Abstract
The ubiquitin–proteasome system (UPS) is the primary proteolytic complex responsible for the elimination of damaged and misfolded intracellular proteins, often formed upon oxidative stress. Parkinson’s disease (PD) is neuropathologically characterized by selective death of dopaminergic neurons in the substantia nigra (SN) and accumulation of intracytoplasmic inclusions of aggregated proteins. Along with mitochondrial dysfunction and oxidative stress, defects in the UPS have been implicated in PD. Glutathione S-transferase pi (GSTP) is a phase II detoxifying enzyme displaying important defensive roles against the accumulation of reactive metabolites that potentiate the aggression of SN neuronal cells, by regulating several processes including S-glutathionylation, modulation of glutathione levels and control of kinase-catalytic activities. In this work we used C57BL/6 wild-type and GSTP knockout mice to elucidate the effect of both MPTP and MG132 in the UPS function and to clarify if the absence of GSTP alters the response of this pathway to the neurotoxin and proteasome inhibitor insults. Our results demonstrate that different components of the UPS have different susceptibilities to oxidative stress. Importantly, when compared to the wild-type, GSTP knockout mice display decreased ubiquitination capacity and overall increased susceptibility to UPS damage and inactivation upon MPTP-induced oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ubiquitin–proteasome system (UPS) is the major non-lysosomal protein degradation pathway within cells [1, 2]. Besides being the primary route for degradation of misfolded intracellular proteins, through the regulation of protein turnover, the UPS is critical for numerous cellular functions including regulation of cell cycle and division, survival and cellular response to stress, apoptosis and intracellular signaling [1, 2].
The degradation of proteins by the UPS is a sequential process involving an initial step of ubiquitin conjugation to the protein substrate followed by the degradation of the tagged protein through the 26S proteasome complex with release of free ubiquitin [1, 3]. In this pathway the ubiquitin-activating enzyme (E1) activates ubiquitin through the formation of a high-energy thiol ester bond between the C-terminal glycine residue of ubiquitin and a specific cysteine in the active site of the E1 enzyme, in an ATP-dependent reaction [3–5]. Subsequently, ubiquitin is transferred to one of several ubiquitin-conjugating enzymes (E2) to which ubiquitin is also linked via a thiol ester bond [6, 7]. Some E2 enzymes catalyze the covalent attachment of ubiquitin to a lysine residue in the substrate proteins directly, whereas other E2 enzymes act in concert with ubiquitin protein ligases (E3) [8]. The combination of E2s and E3s determines the substrate specificity. The ubiquitinated proteins can then be recognized and degraded by the proteasome.
Many neurodegenerative diseases are characterized by accumulation of misfolded protein deposits in affected brain regions, suggesting a failure in the cellular protein degradation pathways [9]. Parkinson’s disease (PD) is neuropathologically characterized by selective death of dopaminergic neurons in the substantia nigra (SN) and by the accumulation of intracytoplasmic inclusions of aggregated proteins [10]. Although the molecular mechanisms underlying neurodegeneration in sporadic PD are still unclear, solid evidence has accumulated implicating mitochondrial dysfunction, oxidative stress and neuro-inflammation in the pathogenesis of the disease [11]. Moreover, defects in the UPS with failure to degrade misfolded and aggregated proteins have been implicated in both familial and sporadic forms of PD [12–15]. Evidences for UPS dysfunction in PD include the mutations in genes associated with protein processing and degradation, namely parkin [16] and ubiquitin C-terminal hydrolase-L1 [17] found in patients with familial PD (reviewed by Dawson and Dawson [13]). Moreover, impairment of UPS has been reported in sporadic PD, with patients displaying impaired proteasomal activity in the SN [12] but not in the striatum (ST) [18].
In experimental models of PD, the involvement of the UPS in neurodegeneration has been a controversial issue. Although inhibition of the UPS has been shown to play a key role in mediating cellular toxicity [19], it was also demonstrated that proteasome inhibition can induce increased expression of neuroprotective factors [20] and protect against dopaminergic cell death [21, 22]. Additionally, it was demonstrated that although the systemic administration of a proteasome inhibitor to rats, transiently decreases proteasome activity, it did not resulted in biochemical, neuropathological or behavioral evidences of lesions to nigrostriatal dopaminergic neurons [23]. The key to these contradicting effects may rely on the fact highlighted in an elegant study by Fornai et al. [24], who demonstrated that continuously administered MPTP induces a PD-like syndrome with the presence of inclusions that are only formed when mild and prolonged inhibition of the mitochondrial respiratory chain causes chronic decreases of the UPS activity, reconciliating the effects of MPTP and proteasome inhibition in a mouse model of PD. This study further dissects the mechanisms of dopaminergic neuronal degeneration dependent on proteasomal dysfunction, investigated in a previous study [25].
Glutathione S-transferase pi (GSTP) belongs to a family of phase II drug metabolizing enzymes that catalyze the detoxification of electrophiles by conjugation with glutathione [26–28]. In previous studies, we showed that GSTP expression is significantly increased in both SN and ST of C57BL/6 mice after a single MPTP injection [29] and that GSTP knockout (GSTP ko) mice are more susceptible to MPTP neurotoxicity than wild-type mice [30]. Furthermore, we [30] and other research groups [31–33] demonstrated that GSTP protects against MPTP-induced toxicity by direct modulation of c-Jun N-terminal kinase (JNK) activity [30–32] and by modulation of the nuclear factor-erythroid 2-related factor2 (Nrf2) pathway through S-glutathionylation of Kelch ECH associating protein 1 Keap1 (manuscript in preparation).
Removal of oxidative-damaged proteins by the UPS is essential for the cells to cope with environmental stresses [34, 35]. As the UPS is known to prefer oxidized proteins to their native forms as substrates this is an important protein quality control mechanism. However, oxidation can impair the components of the UPS both at the level of ubiquitination and of proteasomal degradation, defining the UPS itself as a target of oxidative stress.
Since UPS impairment has been suggested to participate in the process of neurodegeneration in PD by mechanisms not yet completely elucidated, with the present study we aim to further characterize the extent and mechanisms of UPS impairment in a well established MPTP experimental model of PD. Moreover, we also assessed whether the absence of GSTP could be a determinant factor in the failure of the UPS under oxidative stress situations.
The results present herein show that in mice brain, different components of the UPS display different susceptibilities to MPTP-induced oxidative stress and to proteasome inhibition. Importantly, upon MPTP and MG132 insults GSTP ko mice are more susceptible to UPS failure indicating that GSTP has also a role in protecting protein degradation pathways against oxidative stress.
Materials and Methods
Materials
MPTP was purchased from Sigma (St. Louis, MO, USA). Na125I was purchased from Perkin Elmer (Boston, MA, USA). Proteasome inhibitor (MG132), isopeptidase inhibitor (Ubiquitin aldehyde [Ubal]) and the fluorogenic peptide substrates succcinyl-leucine-leucine-valine-tyrosine-7-amido-4-methylcoumarin (Suc-LLVY-AMC), Boc-leucine-arginine-arginine-7-amido-4-methylcoumarin (Boc-LRR-AMC) and Z-leucine-leucine-glutamic acid-7-amido-4-methylcoumarin (Z-LLE-AMC) were purchased from Boston Biochem (Cambridge, MA, USA) and Centricon-10 microconcentrators were from Millipore Corporation (Bedford, MA, USA). Coomassie Plus Protein assay and BCA reagents were obtained from Pierce (IL, USA), and Complete Mini protease inhibitors cocktail from Roche Diagnostics (Penzberg, Germany). Immobilon P membrane was from Millipore and ECL and Hyperfilm ECL were purchased from GE Healthcare Biosciences (Uppsala, Sweden). The following antibodies were used: mouse anti-ubiquitin clone P4D1 (Covance, Princeton, NJ, USA), rabbit anti-E1 (provided by Dr. Fu Shang from Tufts University, Boston, MA, USA) and mouse anti-β-actin (Sigma) and horseradish peroxidase-conjugated secondary anti-mouse or anti-rabbit (Bio-Rad, Hercules, CA, USA). Unless otherwise specified, all other reagents were of the highest analytical grade and were purchased from Sigma.
Animals and Treatment
All procedures involving the use of animals were carried out in accordance with the institutional, Portuguese and European guidelines (Diário da República, 2.ª série N.° 121 of 27 June 2011; and 2010/63/UE European Council Directive) and methods were approved by the Ethical Committee for Animal Experimentation of the Faculty of Pharmacy, University of Lisbon. Twelve-week-old (25–30 g body weight) male C57BL/6 wild-type mice purchased from the Gulbenkian Institute of Science Animal House (Oeiras, Portugal) and C57BL/6 Gstp1/p2 null mice lineage (Cancer Research UK; [36]) re-derived and maintained at the same Animal House, were used. All animals were housed under standardized conditions on a 12 h light–dark cycle, with constant temperature (22–24 °C) and humidity (50–60 %) with free access to a standard diet and water ad libitum.
Mice were intraperitoneally (i.p.) injected with either MPTP at 40 mg/kg, MG132 at 5 mg/kg, or with both MPTP and MG132, at the previous indicated dosages. Control mice received saline alone. At 6 or 24 h after MPTP, MG132 or vehicle administration mice were sacrificed under anesthesia with sodium pentobarbital (50 mg/kg, i.p.) and brain dissected as previously described [30]. Groups of at least three mice were used for each condition (time point/dosage).
Proteasome Activity
Mice midbrain and striatum were dissected and homogenized in 50 mM Tris–HCl pH 7.6. The homogenate was then centrifuged at 15,000 × g, at 4 °C, for 15 min. The protein concentration was assayed from the resulting supernatants with BCA Protein assay reagents. The mixture, containing 20 μg of tissue extract, was incubated at 37 °C for 1 h with the peptide substrates Suc-LLVY-AMC to assess the chymotrypsin-like activity or Z-LLE-AMC for the peptidylglutamyl peptide hydrolase activity or Boc-LRR-AMC for the trypsin-like activity of the proteasome, respectively. The three peptidase activities were determined by monitoring the accumulation of the fluorescent cleaved product 7-amido-4-methylcoumarin, using a temperature-controlled automatic multiwell plate reader (BioTek Synergy Spectrophotometer, Wynooski, VT, USA) at excitation/emission wavelengths of 380/460 nm. Fluorescence was measured every 5 min during 1 h, linear rates calculated as fluorescence intensity per second per microgram of protein (ΔFU/s/μg protein) and expressed as percentage of control samples.
Western Blot Analysis
Mice midbrain and striatum were dissected and tissue extracts were prepared as previously described [29]. Samples were resolved by 10 % SDS-PAGE and transferred onto Imobilon P membrane. Membranes were blocked with 5 % nonfat dry milk in Tris-buffered saline with 0,1 % Tween-20, for 1 h at room temperature and further probed with antibodies to ubiquitin (1:1,000) or E1 (1:1,000), overnight at 4 °C, followed by incubation with secondary antibodies, for 1 h at room temperature. Analysis of β-actin (1:15,000) expression was done in parallel as a loading control. The immunocomplexes were detected by the ECL chemiluminiscent method and visualized with Hyperfilm ECL. Results were quantified using the Gel-Pro Analyzer densitometry analysis software (Media Cybernetics, Maryland, USA).
Preparation of 125I-Labeled Ubiquitin
Ubiquitin was radio-iodinated as described previously [37, 38]. Briefly, ubiquitin was mixed with Na125I in 50 mM Tris–HCl, pH 7.6. The reaction of iodination was initiated by addition of chloramine T, and the vials were shaken for 2 min at room temperature. The reaction was stopped by the addition of sodium meta-bisulfite and sodium iodide. Free 125I and small protein fragments were removed from the protein solution using Centricon-10 microconcentrators.
Determination of Ubiquitin Conjugation Activity
In order to determine the conjugation capacity of the UPS components from midbrain and striatum tissue extracts to catalyze the conjugation of exogenous radio-labeled ubiquitin (125I-Ub) to endogenous protein substrates a modification of an assay described by Hersko and col. 1980 [39] was used. Mice midbrain and striatum were dissected and homogenized in 50 mM Tris–HCl pH 7.6 and sonicated at 4 °C. The soluble fraction obtained after centrifugation at 15,000 × g, at 4 °C, for 15 min was used to perform the assay and its protein content was determined by the Coomassie method.
The conjugation activity assay was performed in a final volume of 25 μl, containing 50 μg of tissue extract and 10 μl conjugation buffer (50 mM Tris–HCl, pH 7.6, 1 mM DTT, 5 mM MgCl2, 34.8 U/ml creatine phosphokinase, 10 mM creatine phosphate, 2 mM ATP, 80 μM MG132, 1 μM Ubal and 0.3 μg of 125I-Ub [approximately 2 × 105 cpm]). Following 30 min of incubation at 37 °C, the reaction was stopped by addition of 25 μl of Laemmli buffer. After incubation at room temperature for 30 min, the proteins were separated by 12 % SDS-PAGE. The formation of de novo ubiquitin conjugates was visualized by autoradiography of dried gels.
Determination of Ubiquitin-Activating and Conjugating Enzymes Activities — Thiol Ester Assay
This assay was carried out as described above for the ubiquitin conjugation assay except that the incubation period was 5 min at 37 °C and the reaction was stopped by addition of 25 μl Laemmli buffer or thiol ester assay buffer (50 mM Tris–HCl, pH 6.8, 4 % SDS, 8 M urea, 10 % glycerol and 0.1 mg/ml bromophenol blue). After incubation at room temperature for 30 min the proteins were separated by SDS-PAGE on 15 % gels. The activities of E1 and E2 were quantified by determining the density of the bands on the autoradiogram of dried gels. Samples treated with β-mercaptoethanol were used as negative controls for the reactions.
Statistical Analysis
Statistical analyses were performed using the software Graphpad Prism 5.0 (San Diego, CA, USA). Data were statistically evaluated for significance using ANOVA with Tukey post-hoc test for multiple comparisons. Differences were considered statistically significant when p < 0.05.
Results
Impairment of Proteasome Activity by MPTP and MG132 in Mice Midbrain and Striatum
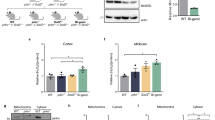
In order to clarify the involvement of proteolytic system impairment in the dopaminergic neuronal damage caused by MPTP-induced oxidative stress we assessed the function of several UPS constituents. We started by measuring the three peptidase activities of the proteasome in wt and GSTP ko mice in both midbrain and striatum regions, at 6 and 24 h post-treatment. We observed significant decreases in the chymotrypsin-like activity of the proteasome in both midbrain (Fig. 1a and b) and striatum (Fig. 1c and d) of wt (Fig. 1a and c) and GSTP ko mice (Fig. 1b and d). In the midbrain of both wt and GSTP ko mice the decrease in this activity was only significant at 24 h after the administration of MPTP and/or MG132 (Fig. 1a and b). In the striatum of wt mice chymotrypsin-like activity was decreased by approximately 30 % at 6 h (p < 0.05) and 40 % at 24 h (p < 0.01), in MG132-treated mice (Fig. 1c). MPTP in turn, led to a 35 % (p < 0.01) decrease in chymotrypsin-like activity at 24 h post-injection (Fig. 1c). The concomitant administration of MPTP and MG132 led to a decrease of approximately 30 % (p < 0.05) in this proteolytic activity at both 6 h and 24 h post-injection (Fig. 1c). Administration of MPTP or MG132 resulted in inhibition of the chymotrypsin-like activity, in the striatum of GSTP ko mice, of approximately 30 % (p < 0.05), at both time-points analyzed (Fig. 1d). Simultaneously administration of MG132 and MPTP reduced the proteasomal activity by approximately 30 % at 6 h (p < 0.05) and 40 % at 24 h (p < 0.01) (Fig. 1d). No significant changes, in neither trypsin-like nor peptidylglutamyl peptide hydrolase activities, were detected (data not shown).
Proteasome activity in mice midbrain and striatum upon MPTP and MG132 administration. Tissue extracts were prepared from midbrains and striata from C57BL/6 wild-type and GSTP ko mice i.p. injected with saline (control), MPTP (40 mg/kg) and/or MG132 (5 mg/kg), and sacrificed 6 or 24 h post-treatment. Proteasome chymotrypsin-like activity in the midbrain (a, b) and striatum (c, d) from wild-type (a, c) and GSTP ko (b, d) was determined using the fluorogenic synthetic peptide substrate Suc-LLVY-AMC. The increase in fluorescence intensity (∆FU) of the enzymatically cleaved product was detected at excitation/emission wavelengths of 380/460 nm. Results were obtained as ∆FU/s/μg protein and then converted to mean percentage activity. Chymotrypsin-like activity in control samples was arbitrarily set as 100 % and its levels in MPTP and/or MG132-treated samples were plotted as percentage of this value. Data presented are mean triplicate values ± SEM of at least three independent experiments. Statistical analysis was carried out using one-way ANOVA with Tukey post-hoc test, where # p < 0.05, ## p < 0.01, ### p < 0.001 relative to wt control and *p < 0.05, **p < 0.01, ***p < 0.001 relative to GSTP ko control
Endogenous Ubiquitin–Protein Conjugates Levels Are Altered in Response to Proteasome Inhibition and MPTP-Induced Oxidative Stress
The levels of endogenous ubiquitin conjugates at a given time-point result from the balance between the ubiquitin conjugation activity and the degradation of the ubiquitin conjugates by the proteasome or deubiquitination by isopeptidases. In order to further assess the effect of MG132 and MPTP-induced oxidative stress on the ubiquitin–proteasome pathway, we determined the levels of endogenous ubiquitin conjugates accumulated in the midbrain and striatum of wt and GSTP ko mice injected with these compounds.
GSTP ko treated with MG132, MPTP or with the combination of MG132 plus MPTP display significantly lower levels of endogenous ubiquitin conjugates accumulation when compared to their wt counterparts, in both midbrain (Fig. 2a and b) and striatum (Fig. 2c and d). However, for GSTP ko mice, only in the striatum the treatments with MG132 or with MPTP induced a significant (p < 0.01) reduction in the levels of the endogenous ubiquitin conjugates (Fig. 2d). The accumulation of ubiquitin conjugates in wt mice, in turn, is significantly affected by the treatment with both compounds, that induced a significant (p < 0.05) increase in accumulation of ubiquitin conjugates in the midbrain (Fig. 2b).
Ubiquitin conjugates levels in the midbrain and striatum of MPTP and MG132-treated C57BL/6 wild-type and GSTP ko mice. Six hours after i.p. administration of saline (control), MG132 (5 mg/kg) and/or MPTP (40 mg/kg), mice were sacrificed and brains were dissected. Endogenous ubiquitin conjugates levels were determined in midbrain (a, b) and striatum (c, d) from wild-type and GSTP ko mice, by Western blot analysis, using a mouse anti-ubiquitin antibody. Analysis of β-actin was done in parallel as a loading control. The ubiquitin (Ub) conjugates levels in control samples, from saline-treated wt mice, were arbitrarily set as 100 % and levels in MPTP and/or MG132-treated samples were calculated and plotted as a percentage of this value. Data shown are mean ± SEM of at least three independent experiments. Statistical analysis was carried out using one-way ANOVA with Tukey post-hoc test where # p < 0.05, ## p < 0.01, relative to wt control; ££ p < 0.01, £££ p < 0.001, relative to wt MG132 plus MPTP; **p < 0.01, relative to GSTP ko control; and two-way ANOVA with Bonferroni’s post-hoc test, where § p < 0.05, §§§ p < 0.001 wild-type vs. corresponding GSTP ko
MPTP and MG132 Induce Alterations in Ubiquitin Conjugation Capacity in Mice Midbrain and Striatum
To assess the function of the enzymes that constitute the endogenous ubiquitin conjugation machinery, the ability to form de novo ubiquitin conjugates was evaluated in tissue extracts from both wild-type and GSTP ko mice, using exogenous radio-labeled ubiquitin (125I-ubiquitin).
As shown in Fig. 3, we detected a significant decrease in the formation of conjugates of ubiquitin with protein substrates in the midbrain of wt mice treated with MG132 (p < 0.05) or with MPTP (p < 0.01) (Fig. 3a and b). Similarly the co-administration of both compounds led to a 55 % (p < 0.01) decrease in the de novo formation of ubiquitin conjugates (Fig. 3a and b). In GSTP ko mice, after injection of both compounds, the conjugation activity is lowered by approximately 60 % (p < 0.05) (Fig. 3a and b). GSTP ko mice exhibited similar results in both brain regions with a 50 % decrease (p < 0.05) in the conjugating activity in the striatum of MG132 plus MPTP treated mice (Fig. 3c and d). However, surprisingly in the striatum of wt mice the administration of MPTP resulted in a significant increase (~65 %, p < 0.001) in the ability to form de novo conjugates (Fig. 3c and d).
Ubiquitin conjugation activity in C57BL/6 wild-type and GSTP ko mice midbrain and striatum upon treatment with MPTP and MG132. C57BL/6 mice were i.p. injected with saline (control), MG132 (5 mg/kg) and/or MPTP (40 mg/kg), euthanized 6 h after treatment and midbrains and striata were dissected. Ubiquitin conjugation activity was determined by incubation of tissue extracts with exogenous 125I-ubiquitin followed by SDS-PAGE analysis. The gels were stained, dried, and subjected to autoradiography. The amount of de novo ubiquitin conjugates formed represents ubiquitin conjugation activity in the midbrain (a, b) and striatum (c, d) of wild-type and GSTP ko mice. The autoradiographs shown are representative of three independent experiments. The ubiquitin conjugates levels in control samples were arbitrarily set as 100 % and the relative levels in MPTP and/or MG132-treated samples was calculated and plotted as a percentage of this value. Data shown are mean ± SEM of three independent experiments. Statistical comparisons were performed using one-way ANOVA with Tukey post-hoc test where # p < 0.05, ## p < 0.01, ### p < 0.001 relative to wild-type control; *p < 0.05, relative to GSTP ko control; £££ p < 0.001, relative to MPTP-treated wild-type; § p < 0.05, §§ p < 0.01, §§§ p < 0.001 wild-type vs. corresponding GSTP ko
Moreover, in the striatum, the alterations in the conjugation activity levels were significantly different in GSTP ko mice when compared to their wt counterparts under the same treatment schedule (Fig. 3c and d).
Ubiquitin-Activating Enzyme Expression and Activity Is Modified by MPTP and MG132 Treatments in the Midbrain and Striatum of Wild-type and GSTP Knock-out Mice
E1 is the rate-limiting enzyme in the ubiquitin conjugation process and its activity has been suggested to be transiently up-regulated in response to oxidative stress [40].
In accordance with the observed significant increase in the de novo formed conjugates in the striatum of wt mice upon MPTP administration (Fig. 3c and d), the E1 activity in this brain region is also up-regulated in mice treated with the neurotoxin (Fig. 4d and f). Furthermore, in the midbrain of wt mice (Fig. 4a and c) and in both the midbrain (Fig. 4b and c) and striatum (Fig. 4e and f) of GSTP ko mice the levels of E1~Ub thiolester are lowered by the treatment with MG132 plus MPTP, which is also in accordance with the data obtained for the de novo ubiquitin conjugates levels in these same brain regions (Fig. 3).
Ubiquitin activating enzyme (E1) and ubiquitin conjugating enzymes (E2s) activities in response to MPTP and MG132 administration. Tissue extracts from C57BL/6 wild-type (a, d) and GSTP ko (b, e) mice midbrains (a, b and c) and striata (d, e and f) were prepared after saline (control), MPTP (40 mg/kg) and/or MG132 (5 mg/kg) i.p. injection, and sacrificed 6 h post-treatment. Ubiquitin activating enzyme (E1) and ubiquitin conjugating enzyme (E2) activities following MPTP/MG132 treatment were determined by thiolester assay using exogenous 125I-ubiquitin. In parallel, as a control for this assay, tissue extracts were exposed to β-mercaptoethanol (β-ME) because E1~Ubiquitin (E1~Ub) and E2~Ubiquitin (E2~Ub) thiolesters are labile to reducing agents. The bands that are not visible in the presence of β-ME are E1~Ub and E2~Ub thiolesters. The autoradiographs shown are representative of three independent experiments using at least three mice per condition. The E1 levels in control samples were arbitrarily set as 100 % and the relative levels in MPTP and/or MG132-treated samples was calculated and plotted as a percentage of this value (c and f). Data shown are mean ± SEM of three independent experiments. Statistical comparisons were performed using one-way ANOVA with Tukey post-hoc test, where # p < 0.05, ## p < 0.01 relative to wild-type control; **p < 0.01, relative to GSTP ko control; §§ p < 0.01, §§§ p < 0.001 wild-type vs. corresponding GSTP ko
Moreover, the E1 protein levels are significantly lower in GSTP ko mice when compared to wt mice under proteasome inhibition or treatment with the neurotoxin, in both brain compartments (Fig. 5b and d). Additionally, whereas in the striatum of wt mice E1 protein levels increase in response to the insults, in GSTP ko mice E1 expression is decreased upon exposure to both compounds (Fig. 5).
Ubiquitin activating enzyme (E1) expression levels in response to MPTP and MG132 administration. C57BL/6 wild-type and GSTP knockout mice were i.p. injected with saline (control), MPTP (40 mg/kg) and/or MG132 (5 mg/kg) and sacrificed 6 h post-treatment. Tissue extracts were prepared from midbrain (a, b) and striatum (c, d) from both wt and GSTP ko mice and subjected to SDS-PAGE. The corresponding blots were probed with a rabbit anti-E1 antibody. Analysis of β-actin was done in parallel as a loading control. The relative levels of E1 in control samples were arbitrarily set as 100 % and the relative levels in MPTP and/or MG132-treated samples were calculated and plotted as a percentage of this value. Data shown are mean ± SEM of at least three independent experiments. Statistical analysis was performed using one-way ANOVA with Tukey post-hoc test where # p < 0.05, ### p < 0.001 relative to wild-type control; **p < 0.01, ***p < 0.001, relative to GSTP ko control; £££ p < 0.001, relative to MPTP-treated wild-type; and two-way ANOVA with Bonferroni’s post-hoc test where § p < 0.05, §§§ p < 0.001 wild-type vs. corresponding GSTP ko
Discussion
In sporadic PD, neuronal cell death is accompanied by the accumulation of misfolded protein deposits in affected brain regions [10], suggesting that a failure in the cellular protein degradation pathways might contribute to the pathogenesis of the disease [1, 14].
The selective removal of oxidative-damaged proteins by the UPS is essential for cells to survive under conditions of environmental stresses [34, 35]; however, the UPS itself is a target of oxidative stress [41].
We have previously observed that both MPTP and MG132 in vivo administration induces oxidative stress as demonstrated by increased levels of reactive oxygen species and protein carbonyls along with decreased glutathione levels (manuscript in preparation). We have also shown that GSTP protects against MPTP-induced neurotoxicity by modulating JNK activity [30] and the Nrf2 antioxidant pathway (manuscript in preparation) in a mouse model of PD. The inhibition of proteasome has been suggested to be related with dopaminergic neuronal degeneration in PD experimental models. However, the comparison between the mechanisms underlying the effects of neurotoxins, such as MPTP, and proteasome inhibitors, such as MG132, has not been thoroughly explored. Therefore, we sought to evaluate the effect of MPTP treatment on the UPS function in wild-type and GSTP ko mice midbrain and striatum and assess whether this effect is reversed, potentiated or not affected when the proteasome pathway is impaired.
Our data, consistent with previous studies [24], show that single-dose injections of MPTP in mice decreased the nigral UPS activity for a short period of time. Here we show that MG132 and MPTP affected the proteasome proper function leading to a decrease in the chymotrypsin-like activity in both wild-type and GSTP ko mice. The proteasome function seems to be altered by the treatments in a similar manner in both wt and ko mice, although in GSTP ko mice treated with MPTP the inhibition of the proteasome in the striatum occurs at the earlier time-point tested when compared to wt mice.
Oxidative damage to the proteasome is one possible mechanism that enhances stress-induced accumulation of ubiquitin conjugates in the cells, which have been reported as sensitive markers of oxidative stress [40, 42–44]. Oxidized proteins are generally preferred proteasome substrates [45, 46] and upon oxidizing conditions the cell can initiate a response that involves the up-regulation of both proteasome activity and ubiquitin conjugation enzymes leading to enhanced intracellular protein degradation. The proteasome has been reported to be more susceptible to oxidative stress-induced inactivation than the enzymes of the ubiquitin conjugation machinery [40, 41, 47]. Therefore, the proteasome can be inactivated and even so the ubiquitin conjugation machinery may still maintain proper function. In this scenario, proteins are tagged for degradation by ubiquitin but are not degraded by the proteasome resulting in an accumulation of ubiquitin conjugates. On the other hand, extensive oxidative stress that compromises both the proteasome and the ubiquitination enzymes results in a decrease in the de novo synthesis of ubiquitin–protein conjugates and in proteasomal degradation.
In fact, in the midbrain of wt mice treated with both MG132 and MPTP we observe impaired proteasome activity (Fig. 1a and b) and accumulation of endogenous ubiquitin conjugates (Fig. 2a and b). This can be explained by the fact that, as a result of different UPS susceptibilities to oxidative stress, proteasomal activity can be affected earlier than the ubiquitination capacity, leading to the accumulation of protein–ubiquitin conjugates. However, as a result of a strong oxidative insult, such as the one inflicted by the concomitant administration of both compounds, the ubiquitination capacity is also ultimately compromised, resulting in the impairment of the de novo conjugates formation observed in Fig. 3.
Moreover, we observed a significant decrease in the levels of endogenous ubiquitin conjugates in the striatum of both wild-type and GSTP ko mice in response to MPTP administration, pointing towards an alteration in the physiological/steady-state function of the UPS induced by MPTP administration (Fig. 2c and d). Notably, ubiquitin–conjugates levels of both control and MG132/MPTP treated mice were significantly lower in GSTP ko mice when compared with their wt counterparts (Fig. 2b and d). Additionally, ubiquitination capacity of GSTP ko mice in the striatum is also significantly decreased when compared to the wild-type mice (Fig. 3c and d). These results indicate that the UPS is more affected by oxidative stress in GSTP ko mice than in wt mice. Moreover, wt mice have the ability to increase ubiquitination, by inducing E1 activity (Fig. 4d and f), as an attempt of the cells to increase the degradation of damaged proteins in response to MPTP-induced oxidative stress.
In accordance, the levels of E1 enzyme, upon treatment with MG132 or MPTP, are also significantly lower in GSTP ko mice when compared to their wt counterparts (Fig. 5). Moreover, in GSTP ko mice, treatment with MPTP, MG132 or with both compounds induce significant decreases in midbrain E1 protein levels (Fig. 5a and b). In contrast, in wt mice both E1 expression and activity tend to increase upon treatment with MPTP, indicating that wt mice have a higher capacity to cope with MPTP-induced oxidative stress and still maintain a functional UPS.
Taylor and collaborators have proposed that activities of E1 and E2 enzymes are regulated by cellular redox status, namely GSSG/GSH ratio [48]. Moreover, an independent report [43] showed that changes in UPS function could be directly related to a decrease in GSH levels and an increase in S-glutathionylated proteins.
Recently obtained results from our group show that MPTP induces a significant decrease in glutathione levels that is accompanied by an increase in S-glutathionylated protein levels in C57BL/6 wild-type mice brain (manuscript in preparation). Structurally, all the enzymes in the ubiquitin conjugation pathway have a cysteine residue in their active sites, and therefore might be subjected to regulation through S-glutathionylation [34]. Glutathionylation, being a reversible process, might protect protein cysteine residues from irreversible oxidative-driven inactivation. Interestingly, GSTP ko mice exhibit lower global levels of S-glutathionylation when compared to wt mice, in response to MPTP and MG132 treatments (manuscript in preparation). This might account for the inability for the up-regulation of E1 activity in GSTP ko mice in response to MPTP-induced oxidative stress.
Here we show that GSTP ko mice under MPTP treatment display impaired ubiquitination capacity and lower expression levels of E1, the rate-limiting enzyme in the ubiquitination process. Since the increase in proteolysis may function as an anti-oxidant mechanism, by enhancing the removal of oxidative-damaged proteins from cells, our results strongly suggest that GSTP ko mice exhibit deficits in the UPS, that may contribute to their higher susceptibility to MPTP-induced neurotoxicity. Taken together, the present results show that GSTP ko mice are more susceptible to UPS failure, under conditions of oxidative stress and proteasome inhibition, reinforcing our previous data and indicating that GSTP also plays a role in protecting protein degradation pathways against oxidative stress.
References
Ciechanover A, Brundin P (2003) The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron 40(2):427–446
Schwartz AL, Ciechanover A (2009) Targeting proteins for destruction by the ubiquitin system: implications for human pathobiology. Annu Rev Pharmacol Toxicol 49:73–96
Glickman MH, Ciechanover A (2002) The ubiquitin–proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82(2):373–428
Haas AL, Rose IA (1982) The mechanism of ubiquitin activating enzyme. A kinetic and equilibrium analysis. J Biol Chem 257(17):10329–10337
Haas AL, Warms JV, Hershko A, Rose IA (1982) Ubiquitin-activating enzyme. Mechanism and role in protein–ubiquitin conjugation. J Biol Chem 257(5):2543–2548
Pickart CM, Rose IA (1985) Functional heterogeneity of ubiquitin carrier proteins. J Biol Chem 260(3):1573–1581
Haas AL, Bright PM (1988) The resolution and characterization of putative ubiquitin carrier protein isozymes from rabbit reticulocytes. J Biol Chem 263(26):13258–13267
Hershko A, Heller H, Elias S, Ciechanover A (1983) Components of ubiquitin–protein ligase system. Resolution, affinity purification, and role in protein breakdown. J Biol Chem 258(13):8206–8214
Taylor JP, Hardy J, Fischbeck KH (2002) Toxic proteins in neurodegenerative disease. Science (New York, NY) 296(5575):1991–1995
Dauer W, Przedborski S (2003) Parkinson's disease: mechanisms and models. Neuron 39(6):889–909
Moore DJ, West AB, Dawson VL, Dawson TM (2005) Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci 28:57–87
McNaught KS, Jenner P (2001) Proteasomal function is impaired in substantia nigra in Parkinson's disease. Neurosci Lett 297(3):191–194
Dawson TM, Dawson VL (2003) Rare genetic mutations shed light on the pathogenesis of Parkinson disease. J Clin Investig 111(2):145–151
Olanow CW, McNaught KS (2006) Ubiquitin–proteasome system and Parkinson's disease. Mov Disord 21(11):1806–1823
McNaught KS, Jnobaptiste R, Jackson T, Jengelley TA (2010) The pattern of neuronal loss and survival may reflect differential expression of proteasome activators in Parkinson's disease. Synapse (New York, NY) 64(3):241–250
Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392(6676):605–608
Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, Harta G, Brownstein MJ, Jonnalagada S, Chernova T, Dehejia A, Lavedan C, Gasser T, Steinbach PJ, Wilkinson KD, Polymeropoulos MH (1998) The ubiquitin pathway in Parkinson's disease. Nature 395(6701):451–452
Furukawa Y, Vigouroux S, Wong H, Guttman M, Rajput AH, Ang L, Briand M, Kish SJ, Briand Y (2002) Brain proteasomal function in sporadic Parkinson's disease and related disorders. Ann Neurol 51(6):779–782
McNaught KS, Perl DP, Brownell AL, Olanow CW (2004) Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson's disease. Ann Neurol 56(1):149–162
Yew EH, Cheung NS, Choy MS, Qi RZ, Lee AY, Peng ZF, Melendez AJ, Manikandan J, Koay ES, Chiu LL, Ng WL, Whiteman M, Kandiah J, Halliwell B (2005) Proteasome inhibition by lactacystin in primary neuronal cells induces both potentially neuroprotective and pro-apoptotic transcriptional responses: a microarray analysis. J Neurochem 94(4):943–956
Inden M, Kondo J, Kitamura Y, Takata K, Nishimura K, Taniguchi T, Sawada H, Shimohama S (2005) Proteasome inhibitors protect against degeneration of nigral dopaminergic neurons in hemiparkinsonian rats. J Pharmacol Sci 97(2):203–211
Sawada H, Kohno R, Kihara T, Izumi Y, Sakka N, Ibi M, Nakanishi M, Nakamizo T, Yamakawa K, Shibasaki H, Yamamoto N, Akaike A, Inden M, Kitamura Y, Taniguchi T, Shimohama S (2004) Proteasome mediates dopaminergic neuronal degeneration, and its inhibition causes alpha-synuclein inclusions. J Biol Chem 279(11):10710–10719
Mathur BN, Neely MD, Dyllick-Brenzinger M, Tandon A, Deutch AY (2007) Systemic administration of a proteasome inhibitor does not cause nigrostriatal dopamine degeneration. Brain Res 1168:83–89
Fornai F, Schluter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, Lazzeri G, Busceti CL, Pontarelli F, Battaglia G, Pellegrini A, Nicoletti F, Ruggieri S, Paparelli A, Sudhof TC (2005) Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin–proteasome system and alpha-synuclein. Proc Natl Acad Sci U S A 102(9):3413–3418
Fornai F, Lenzi P, Gesi M, Ferrucci M, Lazzeri G, Busceti CL, Ruffoli R, Soldani P, Ruggieri S, Alessandri MG, Paparelli A (2003) Fine structure and biochemical mechanisms underlying nigrostriatal inclusions and cell death after proteasome inhibition. J Neurosci 23(26):8955–8966
Henderson CJ, McLaren AW, Moffat GJ, Bacon EJ, Wolf CR (1998) Pi-class glutathione S-transferase: regulation and function. Chem Biol Interact 111–112:69–82
Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88
Henderson CJ, Wolf CR (2011) Knockout and transgenic mice in glutathione transferase research. Drug Metab Rev 43(2):152–164
Castro-Caldas M, Neves Carvalho A, Peixeiro I, Rodrigues E, Lechner MC, Gama MJ (2009) GSTpi expression in MPTP-induced dopaminergic neurodegeneration of C57BL/6 mouse midbrain and striatum. J Mol Neurosci 38(2):114–127
Castro-Caldas M, Carvalho AN, Rodrigues E, Henderson C, Wolf CR, Gama MJ (2012) Glutathione S-transferase pi mediates MPTP-induced c-Jun N-terminal kinase activation in the nigrostriatal pathway. Mol Neurobiol 45(3):466–477
Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, Davis RJ, Ronai Z (1999) Regulation of JNK signaling by GSTp. EMBO J 18(5):1321–1334
Wang T, Arifoglu P, Ronai Z, Tew KD (2001) Glutathione S-transferase P1-1 (GSTP1-1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J Biol Chem 276(24):20999–21003
Smeyne M, Boyd J, Raviie Shepherd K, Jiao Y, Pond BB, Hatler M, Wolf R, Henderson C, Smeyne RJ (2007) GSTpi expression mediates dopaminergic neuron sensitivity in experimental parkinsonism. Proc Natl Acad Sci U S A 104(6):1977–1982
Jahngen-Hodge J, Obin MS, Gong X, Shang F, Nowell TR Jr, Gong J, Abasi H, Blumberg J, Taylor A (1997) Regulation of ubiquitin-conjugating enzymes by glutathione following oxidative stress. J Biol Chem 272(45):28218–28226
Dudek EJ, Shang F, Valverde P, Liu Q, Hobbs M, Taylor A (2005) Selectivity of the ubiquitin pathway for oxidatively modified proteins: relevance to protein precipitation diseases. FASEB J 19(12):1707–1709
Henderson CJ, Smith AG, Ure J, Brown K, Bacon EJ, Wolf CR (1998) Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. Proc Natl Acad Sci U S A 95(9):5275–5280
Jahngen JH, Haas AL, Ciechanover A, Blondin J, Eisenhauer D, Taylor A (1986) The eye lens has an active ubiquitin–protein conjugation system. J Biol Chem 261(29):13760–13767
Marques C, Guo W, Pereira P, Taylor A, Patterson C, Evans PC, Shang F (2006) The triage of damaged proteins: degradation by the ubiquitin–proteasome pathway or repair by molecular chaperones. FASEB J 20(6):741–743
Hershko A, Ciechanover A, Heller H, Haas AL, Rose IA (1980) Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci U S A 77(4):1783–1786
Shang F, Gong X, Taylor A (1997) Activity of ubiquitin-dependent pathway in response to oxidative stress. Ubiquitin-activating enzyme is transiently up-regulated. J Biol Chem 272(37):23086–23093
Zhang X, Zhou J, Fernandes AF, Sparrow JR, Pereira P, Taylor A, Shang F (2008) The proteasome: a target of oxidative damage in cultured human retina pigment epithelial cells. Investig Ophthalmol Vis Sci 49(8):3622–3630
Shang F, Taylor A (1995) Oxidative stress and recovery from oxidative stress are associated with altered ubiquitin conjugating and proteolytic activities in bovine lens epithelial cells. Biochem J 307(Pt 1):297–303
Figueiredo-Pereira ME, Yakushin S, Cohen G (1997) Accumulation of ubiquitinated proteins in mouse neuronal cells induced by oxidative stress. Mol Biol Rep 24(1–2):35–38
Adamo AM, Pasquini LA, Moreno MB, Oteiza PI, Soto EF, Pasquini JM (1999) Effect of oxidant systems on the ubiquitylation of proteins in the central nervous system. J Neurosci Res 55(4):523–531
Davies KJ (2001) Degradation of oxidized proteins by the 20S proteasome. Biochimie 83(3–4):301–310
Grune T, Merker K, Sandig G, Davies KJ (2003) Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem Biophys Res Commun 305(3):709–718
Shang F, Taylor A (2011) Ubiquitin–proteasome pathway and cellular responses to oxidative stress. Free Radic Biol Med 51(1):5–16
Obin M, Shang F, Gong X, Handelman G, Blumberg J, Taylor A (1998) Redox regulation of ubiquitin-conjugating enzymes: mechanistic insights using the thiol-specific oxidant diamide. FASEB J 12(7):561–569
Acknowledgments
This work was supported by Fundação para a Ciência e a Tecnologia (FCT) and FEDER through grants PPCDT/SAU-FCF/58171/2004, PTDC/SAU-MMO/57216/2004 and PEst-OE/SAU/UI4013/2011, and PhD fellowship SFRH/BD/39897/2007 (to ANC).
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. N. Carvalho and C. Marques are joint first authors.
Rights and permissions
About this article
Cite this article
Carvalho, A.N., Marques, C., Rodrigues, E. et al. Ubiquitin–Proteasome System Impairment and MPTP-Induced Oxidative Stress in the Brain of C57BL/6 Wild-type and GSTP Knockout Mice. Mol Neurobiol 47, 662–672 (2013). https://doi.org/10.1007/s12035-012-8368-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-012-8368-4