not completely present human AD, dramatic changes of synaptic associated proteins may contribute to the reproductive experience induced alteration of cognitive functions.
Materials and Methods
Animal
The generation of B6,D2-TgN(Thy1-APPSwe) transgenic mouse line (APP23) has been described previously [33]. The APP23 mice have been bred for three generations with animals of the same background. Generation 4 animals will also be bred with C57Bl/6 (Jackson Laboratory) for five generations to produce a line with a more inbred background for this study. The APP23 line selected for this study expresses 7-fold more APP23, form typical plaques at 9 months, and the formation of plaques increases with age. In this study, all breeders had one or more litters before the age of 5 months. At age of 6 or 12 months, breeders and non-breeders (virgins) were tested for cognitive function. All mice were maintained on a 12 h:12 h reversed-light cycle (lights off at 0800 h) with continuous free access to food chow and water.
Tissue Preparation and Immunohistochemistry
At the end of the behavioral tests, mice were anesthetized and their brains quickly removed and bisected. One hemisphere of brain tissue was fixed with 4 % paraformaldehyde for immunohistochemistry, and another half was frozen at –80 °C for biochemistry and molecular biological analysis. The fixed tissue was serially sectioned (15–30 μm in thickness) in the sagittal plane with a Leica CA 1900 cryostat. Eight to ten sections (about 400 μm apart) were immunostained for Aβ (6E10; 1:250; Invitrogen). The images were digitally documented, then processed with a Leica DMLS complementary software package (MagnaFire SP).
Western Blot Analysis
To extract total protein, brain samples were homogenized in buffer containing 1 % Nonidet P-40, 0.1 % SDS, 50 mM Tris (pH 8.0), 50 mM NaCl, 0.05 % deoxycholate, and protease inhibitors (Boehringer Mannheim). Extracts (20–40 μg protein) were subjected to polyacrylamide gel electrophoresis, and separated proteins were transferred onto nitrocellulose membranes, which were then immunostained with the following primary antibodies: polyclonal anti-ERAB (1:1,000; Novus), and anti-CAMKII (1:500; Santa Cruz), anti-CREB (1:1,000; Cell Signaling), anti-SNAP25 (1:1,000; Abcam), and anti-SYP (1:1,000; Sigma). The membranes were incubated with peroxidase-conjugated secondary antibodies (1:1,000; Santa Cruz), and immunoreactive bands were visualized with an ECL system.
Behavioral Tests
Y Maze
The Y maze was used for initial memory study by detecting spontaneous alternation as well as recognition memory as described by others [62, 63]. A Y-shaped maze with three white plastic arms at a 120° angle from each other was used. Y-maze alternation was done by introducing a mouse in the center of the maze, and the mouse was allowed to freely explore the three arms for 5 min. The number of arm entries and the number of triads were recorded in order to calculate the percentage of alternation. Spontaneous alternation is a natural, hippocampal-dependent behavior of rodents in which they tend not to repeat exploration of a region that has no reward. The percentage spontaneous alternation was defined as the ratio of successive entries into each of the three arms on overlapping triplet sets (actual total alternations) to possible alternation (total arm entries–2) × 100. Total entries were also scored as an index of ambulatory activity in the Y maze. We also performed the two-trial recognition memory test. In details, the mouse starts at the end of one arm (start arm), then chooses one of the other two arms. To test if mice prefer to spend time in novel or known areas, one arm of the Y maze is blocked off and the mouse is allowed to explore the other two arms for 15 min. The mouse was returned to the maze 2 h later with all arms open and scored for 5 min. The amount of time spent in each arm, and the number of entries into each arm were recorded. Seven days later, the test was repeated with a delay time of only 2 min between the trials. The number of arm entries and time in the novelty arm were recorded. An arm entry was recorded when all four limbs were within the arm.
Barnes Maze
The Barnes maze is a dry-land maze test for spatial learning and memory that requires mice to learn the position of a hole that can be used to escape from the bright light [64]. The Barnes table contained a total of 20 holes including one target (escape) hole that leads to a dark cage and 19 empty holes. The latencies to enter the escape box (target latency) and the number of errors (defined as visits to any non-target hole) were recorded by a video camera mounted directly over the center of the maze. A Video Tracking System (ViewPoint Life Sciences) was used to analyze all the parameters mentioned above as well as the distance an animal moved and the amount of time the animal spent ambulating and freezing. In the cued version of the Barnes maze, the location of the tunnel was indicated by a prominent visual cue on the wall next to the target hole for the animal to develop a spatial map of the extra-maze cues and then used to locate the target hole with hidden tunnel. A spatial learning ability test may address reference memory, which refers to learning the rules associated with a task, such as the location of the target hole remaining the same between testing sessions, and the entire testing time. The test includes two parts: spatial learning acquisition and spatial reference memory. For the learning and acquisition, mice were tested daily for 4 days. Mice were engaged in spatial learning acquisition trails, in which each mouse was allowed 3 min to find or be guided into the target hole. The learning trial was repeated four times a day, with 15–20 min between trials. For the spatial memory, mice were recorded for 90 s on day 5 and day 12 as short- and long-term memory trial, respectively. Target latency (time to locate the escape hole), search pattern (number of head poke for each hole nearby the escape hole), and total errors (total number of head deflections into non-target holes) were recorded and analyzed.
Statistical Analysis
All results are expressed as mean ± SEM (standard error of the mean). Statistical calculations were performed with the software Sigmastat (Systat Softwares Inc.). For multi-group comparisons, data were analyzed for statistical significance using two-way analysis of ANOVA. If there were significant values, post-hoc Bonferroni's or Dunnett's multiple comparison tests were subsequently used. The criterion for statistical significance was p < 0.05 in all evaluations.
References
Letenneur L, Gilleron V, Commenges D, Helmer C, Orgogozo JM, Dartigues JF (1999) Are sex and educational level independent predictors of dementia and Alzheimer's disease? Incidence data from the PAQUID project. J Neurol Neurosurg Psychiatry 66(2):177–83
Hy LX, Keller DM (2000) Prevalence of AD among whites: a summary by levels of severity. Neurology 55(2):198–204
Colucci M, Cammarata S, Assini A, Croce R, Clerici F, Novello C, Mazzella L, Dagnino N, Mariani C, Tanganelli P (2006) The number of pregnancies is a risk factor for Alzheimer's disease. Eur J Neurol 13(12):1374–7
McLay RN, Maki PM, Lyketsos CG (2003) Nulliparity and late menopause are associated with decreased cognitive decline. J Neuropsychiatry Clin Neurosci 15(2):161–7
Corbo RM, Gambina G, Ulizzi L, Monini P, Broggio E, Rosano A, Scacchi R (2007) Combined effect of apolipoprotein e genotype and past fertility on age at onset of Alzheimer's disease in women. Dement Geriatr Cogn Disord 24(2):82–5
Ptok U, Barkow K, Heun R (2002) Fertility and number of children in patients with Alzheimer's disease. Arch Womens Ment Health 5(2):83–6
Love G, Torrey N, McNamara I, Morgan M, Banks M, Hester NW, Glasper ER, Devries AC, Kinsley CH, Lambert KG (2005) Maternal experience produces long-lasting behavioral modifications in the rat. Behav Neurosci 119(4):1084–96
Kinsley CH, Madonia L, Gifford GW, Tureski K, Griffin GR, Lowry C, Williams J, Collins J, McLearie H, Lambert KG (1999) Motherhood improves learning and memory. Nature 402:137–8
Pawluski JL, Galea LA (2006) Hippocampal morphology is differentially affected by reproductive experience in the mother. J Neurobiol 66(1):71–81
Price DL, Sisodia SS (1994) Cellular and molecular biology of Alzheimer's disease and animal models. Annu Rev Med 45:435–46, Review
Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M (1999) Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286(5440):735–41
Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE, Carter DB, Tomasselli AG, Parodi LA, Heinrikson RL, Gurney ME (1999) Membrane-anchored aspartyl protease with Alzheimer's disease beta-secretase activity. Nature 402(6761):533–7
Yang L-B, Lindholm K, Yan R, Citron M, Xia W, Konishi Y, Yang XL, Beach T, Sue L, Wang P, Price D, Li R, Shen Y (2003) Elevated β-secretase expression and enzymatic activity detected in sporadic Alzheimer's brains. Nat Med 9:3–4
Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R (2005) Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer's disease animal model. Proc Natl Acad Sci U S A 102(52):19198–203, Epub 2005 Dec 19
Boncristiano S, Calhoun ME, Howard V, Bondolfi L, Kaeser SA, Wiederhold KH, Staufenbiel M, Jucker M (2005) Neocortical synaptic bouton number is maintained despite robust amyloid deposition in APP23 transgenic mice. Neurobiol Aging 26(5):607–13
Wegenast-Braun BM, Fulgencio Maisch A, Eicke D, Radde R, Herzig MC, Staufenbiel M, Jucker M, Calhoun ME (2009) Independent effects of intra- and extracellular Abeta on learning-related gene expression. Am J Pathol 175(1):271–82
Li R, Cui J, Jothishankar B, Shen J, He P, Shen Y (2013) Early reproductive experiences in females make differences in cognitive function later in life. J Alzheimers Dis 34(3):589–94
Amada N, Aihara K, Ravid R, Horie M (2005) Reduction of NR1 and phosphorylated Ca2+/calmodulin-dependent protein kinase II levels in Alzheimer's disease. Neuroreport 16:1809–13
Novak G, Seeman P, Tallerico T (2006) Increased expression of calcium/calmodulin-dependent protein kinase IIbeta in frontal cortex in schizophrenia and depression. Synapse 59:61–8
Suda S, Segi-Nishida E, Newton SS, Duman RS (2008) A postpartum model in rat: behavioral and gene expression changes induced by ovarian steroid deprivation. Biol Psychiatry 64:311–9
Takahashi E, Niimi K, Itakura C (2009) Enhanced CaMKII activity and spatial cognitive function in SAMP6 mice. Behav Neurosci 123:527–32
Hardingham GE, Bading H (2003) The Yin and Yang of NMDA receptor signalling. Trends Neurosci 26:81–9
Luine V, Frankfurt M (2012) Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience October 16
Lonze BE, Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35:605–23
Jin SH, Blendy JA, Thomas SA (2005) Cyclic AMP response element-binding protein is required for normal maternal nurturing behavior. Neuroscience 133:647–55
Hovorkova P, Kristofikova Z, Horinek A, Ripova D, Majer E, Zach P, Sellinger P, Ricny J (2008) Lateralization of 17beta-hydroxysteroid dehydrogenase type 10 in hippocampi of demented and psychotic people. Dement Geriatr Cogn Disord 26(3):193–8
Kristofiková Z, Bocková M, Hegnerová K, Bartos A, Klaschka J, Rícný J, Rípová D, Homola J (2009) Enhanced levels of mitochondrial enzyme 17beta-hydroxysteroid dehydrogenase type 10 in patients with Alzheimer disease and multiple sclerosis. Mol Biosyst 5(10):1174–9
He XY, Wen GY, Merz G, Lin D, Yang YZ, Mehta P, Schulz H, Yang SY (2002) Abundant type 10 17 beta-hydroxysteroid dehydrogenase in the hippocampus of mouse Alzheimer's disease model. Brain Res Mol Brain Res 99(1):46–53
Yang SY, He XY, Miller D (2007) HSD17B10: a gene involved in cognitive function through metabolism of isoleucine and neuroactive steroids. Mol Genet Metab 92(1–2):36–42
Wharton W, Hirshman E, Merritt P, Doyle L, Paris S, Gleason C (2008) Oral contraceptives and androgenicity: influences on visuospatial task performance in younger individuals. Exp Clin Psychopharmacol 16(2):156–64
Griksiene R, Ruksenas O (2011) Effects of hormonal contraceptives on mental rotation and verbal fluency. Psychoneuroendocrinology 36(8):1239–48
Moran PM, Higgins LS, Cordell B, Moser PC (1995) Age-related learning deficits in transgenic mice expressing the 751-amino acid isoform of human beta-amyloid precursor protein. Proc Natl Acad Sci U S A 92(12):5341–5345
Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Bürki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B (1997) Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci U S A 94(24):13287–92
Korneyev AY (1998) Stress-induced tau phosphorylation in mouse strains with different brain Erk 1 + 2 immunoreactivity. Neurochem Res 23:1539–1543
Okawa Y, Ishiguro K, Fujita SC (2003) Stress-induced hyperphosphorylation of tau in the mouse brain. FEBS Lett 535:183–189
Silva AJ, Kogan JH, Frankland PW, Kida S (1998) CREB and memory. Annu Rev Neurosci 21:127–48
Sobow T, Kloszewska I (2004) Parity, number of pregnancies, and the age of onset of Alzheimer's disease. J Neuropsychiatry Clin Neurosci 16(1):120–1
Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S (2003) Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 299:117–20
Prange-Kiel J, Rune GM (2006) Direct and indirect effect of estrogen on rat hippocampus. Neuroscience 138:765–772
Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, Miyamoto O, Itano T, Matsui H (2003) Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci 6:384–90
Henry JD, Rendell PG (2007) A review of the impact of pregnancy on memory function. J Clin Exp Neuropsychol 29:793–803
de Groot RH, Hornstra G, Roozendaal N, Jolles J, de Groot RHM, Hornstra G, Roozendaal N, Jolles J (2003) Memory performance, but not information processing speed, may be reduced during early pregnancy. J Clin Exp Neuropsychol 25:482–8
Henry JF, Sherwin BB (2012) Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci 126:73–85
Sharp K, Brindle PM, Brown MW, Turner GM (1993) Memory loss during pregnancy. Br J Obstet Gynaecol 100:209–15
Buckwalter JG, Buckwalter DK, Bluestein BW, Stanczyk FZ (2001) Pregnancy and post partum: changes in cognition and mood. Prog Brain Res 133:303–19, Review
McDowall J, Moriarty R (2000) Implicit and explicit memory in pregnant women: an analysis of data-driven and conceptually driven processes. Q J Exp Psychol 53:729–40
Christensen H, Leach LS, Mackinnon A (2010) Cognition in pregnancy and motherhood: prospective cohort study. Br J Psychiatry 196:126–32
Lemaire V, Billard JM, Dutar P, George O, Piazza PV, Epelbaum J, Le Moal M, Mayo W (2006) Motherhood-induced memory improvement persists across lifespan in rats but is abolished by a gestational stress. Eur J Neurosci 23:3368–74
Paris JJ, Frye CA (2008) Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction 136:105
Darnaudéry M, Perez-Martin M, Del Favero F, Gomez-Roldan C, Garcia-Segura LM, Maccari S (2007) Early motherhood in rats is associated with a modification of hippocampal function. Psychoneuroendocrinology 32:803–12
Gatewood JD, Morgan MD, Eaton M, McNamara IM, Stevens LF, Macbeth AH, Meyer EA, Lomas LM, Kozub FJ, Lambert KG, Kinsley CH (2005) Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Res Bull 66:91–8
Heys M, Jiang C, Cheng KK, Zhang W, Au Yeung SL, Lam TH, Leung GM, Schooling CM (2011) Lifelong endogenous estrogen exposure and later adulthood cognitive function in a population of naturally postmenopausal women from Southern China: the Guangzhou Biobank Cohort Study. Psychoneuroendocrinology 36(6):864–73
Laughlin GA, Kritz-Silverstein D, Barrett-Connor E (2010) Higher endogenous estrogens predict four year decline in verbal fluency in postmenopausal women: the Rancho Bernardo Study. Clin Endocrinol (Oxf) 72(1):99–106
Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ (1994) Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79(1):59–68
Scott BR (2012) Cyclic AMP response element-binding protein (CREB) phosphorylation: a mechanistic marker in the development of memory enhancing Alzheimer's disease therapeutics. Biochem Pharmacol 83(6):705–14
Ninan I, Arancio O (2004) Presynaptic CaMKII is necessary for synaptic plasticity in cultured hippocampal neurons. Neuron 42:129–141
Giese KP, Fedorov NB, Filipkowski RK, Silva AJ (1998) Autophosphorylation at Thr286 of the alpha calcium–calmodulin kinase II in LTP and learning. Science 279:870–873
Lisman J, Schulman H, Cline H (2008) The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 3:175–190
Min D, Guo F, Zhu S, Xu X, Mao X, Cao Y, Lv X, Gao Q, Wang L, Chen T, Shaw C, Hao L, Cai J (2013) The alterations of Ca(2+)/calmodulin/CaMKII/Ca(V)1.2 signaling in experimental models of Alzheimer's disease and vascular dementia. Neurosci Lett Feb 8
Di G, Zheng Y (2013) Effects of high-speed railway noise on the synaptic ultrastructure and phosphorylated-CaMKII expression in the central nervous system of SD rats. Environ Toxicol Pharmacol 35(1):93–9
Narita M, Matsumura Y, Ozaki S, Ise Y, Yajima Y, Suzuki T (2004) Role of the calcium/calmodulin-dependent protein kinase ii (CaMKII) in the morphine-induced pharmacological effects in the mouse. Neuroscience 126(2):415–21
Paul CM, Magda G, Abel S (2009) Spatial memory: theoretical basis and comparative review on experimental methods in rodents. Behav Brain Res 203(2):151–64
Olton DS (1979) Mazes, maps, and memory. Am Psychol 34:583–596
Barnes CA (1979) Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol 93(1):74–104
Acknowledgments
This work was supported by grants from the Alzheimer's Association IIRG-07-59510, American Health Assistance Foundation Grant G2006-118, NIH R01AG032441, NIH R01AG025888. We thank Mr. Alex Bishop for editing and proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, J., Jothishankar, B., He, P. et al. Amyloid Precursor Protein Mutation Disrupts Reproductive Experience-Enhanced Normal Cognitive Development in a Mouse Model of Alzheimer's Disease. Mol Neurobiol 49, 103–112 (2014). https://doi.org/10.1007/s12035-013-8503-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8503-x