Abstract
Brain-derived neurotrophic factor (BDNF), belonging to the neurotrophic family of growth factors, has a widespread distribution in the central and peripheral nervous systems. In central motor structures including the motor cortex, cerebellum, basal ganglia, and spinal cord, BDNF exerts both neurotrophic and direct electrophysiological effects via a high-affinity tyrosine receptor kinase B receptor and a common low-affinity p75 neurotrophin receptor. The underlying signaling pathways mainly involve mitogen-activated protein kinase cascades, phosphatidylinositol 3-kinase pathway, and phospholipase C-γ pathway. The loss of BDNF usually leads to neurodegeneration in these motor centers and eventually results in several severe motor diseases, such as amyotrophic lateral sclerosis, spinocerebellar ataxias, Parkinson’s disease, Huntington’s disease, as well as vestibular syndrome. In this review, we summarize the recent understanding of functions of BDNF in motor structures and suggest that BDNF may be a potent candidate for the treatment of these neurodegenerative motor diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain-derived neurotrophic factor (BDNF) is the second found member of the neurotrophic family of growth factors and was first purified by Barde et al. from pig brain in 1982 [1] and cloned by the same group in 1989 [2]. Constituted by homodimeric proteins (monomeric size of mature protein, 112 amino acids; molecular weight, approximately 13 kDa), BDNF is initially synthesized as a glycosylated precursor, proBDNF, and then cleaved to form mature BDNF intracellularly or extracellularly [3]. Both proBDNF and mature BDNF are biologically active and have distinct biological functions [3].
BDNF has a rich distribution in the central and peripheral nervous systems and plays neurotrophic and other physiological roles in various brain regions. Links between BDNF and many diseases, such as depression, Alzheimer’s disease, epilepsy, and drug addiction, have been well revealed and attract great attention [4–6]. However, the role of BDNF in motor system and motor control, one of major functions of the brain and basis for executions of behaviors, as well as in various neurodegenerative motor diseases, has not been fully known. Actually, accumulating data indicate that BDNF also holds a key position in central motor structures and ablation of BDNF could lead to severe motor deficits. In this review, we mainly focus on the function of BDNF in motor-related neurons. Progress in experimental and clinical studies on connections between BDNF and motor diseases is also summarized and discussed.
Neurotrophic Effect of BDNF on Motor-Related Neurons and its Underlying Signaling Pathways
BDNF mRNA is extensively distributed in motor-related neurons in the brain, such as neurons in the cerebellum, basal ganglia, brain stem, and even the spinal cord [7]. As expected, several lines of evidence indicate that BDNF has a significant neurotrophic action on these motor-related neurons. The growth cones of cultured spinal neurons show a chemotropic behavior in the presence of BDNF gradients, hinting a rapid modulation of neuronal morphology and motility by BDNF [8], and treatment with BDNF even prevents the death of spinal motoneurons in vivo [9]. Applying BDNF to lesioned corticospinal neurons significantly increases collateral growth [10]. In the cerebellum, BDNF, acting as a survival factor, regulates morphologic development and differentiation of cerebellar granule and Purkinje cells and thereby affects neural patterning in the cerebellum [11]. BDNF has also been proved to provide neurotrophic support for dopaminergic neurons in both the ventral tegmental area and the medial substantia nigra pars compacta [12]. BDNF loss elicits an array of striatal developmental malformations, including morphological damage and motor deficits [13].
There are two distinct categories of transmembrane receptor for BDNF in the mammalian brain, namely, a high-affinity tyrosine receptor kinase B (TrkB) receptor and a common low-affinity p75 neurotrophin receptor (p75NTR) [14], respectively. TrkB is one of the three members in the Trk family of receptor tyrosine kinase and is activated by BDNF and neurotrophin-4 [15]. In contrast with the specificity displayed by TrkB, p75NTR binds to each of the neurotrophins with approximately equal affinity [14]. However, proBDNF binds to p75NTR with higher affinity than BDNF [3]. Both TrkB and p75NTR receptors are abundantly and extensively expressed in the developing and adult mammalian brain, including many motor structures, yet play different physiological functions via different coupled signaling pathways [14].
TrkB Receptors Mediated Signaling Pathways in Motor-Related Neurons
It has been well known that binding of BDNF to TrkB promotes receptor homodimerization, and then induces tyrosine phosphorylations in the cytoplasmic domain [15]. Serving as a scaffold, the phosphorylated TrkB recruits different adaptor proteins and enzymes in turn, leading to the activation of parallel downstream signaling transduction pathways [15]. In the central nervous system, three main signaling cascades coupled to TrkB receptors, including mitogen-activated protein kinase (MAPK) cascades, phosphatidylinositol 3-kinase (PI3K) pathway, and phospholipase C-γ (PLC-γ) pathway, have been identified [15] (Fig. 1). These signaling pathways have also been found in various central motor structures. Through these intracellular signaling transductions, BDNF plays a survival role and exerts multiple physiological functions in motor-related neurons.
BDNF, BDNF receptors, and its underlying signal transduction pathways in the motor control system. The effects of BDNF are mediated through two different sorts of transmembrane receptors, a high-affinity TrkB and a common low-affinity p75NTR. Three main signaling cascades, including MAPK cascades, PI3K pathway, and PLC-γ pathway, identified in the central nervous system are involved in signal transduction in the spinal cord (red line). In the cerebellum (green line), bound by BDNF, TrkB phosphorylates the PLC–PKC axis and MAPK route to promote cell survival. In the basal ganglia (blue line), there are two pathways triggered by BDNF, a Ras–MEK–MAPK axis and a PI3K/Akt axis. In contrast with the Trk receptors, p75NTR depends on the NF-κB and Akt pathways to promote cell survival and on caspase, JNK, and FADD to promote apoptosis. Akt also known as protein kinase B, BDNF brain-derived neurotrophic factor, p75NTR p75 neurotrophin receptor, CREB cAMP response element binding protein, DAG dystrophin-associated glycoprotein, ER endoplasmic reticulum, FADD Fas-associated death domain, FKHRL1 the forkhead transcription factor, IκB inhibitor of kappa B, JNK c-Jun N-terminal kinase, MAPK mitogen-activated protein kinase, MEK also known as MAPKK, NF-κB the nuclear factor-kappa B, NMDA-R N-methyl-d-aspartate receptor, PI3K phosphatidylinositol 3-kinase, PKC protein kinase C, PLC-γ phospholipase C-γ, Ras a GTPase, Rsks ribosomal S6 kinase family, BAD Bcl-xL/Bcl-2-associated death promoter, TrkB tyrosine receptor kinase B
The cerebellum is a classic subcortical motor structure that contributes to coordination and accuracy of movements as well as motor learning [16]. Damage to the cerebellum produces disorders in fine movement, equilibrium, posture, and motor learning [16]. In the cerebellar cortical circuit, granule cells are the smallest but most numerous neurons and exert an excitatory effect on Purkinje cells, the principal (projection) neurons of the cerebellar cortex [16]. It has been reported that, in granule cells, TrkB receptor signaling triggered by BDNF is coupled to the PLC-γ axis and the activated PLC-γ hydrolyses its substrate to generate inositol triphosphate and diacylglycerol. Subsequently, intracellular calcium ions are released from internal stores and protein kinase C (PKC) is activated to promote cell survival [17, 18]. In addition, BDNF can also trigger the Ras (a GTPase)–mitogen-activated protein kinase kinase (MEK)–MAPK cascade in cerebellar granule neurons. Then, MAPK-activated ribosomal S6 kinases phosphorylate B cell lymphoma-extra large (Bcl-xL)/Bcl-2-associated death promoter (BAD) and the cAMP response element binding protein to promote cell survival [19].
Basal ganglia constitutes another important subcortical side loop responsible for the initiation of voluntary movements and procedural learning [20]. Its dysfunction causes several severe motor disorders including Parkinson’s disease (PD) and Huntington’s disease (HD) [21]. In the striatum and substantia nigra, TrkB receptors bound to BDNF initiate two dominating anti-apoptotic signaling cascades, PI3K/Akt (also known as protein kinase B) axis or MAPK/extracellular signal-regulated kinase (ERK) pathway. Subsequently, the phosphorylated BAD at Ser136 or Ser112 promotes the release of Bcl-2 and Bcl-xL, leading to the inhibition of the apoptotic pathways [22]. Thus, BDNF enhances the survival of the degenerating dopaminergic neurons [22].
In spinal motoneurons, the final common path for motor commands from various high motor centers and reflexive signals from the spinal cord itself [23], BDNF contributes to the restoration of locomotive function through the activation of the ERK signaling pathway coupled to TrkB receptors [24]. Another study also reveals that the PI3K/Akt signaling cascade is involved in the survival of the spinal motoneurons responding to BDNF. In the cascade, the activated signal molecules consist of BAD, inhibitor of kappa B, and the forkhead transcription factor, FKHRL1 [25]. In neonatal rat spinal cord slices probed with electrophysiological recordings, it has been reported that BDNF can phosphorylate N-methyl-d-aspartate (NMDA) receptors to facilitate synaptic efficacy via the MEK-ERK and PLC-γ/PKC pathways [26].
p75NTR Receptors Mediated Signal Transduction Pathways in Motor-Related Neurons
Unlike the Trk receptors, p75NTR plays a dual role in cell survival, either protective or apoptotic [27], suggesting that its signaling pathways are quite distinct from those activated by the Trk receptors. Nevertheless, the pro-apoptotic pathways are predominant [27]. The signaling mechanism of p75NTR in motor-related neurons remains to be ambiguous up to date. The pro-survival role of p75NTR in motor structures is thought to rely on the nuclear factor-kappa B and Akt pathways [27], whereas the precise signaling pathway to promote apoptosis may be independent of Trk signaling cascades and involves caspase, c-Jun N-terminal kinase [27], and Fas-associated death domain protein [28].
Direct Electrophysiological Effects of BDNF on Motor-Related Neurons
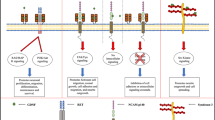
The neurotrophic function of BDNF is so important that its direct electrophysiological effect has been almost ignored. However, there are studies revealing that BDNF plays a direct and vital role on neuronal excitability, synaptic transmission, and even synaptic plasticity in motor-related neurons (Fig. 2).
Schematic illustration of the electrophysiological effects of BDNF on central motor structures. BDNF plays a direct and vital role on neuronal excitability, synaptic transmission, and synaptic plasticity in neurons of central motor structures, including the cerebellum, spinal cord, basal ganglia, as well as motor cortex. In the cerebellum (green dashed box), BDNF couples to the TrkB receptor to increase neuronal excitability, dually affect GABAA IPSCs, enhance GABAA mIPSCs postsynaptically, as well as promote STP in PF-PC synapses presynaptically. In the spinal cord (red dashed box), BDNF induces an enhancement of neuronal excitability and an initial brief facilitation followed by a long-lasting depression of the DR-evoked glutamatergic EPSPs. In the basal ganglia (blue dashed box), neuronal excitability and LTP in corticostriatal synapses are promoted by BDNF. In the motor cortex (purple dashed box), when transcranial direct current stimulation is delivered to the M1, BDNF promotes NMDA-dependent LTP. Cdk5 cyclin-dependent kinase 5, DR dorsal root, EPSPs excitatory postsynaptic potentials, GABA A -R γ-aminobutyric acid receptor, Glu-R glutamine receptor, IPSCs inhibitory postsynaptic currents, KA-R kainate receptor, KCC2 K+–Cl− cotransporter 2, LTP long-term potentiation, M1 primary motor cortex, NMDA-R N-methyl-d-aspartate receptor, PC Purkinje cell, PF parallel fiber, STP short-term potentiation
BDNF can potentiate the spontaneous firing activity of neurons in the spinal cord ventral horn, cerebellum, as well as basal ganglia. Chronic BDNF treatment enhances the excitability of spinal cord neurons by activating Na+ and Ca2+ channels and then improves hind limb locomotion [29]. BDNF also elicits a rapid depolarization of cerebellar Purkinje cells by activating Na+ channels [30]. Chronic BDNF infusion increases firing rate and burst activity of active dopaminergic neurons and even initiates action potentials in the silent dopaminergic neurons in the substantia nigra pars compacta and striatum [31].
In addition, BDNF has an important effect on synaptic transmission and plasticity in the motor cortex, subcortical motor structures, as well as peripheral neuromuscular junctions. Human BDNF val66met polymorphism, a single-nucleotide polymorphism with a substitution of valine-to-methionine at codon 66, is related to abnormal training-induced motor cortex plasticity with a decrease of motor-evoked potential and motor map reorganization in corticospinal output [32]. When transcranial direct current stimulation is delivered to the primary motor cortex, activity-dependent BDNF secretion promotes NMDA-dependent long-term potentiation (LTP)/long-term depression and motor skill learning [33].
In the cerebellum, BDNF superfusion is effective in enhancing the amplitude of GABAergic miniature inhibitory postsynaptic currents (mIPSCs) as a result of co-activation of TrkB and Src (nonreceptor tyrosine kinases) in Purkinje cells at postnatal days 13–16 [34]. In addition, depending on the involvement of K+–Cl− cotransporter and Ca2+ or not, BDNF inhibits or potentiates GABAergic transmission (GABAA IPSCs) in a cyclin-dependent kinase 5-dependent manner in cerebellar Purkinje neurons [35]. Since the short-term and long-term change of Cl− concentration in Purkinje cells is a key factor of GABAergic transmission, BDNF may modulate ionic plasticity of cerebellar Purkinje neurons [35]. Moreover, paired-pulse facilitation, a form of short-term plasticity (STP), is significantly decreased in BDNF knockout mice at parallel fiber to Purkinje cell (PF-PC) synapses in the cerebellar cortex. This BDNF-mediated STP at PF-PC synapses is mediated by presynaptic TrkB receptors [36]
BDNF enhances NMDA receptor-mediated excitatory postsynaptic potentials (EPSPs) on the medium spiny neurons of adult mice striatum and induces the production of LTP in corticostriatal synapses [37]. Experiments in the HD mouse model indicate that a complete integrity in the BDNF pathway is critical for the formation of LTP in corticostriatal circuitry [38].
Moreover, on spinal motoneurons in the neonatal (younger than 1 week) rat, BDNF induces an initial brief facilitation and a subsequent long-lasting depression of dorsal root-evoked monosynaptic EPSPs, which requires the involvement of postsynaptic and presynaptic NMDA receptors, respectively [39]. In the periphery, acute treatment of BDNF to cultured neuromuscular synapses within several minutes rapidly increases the frequency and amplitude of excitatory postsynaptic currents. In this process, canonical transient receptor potential channels are required specifically for BDNF-induced Ca2+ elevation and full synaptic potentiation [40, 41].
The direct electrophysiological effects of BDNF on motor-related neurons in various motor structures are usually mediated by TrkB receptors. However, the common ionic mechanisms underlying BDNF electrophysiological functions are complicated (Fig. 2) and their coupled signaling transduction pathways are still largely unknown. Presumably, via regulating neuronal excitability, synaptic transmission, and synaptic plasticity, BNDF may be consequently and functionally involved in the modulation of motor behaviors and motor learning. Therefore, in addition to neurotrophic effects, these electrophysiological actions and mechanisms of BDNF on motor-related neurons may also contribute to the physiological and pathophysiological significance of BDNF modulation on motor behaviors and motor functions.
BDNF and Motor Diseases
Many severe motor diseases, including amyotrophic lateral sclerosis (ALS), spinocerebellar ataxias (SCAs), PD, HD, as well as vestibular syndrome that belong to neurodegenerative diseases, seriously affect the normal human life, and many of them are even life-threatening. However, most treatments for these motor diseases so far are temporary medical palliatives and ineffective in stopping or reversing the degenerative process. Thus, neuroprotective therapies become a new trend against neurodegeneration, and BDNF, the most abundant neurotrophin in the brain, may be one of the potent candidates used for experimental, preclinical, and even clinical treatment for these neurodegenerative motor diseases (Fig. 3) [6, 42].
The distribution of BDNF in central motor structures and its related motor diseases. BDNF (red dots) has a wide distribution in the central and peripheral nervous systems and plays neurotrophic and other physiological roles in various brain regions, including almost all hierarchical structures in the motor control system, such as the cerebral motor cortex, cerebellum, basal ganglia, brain stem, and spinal cord. The loss of BDNF is associated with many severe motor diseases, including ALS, SCAs, PD, HD, as well as vestibular syndrome, and BDNF is a potential candidate for the treatment of these motor discords. ALS amyotrophic lateral sclerosis, HD Huntington’s disease, PD Parkinson’s disease, SCAs spinocerebellar ataxias
Amyotrophic Lateral Sclerosis
ALS is a devastating paralyzing disorder with typical feature of rapidly progressive degeneration of cortical, bulbar, and spinal motor neurons. Its clinical symptoms manifest as generalized muscle weakness and atrophy, speech and swallowing disabilities, and progressive paralysis until death caused by respiratory failure [43]. Yet the precise pathogenesis of the disease still remains enigmatic in most instances [44]. However, as ALS results from the degeneration of lower and upper motoneurons, neurotrophic growth factors, including BDNF, have long been considered as a therapeutic candidate for the disease.
It has been proved that a great mass of spinal motoneurons and upper motor structures degenerated in ALS express both BDNF and TrkB receptors [45]. Moreover, the expressions of BDNF and TrkB receptors exhibit many differences between controls and ALS patients. In ALS patients, 75 % of spinal motoneurons degenerate and the remaining ones exhibit decreased BDNF level [45]. Consistently, the high-affinity functional receptors for BDNF, TrkB receptors, are much less phosphorylated on tyrosine residues in ALS spinal cords than those in controls [46].
Furthermore, preclinical animal experiments have demonstrated that BDNF prevents lesion-induced degeneration of spinal motoneurons [47] and natural and experimental motoneuron disease characterized by slowed motor deterioration and motor axon degeneration in wobbler mice [48]. In addition, by using the gene manipulation technique, both p75NTR [49] and BDNF/TrkB [50] signaling have been proven to be effective in the treatment of ALS in animal models. Also, a latest study has found that truncated TrkB.T1 (one of alternative splices lacking the intracellular tyrosine kinase domain) deletion significantly slows the onset of motoneuron degeneration in a mouse model of ALS [51].
Besides a number of preclinical trials, BDNF has also been administered to groups of ALS patients. During a phase I through II research, it seemed that BDNF increased survival and retarded the loss of pulmonary function in ALS patients [52]. In the major multicenter clinical therapy trial of 1,135 ALS patients reported by the BDNF Study Group in 1999 (phase III), although intrathecal or subcutaneous BDNF injection to ALS patients brought about no significant improvement on the overall survival or benefit for the primary end points, subcutaneous infusion of BDNF did show statistical amelioration in subgroups of patients with early respiratory damage [46, 52].
The cause of this obscure efficacy of BDNF therapy on survival in clinical trials remains unclear to date. At least part of the reasons can be ascribed to the short half-life, immunogenicity, dose-dependent dual effect on neuronal survival versus apoptosis, undesired toxicity, limited ability to cross the blood–brain barrier after systemic delivery of these proteins and weak motoneuron responsiveness to endogenous TrkB.T1 in vivo [51, 53]. Therefore, new administration strategies such as secretion of BDNF from transplanted stem cells and Trk receptor transactivation may be used to improve the curative effect on ALS [51, 54].
Spinocerebellar Ataxias
SCAs are a group of progressive degenerative disorders characterized by incoordination of gait and often associated with poor coordination of hands, speech, and eye movements [55]. To date, over 30 SCAs have been identified by different loci of autosomal dominant SCA genes and have been named in chronological order of their discovery from SCA1 to SCA30 [55]. Although the specific pathogenic mechanisms of SCAs are still not fully understood, it has been recognized that SCAs generally result from the slow atrophy of the cerebellum, particularly the Purkinje cells.
Accumulating studies highlight the involvement of BDNF in the pathogenesis of SCAs. In the cerebellum of SCA6, in which Purkinje cell predominant neuronal loss is the characteristic neuropathology, BDNF mRNA is significant suppressed and BDNF protein forms abnormal definable granules [56]. The reduced BDNF gene expressions are also observed in SCA1 mouse models [57]. Furthermore, it has been reported that BDNF/TrkB interaction is present in both developing and adult cerebellum and distributes in the cerebellar cortex as well as the cerebellar nuclei, providing a powerful neuroanatomical basis for the regulation of BDNF in SCAs [58, 59]. In addition, numerous studies demonstrate a critical role of BDNF signaling in the maintenance of synaptic plasticity of Purkinje cells in the mature cerebellum [36], which also indicates possibilities of BDNF to counter the pathogenesis of SCAs. As an important endogenous synaptic modulator, BDNF regulates short-term synaptic plasticity as well as synaptic ultrastructure at the PF-PC synapses [36]. In vivo application of an antibody against BDNF decreases PF-PC synaptic strength [60]. Moreover, exogenous BDNF application rescues the abnormal dendritic morphology of Purkinje cells in IP3 receptor type 1 knockout mice, suggesting a novel role of BDNF produced in cerebellar granule cells on shaping the dendritic arborization of Purkinje cells [61].
The effect of BDNF on SCAs has already been investigated in animal models. There is evidence that the BDNF knockout and TrkB conditional knockout mice display deficits in motor coordination and balance, similar to the clinical symptoms of SCAs [62, 63]. Moreover, by increasing BDNF expression levels, motor behaviors of cerebellar ataxia mice models have been improved [57, 64]. Although BDNF has not yet been applied in clinical trials, considering that no effective therapy for SCAs has been discovered to date, BDNF, as a neurotrophic factor, may be a promising therapeutic agent for SCAs in the future [65].
Parkinson’s Disease
PD is a very common progressive neurodegenerative disorder characterized by selective degeneration of nigral dopaminergic neurons and perturbation of striatal circuits involved in motor control. The motor symptoms of PD include tremor, rigidity, akinesia, and postural instability. For the past 40 years, the most widely used clinical drug has always been the dopamine precursor levodopa. However, levodopa cannot halt the progressive degeneration of dopaminergic neurons and is limited by an increasingly narrow therapeutic window and onset of dyskinesia [66]. Thus, many current studies devote to the search for neuroprotective therapies that may stop or reverse the degenerative process in PD.
BDNF receptor complexes, TrkB and p75NTR, abundantly express in the striatum and substantia nigra [67, 68], providing a therapeutic opportunity of functional exogenous administration of BDNF. Early studies demonstrated that BDNF might support the survival of nigral dopaminergic neurons [12]. It has also been reported recently that BDNF signaling via its TrkB receptor tyrosine kinase is important for the survival of nigrostriatal dopaminergic neurons in an aging brain [69]. Moreover, BDNF/TrkB signaling protects dopaminergic cells from cell death induced by 6-hydroxydopamine (6-OHDA), regardless of whether the treatment is given before, during, or after toxin application. BDNF even shows an upregulation of the dopaminergic phenotype at transcriptional level [70]. All these studies demonstrate a possibility of application of BDNF as a neuroprotective therapy to stop or reverse the degenerative process in PD.
In fact, in PD patients, decreased BDNF protein and its mRNA have been identified in their substantia nigra [71, 72]. Moreover, the decreased level of BDNF correlates positively with the severity of dopaminergic neurodegeneration [73], indicating a causal relationship between BDNF level and dopamine neuronal loss in PD patients. Besides, Karamohamed et al. have reported that the genetic polymorphism of BDNF shows a significant influence on PD onset age, confirming the involvement of BDNF in the pathogenesis of PD [74].
Referring to the efficacy of BDNF therapy in rodent PD models, BDNF has been observed to retard the loss of nigrostriatal dopaminergic axons and to prevent the motor damage associated with PD after 6-OHDA or 1-methyl-4-phenylpyridinium (MPP)-induced lesion [75]. Furthermore, in MPP-induced Parkinsonism in nonhuman primates, intrathecal infusion of BDNF reduces loss of dopamine neurons and ameliorates symptoms [76]. The efficacy of BDNF in the treatment of PD has not been assessed in clinical research so far. Maybe an effective and safe method of delivering BDNF to the brain in the future would allow the evaluation of whether BDNF could be a potent therapeutic drug for PD in humans [42].
Huntington’s Disease
HD is a neurodegenerative disease caused by mutation in the huntingtin gene, which preferentially induces death of medium spiny neurons of the striatum and cerebral cortical neurons, finally leading to abnormal involuntary writhing movements characterized by chorea [6]. Preliminary study based on brain tissue samples of HD patients at autopsy indicated the region-specific downregulation of BDNF in the caudate and putamen [77]. Currently, a systematic and quantitative assessment of BDNF levels in postmortem human cerebral cortex samples confirms the impaired production of this neurotrophin in the brains of HD patients [6, 78]. Zuccato et al. propose that there exists a mechanistic link between BDNF and huntingtin, and the reduction in BDNF levels in HD is partly due to reduced wild-type huntingtin stimulatory activity [78]. Actually, huntingtin stimulates BDNF gene transcription through BDNF promoter II [6, 78]. Additionally, BDNF produced in the cerebral cortex can be anterogradely transported along the corticostriatal tract to the medium spiny neurons [7, 79]. Thus, the loss of huntingtin-mediated BDNF levels from cortical afferents to striatal neurons leads to insufficient neurotrophic action on striatal neurons, which is also responsible for the progressive neurodegeneration in the hallmark pathology of HD [78].
The findings previously described indicate that BDNF depletion is involved in the pathology of HD and that leads to preclinical studies aimed at evaluating the effect of BDNF in the treatment of HD. In studies with rodent HD models, BDNF has been found to be effective in both rescue of neuronal damage and motor symptoms improvement [6]. Firstly, daily intrastriatal administration of BDNF in HD mice partly survives enkephalinergic striatal neurons by its trophic benefit [80]. Secondly, therapies that can increase BDNF expression are able to improve motor deficits associated with the HD phenotype assessed by open field, rotarod, and balance beam tests [81, 82]. Moreover, conditional overexpression of BDNF suppresses HD pathology and extends life span in HD models [38, 83]. However, BDNF therapy has still not been examined in nonhuman primate models of HD, although it remains a reasonable candidate for therapy. And further clinical studies are still required to evaluate the efficacy of BDNF treatment in human HD.
Vestibular Syndrome
The vestibular syndrome contains graduated levels of damage from basic reflex dysfunction like ocular–motor or postural problems to higher-order perceptual deficits, and it is aggravated by neurovegetative disorders such as nausea and vomiting [84, 85]. Immunohistochemical studies demonstrate that, in adult rats, BDNF and TrkB receptors are expressed in both the inner ear and the vestibular ganglion neurons [68, 86]. The study on BDNF+/− and BDNF−/− mice in vivo and ELISA analysis provide evidence supporting the neurotrophic hypothesis of BDNF on vestibular neurons in embryonic and early postnatal stages [87, 88]. In BDNF or TrkB null mice, the vestibular ganglion cells are reduced age-dependently and there is no innervation of vestibular afferents to the vestibular nucleus [87], suggesting that BDNF/TrkB signaling plays a significant role in the survival of vestibular neurons and the regulation of vestibular function in later developmental stages [89, 90]. And the extensive loss of vestibular ganglion neurons in the BDNF mutant mice causes the deficiency in balance and coordination of movement at the age of 1 week [89]. These results from BDNF mutant animals suggest that the normal expression and secretion of BNDF is essential in maintaining basic vestibular functions.
Moreover, accumulating studies indicate a role of BDNF in counteracting vestibular disorder, especially in vestibular compensation, the recovery process to regain balance control and minimize dizziness symptoms following peripheral vestibular lesion. The level of BDNF in both lateral vestibular nucleus and medial vestibular nucleus elevates after unilateral labyrinthectomy, supporting the hypothesis that BDNF is involved in neuronal reorganization that allows vestibular compensation [91, 92]. Delivery of an antisense oligonucleotide to BDNF in the ipsilateral vestibular nucleus complex of guinea pigs delays vestibular compensation [93], whereas intra-vestibular nucleus administration of BDNF enhances the recovery from peripheral vestibular damage in guinea pig [94]. Therefore, through its positive involvement in the vestibular compensation process, BDNF could lead to progressive recovery of the vestibular syndrome.
Conclusion
BDNF plays a pivotal neurotrophic role in the central nervous system, including almost all hierarchical structures in the central motor system. Besides, BDNF also directly modulates electrophysiological activities of neurons in these central motor structures and even influences ionic, synaptic, and neuronal plasticity, although its underlying mechanisms still need to be further investigated. Considering that most of severe motor diseases, including the ALS, SCAs, PD, HD, and vestibular syndrome, are neurodegenerative disorders, BDNF may be considered as a potential therapeutic agent. The common mechanism of BDNF treatment derives from its pro-survival and anti-apoptotic pathways coupled to TrkB and p75NTR receptors which lead to an improvement of neuronal population survival and effective protection against neuronal cell degeneration in these central motor structures [65]. However, besides neurotrophic function, the direct electrophysiological effect of BDNF on motor-related neurons is also an important aspect need to be considered in therapeutic strategy.
Although BDNF treatment ameliorates the symptoms of motor diseases from in vitro observations and preliminary success in animal models of PD, HD, etc. [65, 77], the efficacy of BDNF treatment in clinical trials is currently insignificant. In the clinic, it is a very difficult task to effectively administer BDNF in humans, and previous large-scale clinical trials targeting at BDNF delivery have not been very successful, probably due to difficulty in brain–blood barrier crossing, degradation, and side effects. However, several potential safe and efficient delivery systems for BDNF treatment in humans are expected to be developed, such as chronic intraparenchymal infusion of BDNF via implantable hardware [95], BDNF gene delivery to the brain using viral vectors [96], the secretion of BDNF from transplanted stem cells [54], and peripheral administration of short peptide mimetics of BDNF which can cross the blood–brain barrier [97]. The study and effort on BDNF delivery and stability in clinical treatment attract more interest and are in the ascendant; the novel delivery strategies may greatly promote the future therapeutic uses of BDNF in motor diseases.
References
Barde YA, Edgar D, Thoenen H (1982) Purification of a new neurotrophic factor from mammalian brain. EMBO J 1:549–53
Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P et al (1989) Molecular cloning and expression of brain-derived neurotrophic factor. Nature 341:149–52
Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14:7–23
Binder DK (2004) The role of BDNF in epilepsy and other diseases of the mature nervous system. Adv Exp Med Biol 548:34–56
Castren E, Voikar V, Rantamaki T (2007) Role of neurotrophic factors in depression. Curr Opin Pharmacol 7:18–21
Zuccato C, Cattaneo E (2009) Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol 5:311–22
Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL et al (1997) Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 389:856–60
Ming GL, Wong ST, Henley J, Yuan XB, Song HJ, Spitzer NC et al (2002) Adaptation in the chemotactic guidance of nerve growth cones. Nature 417:411–8
Oppenheim RW, Yin QW, Prevette D, Yan Q (1992) Brain-derived neurotrophic factor rescues developing avian motoneurons from cell death. Nature 360:755–7
Vavrek R, Girgis J, Tetzlaff W, Hiebert GW, Fouad K (2006) BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain 129:1534–45
Schwartz PM, Borghesani PR, Levy RL, Pomeroy SL, Segal RA (1997) Abnormal cerebellar development and foliation in BDNF−/− mice reveals a role for neurotrophins in CNS patterning. Neuron 19:269–81
Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP et al (1991) BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 350:230–2
Li Y, Yui D, Luikart BW, McKay RM, Rubenstein JL, Parada LF (2012) Conditional ablation of brain-derived neurotrophic factor-TrkB signaling impairs striatal neuron development. Proc Natl Acad Sci U S A 109:15491–6
Chao MV, Hempstead BL (1995) p75 and Trk: a two-receptor system. Trends Neurosci 18:321–6
Huang EJ, Reichardt LF (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72:609–42
Ito M (2011) The cerebellum: brain for an implicit self. FT Press Science, Upper Saddle River
Ortega F, Perez-Sen R, Morente V, Delicado EG, Miras-Portugal MT (2010) P2X7, NMDA and BDNF receptors converge on GSK3 phosphorylation and cooperate to promote survival in cerebellar granule neurons. Cell Mol Life Sci 67:1723–33
Zirrgiebel U, Ohga Y, Carter B, Berninger B, Inagaki N, Thoenen H et al (1995) Characterization of TrkB receptor-mediated signaling pathways in rat cerebellar granule neurons: involvement of protein kinase C in neuronal survival. J Neurochem 65:2241–50
Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME (1999) Cell survival promoted by the Ras–MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286:1358–62
Middleton FA, Strick PL (2000) Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn 42:183–200
DeLong MR (1990) Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13:281–5
Lui NP, Chen LW, Yung WH, Chan YS, Yung KK (2012) Endogenous repair by the activation of cell survival signalling cascades during the early stages of rat parkinsonism. PLoS One 7:e51294
Bizzi E, Tresch MC, Saltiel P, D’Avella A (2000) New perspectives on spinal motor systems. Nat Rev Neurosci 1:101–8
Gao L, Li LH, Xing RX, Ou S, Liu GD, Wang YP et al (2012) Gastrocnemius-derived BDNF promotes motor function recovery in spinal cord transected rats. Growth Factors 30:167–75
Hu P, Kalb RG (2003) BDNF heightens the sensitivity of motor neurons to excitotoxic insults through activation of TrkB. J Neurochem 84:1421–30
Slack SE, Pezet S, McMahon SB, Thompson SW, Malcangio M (2004) Brain-derived neurotrophic factor induces NMDA receptor subunit one phosphorylation via ERK and PKC in the rat spinal cord. Eur J Neurosci 20:1769–78
Roux PP, Barker PA (2002) Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol 67:203–33
Florez-McClure ML, Linseman DA, Chu CT, Barker PA, Bouchard RJ, Le SS et al (2004) The p75 neurotrophin receptor can induce autophagy and death of cerebellar Purkinje neurons. J Neurosci 24:4498–509
Boyce VS, Park J, Gage FH, Mendell LM (2012) Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. Eur J Neurosci 35:221–32
Kafitz KW, Rose CR, Thoenen H, Konnerth A (1999) Neurotrophin-evoked rapid excitation through TrkB receptors. Nature 401:918–21
Shen RY, Altar CA, Chiodo LA (1994) Brain-derived neurotrophic factor increases the electrical activity of pars compacta dopamine neurons in vivo. Proc Natl Acad Sci U S A 91:8920–4
Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R et al (2006) BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci 9:735–7
Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG et al (2010) Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66:198–204
Boxall AR (2000) GABAergic mIPSCs in rat cerebellar Purkinje cells are modulated by TrkB and mGluR1-mediated stimulation of Src. J Physiol 524(Pt 3):677–84
Huang Y, Ko H, Cheung ZH, Yung KK, Yao T, Wang JJ et al (2012) Dual actions of brain-derived neurotrophic factor on GABAergic transmission in cerebellar Purkinje neurons. Exp Neurol 233:791–8
Carter AR, Chen C, Schwartz PM, Segal RA (2002) Brain-derived neurotrophic factor modulates cerebellar plasticity and synaptic ultrastructure. J Neurosci 22:1316–27
Jia Y, Gall CM, Lynch G (2010) Presynaptic BDNF promotes postsynaptic long-term potentiation in the dorsal striatum. J Neurosci 30:14440–5
Giralt A, Carreton O, Lao-Peregrin C, Martin ED, Alberch J (2011) Conditional BDNF release under pathological conditions improves Huntington’s disease pathology by delaying neuronal dysfunction. Mol Neurodegener 6:71
Arvanian VL, Mendell LM (2001) Acute modulation of synaptic transmission to motoneurons by BDNF in the neonatal rat spinal cord. Eur J Neurosci 14:1800–8
Lohof AM, Ip NY, Poo MM (1993) Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature 363:350–3
McGurk JS, Shim S, Kim JY, Wen Z, Song H, Ming GL (2011) Postsynaptic TRPC1 function contributes to BDNF-induced synaptic potentiation at the developing neuromuscular junction. J Neurosci 31:14754–62
Nagahara AH, Tuszynski MH (2011) Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov 10:209–19
Moore DH, Katz JS, Miller RG (2011) A review of clinical trial designs in amyotrophic lateral sclerosis. Neurodegenerative Disease Management 1:481–490
Cozzolino M, Ferri A, Carri MT (2008) Amyotrophic lateral sclerosis: from current developments in the laboratory to clinical implications. Antioxid Redox Signal 10:405–43
Nishio T, Sunohara N, Furukawa S (1998) Neutrophin switching in spinal motoneurons of amyotrophic lateral sclerosis. Neuroreport 9:1661–5
Ekestern E (2004) Neurotrophic factors and amyotrophic lateral sclerosis. Neurodegener Dis 1:88–100
Tuszynski MH, Mafong E, Meyer S (1996) Central infusions of brain-derived neurotrophic factor and neurotrophin-4/5, but not nerve growth factor and neurotrophin-3, prevent loss of the cholinergic phenotype in injured adult motor neurons. Neuroscience 71:761–71
Mitsumoto H, Ikeda K, Klinkosz B, Cedarbaum JM, Wong V, Lindsay RM (1994) Arrest of motor neuron disease in wobbler mice cotreated with CNTF and BDNF. Science 265:1107–10
Turner BJ, Cheah IK, Macfarlane KJ, Lopes EC, Petratos S, Langford SJ et al (2003) Antisense peptide nucleic acid-mediated knockdown of the p75 neurotrophin receptor delays motor neuron disease in mutant SOD1 transgenic mice. J Neurochem 87:752–63
Zhai J, Zhou W, Li J, Hayworth CR, Zhang L, Misawa H et al (2011) The in vivo contribution of motor neuron TrkB receptors to mutant SOD1 motor neuron disease. Hum Mol Genet 20:4116–31
Yanpallewar SU, Barrick CA, Buckley H, Becker J, Tessarollo L (2012) Deletion of the BDNF truncated receptor TrkB.T1 delays disease onset in a mouse model of amyotrophic lateral sclerosis. PLoS One 7:e39946
Ochs G, Penn RD, York M, Giess R, Beck M, Tonn J et al (2000) A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:201–6
Beck M, Flachenecker P, Magnus T, Giess R, Reiners K, Toyka KV et al (2005) Autonomic dysfunction in ALS: a preliminary study on the effects of intrathecal BDNF. Amyotroph Lateral Scler Other Motor Neuron Disord 6:100–3
Nicaise C, Mitrecic D, Pochet R (2011) Brain and spinal cord affected by amyotrophic lateral sclerosis induce differential growth factors expression in rat mesenchymal and neural stem cells. Neuropathol Appl Neurobiol 37:179–88
Schols L, Bauer P, Schmidt T, Schulte T, Riess O (2004) Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol 3:291–304
Takahashi M, Ishikawa K, Sato N, Obayashi M, Niimi Y, Ishiguro T et al (2012) Reduced brain-derived neurotrophic factor (BDNF) mRNA expression and presence of BDNF-immunoreactive granules in the spinocerebellar ataxia type 6 (SCA6) cerebellum. Neuropathology 32:595–603
Hourez R, Servais L, Orduz D, Gall D, Millard I, de Kerchove d’Exaerde A et al (2011) Aminopyridines correct early dysfunction and delay neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J Neurosci 31:11795–807
Dieni S, Rees S (2002) Distribution of brain-derived neurotrophic factor and TrkB receptor proteins in the fetal and postnatal hippocampus and cerebellum of the guinea pig. J Comp Neurol 454:229–40
Ringstedt T, Lagercrantz H, Persson H (1993) Expression of members of the trk family in the developing postnatal rat brain. Brain Res Dev Brain Res 72:119–31
Furutani K, Okubo Y, Kakizawa S, Iino M (2006) Postsynaptic inositol 1,4,5-trisphosphate signaling maintains presynaptic function of parallel fiber–Purkinje cell synapses via BDNF. Proc Natl Acad Sci U S A 103:8528–33
Hisatsune C, Kuroda Y, Akagi T, Torashima T, Hirai H, Hashikawa T et al (2006) Inositol 1,4,5-trisphosphate receptor type 1 in granule cells, not in Purkinje cells, regulates the dendritic morphology of Purkinje cells through brain-derived neurotrophic factor production. J Neurosci 26:10916–24
Johnson EM, Craig ET, Yeh HH (2007) TrkB is necessary for pruning at the climbing fibre–Purkinje cell synapse in the developing murine cerebellum. J Physiol 582:629–46
Rico B, Xu B, Reichardt LF (2002) TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat Neurosci 5:225–33
Jones J, Jaramillo-Merchan J, Bueno C, Pastor D, Viso-Leon M, Martinez S (2010) Mesenchymal stem cells rescue Purkinje cells and improve motor functions in a mouse model of cerebellar ataxia. Neurobiol Dis 40:415–23
Ohta H, Arai S, Akita K, Ohta T, Fukuda S (2011) Neurotrophic effects of a cyanine dye via the PI3K–Akt pathway: attenuation of motor discoordination and neurodegeneration in an ataxic animal model. PLoS One 6:e17137
Yuan H, Zhang ZW, Liang LW, Shen Q, Wang XD, Ren SM et al (2010) Treatment strategies for Parkinson’s disease. Neurosci Bull 26:66–76
Marco S, Canudas AM, Canals JM, Gavalda N, Perez-Navarro E, Alberch J (2002) Excitatory amino acids differentially regulate the expression of GDNF, neurturin, and their receptors in the adult rat striatum. Exp Neurol 174:243–52
Yan Q, Rosenfeld RD, Matheson CR, Hawkins N, Lopez OT, Bennett L et al (1997) Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience 78:431–48
Baydyuk M, Nguyen MT, Xu B (2011) Chronic deprivation of TrkB signaling leads to selective late-onset nigrostriatal dopaminergic degeneration. Exp Neurol 228:118–25
Stahl K, Mylonakou MN, Skare O, Amiry-Moghaddam M, Torp R (2011) Cytoprotective effects of growth factors: BDNF more potent than GDNF in an organotypic culture model of Parkinson’s disease. Brain Res 1378:105–18
Howells DW, Porritt MJ, Wong JY, Batchelor PE, Kalnins R, Hughes AJ et al (2000) Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp Neurol 166:127–35
Scalzo P, Kummer A, Bretas TL, Cardoso F, Teixeira AL (2010) Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J Neurol 257:540–5
Ziebell M, Khalid U, Klein AB, Aznar S, Thomsen G, Jensen P et al (2012) Striatal dopamine transporter binding correlates with serum BDNF levels in patients with striatal dopaminergic neurodegeneration. Neurobiol Aging 33(428):e1–5
Karamohamed S, Latourelle JC, Racette BA, Perlmutter JS, Wooten GF, Lew M et al (2005) BDNF genetic variants are associated with onset age of familial Parkinson disease: GenePD Study. Neurology 65:1823–5
Levivier M, Przedborski S, Bencsics C, Kang UJ (1995) Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson’s disease. J Neurosci 15:7810–20
Tsukahara T, Takeda M, Shimohama S, Ohara O, Hashimoto N (1995) Effects of brain-derived neurotrophic factor on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in monkeys. Neurosurgery 37:733–9, discussion 739–741
Ferrer I, Goutan E, Marin C, Rey MJ, Ribalta T (2000) Brain-derived neurotrophic factor in Huntington disease. Brain Res 866:257–61
Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L et al (2001) Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science 293:493–8
Baquet ZC, Bickford PC, Jones KR (2005) Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci 25:6251–9
Reiner A, Wang HB, Del Mar N, Sakata K, Yoo W, Deng YP (2012) BDNF may play a differential role in the protective effect of the mGluR2/3 agonist LY379268 on striatal projection neurons in R6/2 Huntington’s disease mice. Brain Res 1473:161–72
Dey ND, Bombard MC, Roland BP, Davidson S, Lu M, Rossignol J et al (2010) Genetically engineered mesenchymal stem cells reduce behavioral deficits in the YAC 128 mouse model of Huntington’s disease. Behav Brain Res 214:193–200
Hathorn T, Snyder-Keller A, Messer A (2011) Nicotinamide improves motor deficits and upregulates PGC-1alpha and BDNF gene expression in a mouse model of Huntington’s disease. Neurobiol Dis 41:43–50
Xie Y, Hayden MR, Xu B (2010) BDNF overexpression in the forebrain rescues Huntington’s disease phenotypes in YAC128 mice. J Neurosci 30:14708–18
Borel L, Lopez C, Peruch P, Lacour M (2008) Vestibular syndrome: a change in internal spatial representation. Neurophysiol Clin 38:375–89
Lacour M, Tighilet B (2010) Plastic events in the vestibular nuclei during vestibular compensation: the brain orchestration of a “deafferentation” code. Restor Neurol Neurosci 28:19–35
Pirvola U, Arumae U, Moshnyakov M, Palgi J, Saarma M, Ylikoski J (1994) Coordinated expression and function of neurotrophins and their receptors in the rat inner ear during target innervation. Hear Res 75:131–44
Bianchi LM, Conover JC, Fritzsch B, DeChiara T, Lindsay RM, Yancopoulos GD (1996) Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF null mutant mice. Development 122:1965–73
Montcouquiol ME, Sans NA, Travo C, Sans A, Valat J (2000) Detection and localization of BDNF in vestibular nuclei during the postnatal development of the rat. Neuroreport 11:1401–5
Ernfors P, Van De Water T, Loring J, Jaenisch R (1995) Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron 14:1153–64
Zheng JL, Stewart RR, Gao WQ (1995) Neurotrophin-4/5, brain-derived neurotrophic factor, and neurotrophin-3 promote survival of cultured vestibular ganglion neurons and protect them against neurotoxicity of ototoxins. J Neurobiol 28:330–40
Li YX, Hashimoto T, Tokuyama W, Miyashita Y, Okuno H (2001) Spatiotemporal dynamics of brain-derived neurotrophic factor mRNA induction in the vestibulo-olivary network during vestibular compensation. J Neurosci 21:2738–48
Smith PF, Curthoys IS (1989) Mechanisms of recovery following unilateral labyrinthectomy: a review. Brain Res Brain Res Rev 14:155–80
Bolger C, Sansom AJ, Smith PF, Darlington CL (1999) An antisense oligonucleotide to brain-derived neurotrophic factor delays postural compensation following unilateral labyrinthectomy in guinea pig. Neuroreport 10:1485–8
Maingay MG, Sansom AJ, Kerr DR, Smith PF, Darlington CL (2000) The effects of intra-vestibular nucleus administration of brain-derived neurotrophic factor (BDNF) on recovery from peripheral vestibular damage in guinea pig. Neuroreport 11:2429–32
Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L et al (2000) Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science 290:767–73
Gasmi M, Brandon EP, Herzog CD, Wilson A, Bishop KM, Hofer EK et al (2007) AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of CERE-120 for Parkinson’s disease. Neurobiol Dis 27:67–76
O’Leary PD, Hughes RA (2003) Design of potent peptide mimetics of brain-derived neurotrophic factor. J Biol Chem 278:25738–44
Acknowledgments
This work was supported by grants 31070959, 31071021, and 31171050 and the NSFC/RGC Joint Research Scheme 30931160433 from the National Natural Science Foundation of China; grant 2900336 from the Research Grants Council of Hong Kong, China; RFDP grant 20100091110016 and NCET Program from the State Educational Ministry of China; and grant BK2011014 from the Natural Science Foundation of Jiangsu Province, China.
Conflict of Interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
He, YY., Zhang, XY., Yung, WH. et al. Role of BDNF in Central Motor Structures and Motor Diseases. Mol Neurobiol 48, 783–793 (2013). https://doi.org/10.1007/s12035-013-8466-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8466-y