Abstract
Meckel–Gruber syndrome (MKS) is a severe autosomal recessively inherited disorder characterized by developmental defects of the central nervous system that comprise neural tube defects that most commonly present as occipital encephalocele. MKS is considered to be the most common syndromic form of neural tube defect. MKS is genetically heterogeneous with six known disease genes: MKS1, MKS2/TMEM216, MKS3/TMEM67, RPGRIP1L, CEP290, and CC2D2A with the encoded proteins all implicated in the correct function of primary cilia. Primary cilia are microtubule-based organelles that project from the apical surface of most epithelial cell types. Recent progress has implicated the involvement of cilia in the Wnt and Shh signaling pathways and has led to an understanding of their role in normal mammalian neurodevelopment. The aim of this review is to provide an overview of the molecular genetics of the human disorder, and to assess recent insights into the etiology and molecular cell biology of severe ciliopathies from mammalian animal models of MKS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary cilia are evolutionarily conserved microtubule-based organelles that play important roles in many physiological processes, including cell and fluid movement, development and chemo-, mechano-, and photosensation. In recent years, an ever-increasing number of inherited diseases, of previously unknown etiology, have been shown to arise from defects in the structure and function of primary cilia (reviewed in [1, 2]). Meckel–Gruber syndrome (MKS, MIM number #249000) is a pleiotropic, autosomal recessive developmental disorder which is caused by dysfunction of primary cilia during early embryogenesis [2]. Clinical features of MKS include syndromic forms of neural tube defects (NTDs) [3], midline orofacial clefting anomalies (cleft lip with and without cleft palate), microphthalmos, iris coloboma, and coloboma of the optic nerve [4, 5]. Hydrocephaly and ventriculomegaly are also common but very variable clinical features of this ciliopathy. Although MKS is the most common syndromic NTD [3], the molecular pathogenesis remains unclear. However, it is now clear that primary cilia have a crucial role as a specialized compartment for extracellular signal transduction that responds to Hedgehog (Hh), platelet-derived growth factor (PDGF) and Wnt ligands [6–8].
In the following sections, we review the clinical features and molecular genetics of Meckel–Gruber syndrome, which includes the emerging genetic evidence that the extensive phenotypic variability of the disorder can be, in part, explained by the epistatic effect of modifier alleles. The structure and function of the primary cilia, particularly in the context of mediating signal transduction pathways, is also briefly discussed. We then discuss the possible functional roles of each of the six known MKS proteins in some detail, and develop these findings in the light of the neurodevelopmental phenotypes in several mammalian models of MKS.
Clinical Aspects of Meckel–Gruber Syndrome and Severe Ciliopathies
History
In 1684, Mr. Christopher Krahe described and illustrated a child with severe congenital malformations born in Denmark on Friday, February 29, 1684 [9], possibly the first known description of Meckel–Gruber syndrome. Erwin J. O. Kompanje reviewed the case report, and thought Meckel–Gruber syndrome (MKS) to be the most obvious diagnosis [10]. However, the great German anatomist Johann Friedrich Meckel the Younger first described the clinical features that are now unequivocally identified as Meckel syndrome in 1822 [11]. He reported two newborn children, one male, one female, that both presented with occipital encephalocele, polydactyly, cleft palate, and large cystic kidneys. Subsequently, in 1934, Georg Benno Gruber described a further 16 cases of newborn children with encephalocele and polycystic kidneys, eight of whom had polydactyly [12], naming the condition dysencephalia splanchnocystica. In the late 1960s, John Marius Opitz further delineated the syndrome [13] and it has since been referred to as Meckel–Gruber syndrome.
Clinical Features of Meckel–Gruber Syndrome
Meckel–Gruber syndrome (MKS) is neonatal lethal autosomal recessive disorder. The classic triad of clinical features for MKS, as first described by Meckel, are occipital encephalocele, cystic kidneys, and fibrotic changes to the liver. All MKS patients have cystic dysplasia of the kidneys, and as a result, the kidneys tend to be grossly enlarged but with varying degrees of cyst formation. Associated defects such as agenesis, atresia, and hypoplasia of the ureter or bladder, and a horseshoe kidney have been reported [14]. However, the clinical phenotype has been extended and altered since the disorder was first characterized and there is now known to be broad phenotypic variation [15], and even pleiotropy for the mutations in individual MKS genes [16–18]. Other frequent and variable features include postaxial polydactyly, skeletal dysplasia, microphthalmia, genital anomalies, cleft lip and palate, and heart defects [19]. Laterality defects, including complete or partial situs inversus and dextrocardia, have also been reported [20].
MKS is considered to be the most common syndromic form of neural tube defect [3]. Occipital encephalocele is reported to be present in approximately 90% of cases of MKS in Finland [19]. Other central nervous system (CNS) abnormalities associated with MKS are arhinencephaly, holoprosencephaly, agenesis corpus callosum, fused thalami, hypoplasia of the third ventricle, micropthalmia, small or absent pituitary gland, microcephaly, micrognathia, rhombic roof dysgenesis, absent brain stem tectum, agenesis–dysgenesis of the cerebellar vermis, elongated brain stem, aqueductal stenosis, and polymicrogyria [21, 22]. Ahdab-Barmada and Claassen [22] suggested that a distinctive triad of CNS malformations (prosencephalic dysgenesis, occipital encephalocele, and rhombic roof dysgenesis) should be included in the diagnostic criteria of MKS. Herriot et al. [23] suggested that the Dandy–Walker malformation (DWM) should be accepted as one of the malformations associated with MKS. It had been debated that DWM coexisting with typical features of MKS should, perhaps, be treated as a different recessive syndrome. However, we now know, with the benefit of hindsight and molecular diagnosis, that such cases overlap with the neurodevelopmental condition Joubert syndrome (JS, MIM number #213300), and that MKS and JS are allelic conditions [16–18, 24]. JS is characterized by a hypoplastic cerebellar vermis, elongated and thickened superior cerebellar peduncles and a deep interpeduncular fossa. In combination, these features can be visualized on axial brain MRI as the so-called “molar tooth sign” which is considered to be diagnostic for JS [25].Clinical features include severe developmental delay, ataxia, hypotonia, abnormal breathing pattern and some features overlapping with MKS that include polydactyly, iris coloboma, and cystic kidneys.
Incidence and Differential Diagnoses
The incidence of MKS varies greatly between different populations. As the syndrome is rare in some ethnic backgrounds and not well-known to doctors and pathologists, estimates should be treated with caution as some cases may be either misdiagnosed or unrecorded. The incidence is estimated to be 1:13,250 in the USA [26] and 1:140,000 in the UK [27]. Meckel–Gruber syndrome forms part of the Finnish disease heritage, a group of about 36 monogenic diseases that are more frequent in Finland than in any other population. The incidence in the Finnish population is estimated to be significantly higher at 1:9000 [19]. Our own estimates of cases in the UK city of Bradford, over the years 1996-2005, give an incidence as high as 1:3,000 (unpublished data). Antenatal presentation of Bardet–Biedl syndrome (BBS, MIM number #209900) is one possible differential diagnosis for MKS [28]. BBS is also a ciliopathy [1], characterized in adults by obesity, moderate mental retardation, retinitis pigmentosa, hypogonadism, and renal failure. Conversely, mutations in the MKS genes are both a rare cause BBS, but can also act as modifier alleles in an epistatic effect on mutations in known BBS genes (see below) [29]. Differential diagnoses for MKS can also be etiologically unrelated to ciliopathies and include trisomy 13 (Patau syndrome) and Smith–Lemli–Opitz syndrome (MIM number #270400), both characterized by holoprosencephaly and polydactyly.
The Structure of Primary Cilia and Intraflagellar Transport
Cilia (and flagella) are evolutionarily conserved microtubule-based organelles, that project from the apical surface of most interphase and non-dividing cells. The “stalk” of the cilium, known as the axoneme, is formed of nine microtubule doublets. Each doublet comprises of A and B strands which consist of 13 and 10 tubulin protofilaments, respectively. Post-translational modification of the tubulins, including acetylation and poly-glutamylation, ensure that these microtubules are not as dynamically unstable as those elsewhere in the cytoskeleton. The ciliary axoneme is surrounded by the ciliary matrix and encased by the ciliary membrane. Each cilium arises from a basal body, which is derived from the older, so-called “mother” centriole in a centriole pair. The basal body is a cylindrical structure lying perpendicular to the cell membrane and surrounded by pericentriolar matrix, and it is formed of nine microtubule triplets composed of A, B, and C microtubules (Fig. 1a). All three types of microtubule project into the proximal region of the cilium in an area that is called the transition zone. These transition zone microtubules extend from the basal body to the cell membrane to form a structure that is thought to act as a gatekeeper, restricting, and regulating the access of proteins to the cilium, and the cilium is considered a specific and isolated cellular compartment [30]. A recent study has suggested that the centrosomal protein CEP290, mutated in MKS and other ciliopathies, acts as a molecular linker between the transition zone microtubules and the plasma membrane, so ensuring basal body docking at the apical cell surface and subsequent ciliogenesis [31].
Schematic representations of the organization of primary cilia and localization of MKS proteins. a Schematic representation of the primary cilium, basal body, intraflagellar transport (IFT) and localization of MKS proteins. Anterograde IFT particles are moved from the base of the organelle out to the distal ciliary tip by heterotrimeric kinesin-II motor protein complex. Retrograde transport of IFT particles is mediated by cytoplasmic dynein 1b complex. The motors move along the B tubules of the outer doublet microtubules (MT). The movement is continuous from end-to-end. Sonic hedgehog (Shh) signaling is mediated by the receptor Patched and co-receptor Smoothened. Both proteins localize to the specialized ciliary membrane that is continuous with the plasma membrane of the cell. The receptor TMEM67/meckelin and the putative co-receptor TMEM216 also both localize to the ciliary membrane, and are possible mediators of Wnt signaling. Mutations in TMEM67 and TMEM216 are causes of Meckel–Gruber syndrome. The other four known MKS proteins (CEP290, CC2D2A, MKS1, and RPGRIP1L) predominantly localized to the basal body. b Confocal immunofluorescence microscopy of primary cilia, visualized on the surface of ciliated kidney epithelial cells (top and middle panels), costaining for acetylated alpha-tubulin (left panels) and the MKS transmembrane receptor TMEM67 (also known as meckelin; right panels). Acetylated alpha-tubulin is specific for stable microtubules in the ciliary axoneme (indicated by the brace, top panel). TMEM67/meckelin has a punctuate distribution along on the cilium and at the basal body (arrow). The bottom panel is an x–z optical slice from a volume rendered from confocal images, indicating the apical position of the primary cilium at the surface of the cell. Scales bars 5 μm. c Schematic representations of two of the basal body MKS proteins, MKS1 and RPGRIP1L. Amino acid positions of the indicated protein domains are indicated. Sizes are not to scale. d Schematic representations of TMEM216 and TMEM67/meckelin. Amino acid positions of the indicated protein domains are indicated, including transmembrane domains traversing the ciliary membrane (CM). Sizes are not to scale
Motile cilia consist of nine microtubule doublets with dynein arms, extending from a mother centriole, and containing a central microtubule pair that is connected to the outer doublets by radial spokes (Fig. 1b). Motile cilia have dynein arm projections between neighboring microtubules, which are crucial for their motile function along with the central microtubule pair and radial spokes. Motile cilia are typically found on the surface of multi-ciliated cells lining the organ cavities including ependymal cells lining the brain ventricles, efferent ducts or oviducts, and in the respiratory epithelium. Motile cilia with a “9 + 2” microtubule doublet arrangement beat in a coordinated fashion to generate unidirectional fluid flow [32]. When motile cilia function incorrectly, resulting pathologies include chronic respiratory disease, infertility, situs defects, and hydrocephalus. In contrast, primary, immotile cilia or “9 + 0” cilia lack the central pair of microtubules (Fig. 1b). 9 + 0 cilia protrude from the apical surface of most interphase and non-dividing cells in vertebrates and occur with only one cilium per cell [33]. Primary cilia have been linked to mechano- and chemo-sensation, and recent studies suggest they have a role in sensing morphogenic signals [34]. However, the differences between motile and immotile cilia should not be overstated, since motile cilia clearly also have a sensory role [35]. Furthermore, nodal cilia at the early embryonic structure called the ventral node in human and mouse, are motile but possess the 9 + 0 microtubule doublet arrangement typical of primary cilia. Nodal cilia have been proposed to establish left–right asymmetry during early embryogenesis by controlling fluid flow with a coordinated clockwise beating movement [36, 37].
Since the ciliary matrix is an extension of the cell cytoplasm but is devoid of ribosomes, all proteins involved in cilia biogenesis or function must be transported into and away from the cilium by a specialized trafficking system known as intraflagellar transport (IFT [38, 39]). IFT transportation is facilitated by kinesin or dynein microtubule-based motors [40], each moving in a single direction towards either the plus (anterograde) or minus (retrograde) end of the microtubule (Fig. 1c). All cilial/flagellar microtubules are orientated with their plus end at the distal tip [41]. Kinesins are responsible for powering anterograde transport [42] and dyneins for retrograde transport [43]. Both anterograde and retrograde transport are paramount for the formation and maintenance of cilia and flagella. Anterograde transport is responsible for moving the building blocks of the cilium along the axoneme, and retrograde transport allows disassembly (resorption) of the cilium prior to mitosis or the recycling of the components necessary for anterograde transport. IFT proteins are also involved in the delivery of proteins from the Golgi apparatus to the cilium[39], mediated by BBS proteins [44], and small GTPases of the Rab, Arf, and Arl subfamilies of the Ras superfamily [45]
Role of Primary Cilia in Signal Transduction Pathways and Neurodevelopment
Many epithelial cells have a single primary cilium (Fig. 1d), where they are proposed to act as “antennae” of the external environment (reviewed in [46, 47]). There is now considerable evidence that the development of the vertebrate embryo is dependent on a number of signaling pathways that converge at the primary cilium to control cell fate, the patterning of the tissues and morphogenesis. In the following section, we review briefly the mechanisms of the Sonic hedgehog (Shh) and Wnt signaling pathways, which are the best-studied to date, and discuss the role of primary cilia in these and other pathways.
Mechanosensation by the Primary Cilium and Calcium Signaling
In kidney cells, cilia have been proposed to mediate mechanosensory signals caused by apical fluid shear stress [48], which flattens the cilium and its surrounding plasma membrane, allowing a Ca2+ influx which in turn brings about an intracellular Ca2+ signaling response [49]. The proteins polycystin-1 (PC1) and polycystin-2 (PC2), which when mutated can both cause autosomal dominant polycystic kidney disease (ADPKD), are thought to mediate this mechanosensory process. PC1, a probable receptor, senses the fluid flow that induces bending of the cilium through conformational changes of its large extracellular domain, whereas PC2 is a transient receptor potential-like cation channel [49]. Together, PC1 and PC2 function in the cilium to allow mechanosensation, and a model has emerged, known as the flow hypothesis, that suggests that abnormal response to tubular lumen fluid flow leads to the pathogenesis of PKD and nephronopthisis (NPHP)[49, 50]. A similar flow hypothesis has been suggested to regulate switching between canonical and non-canonical Wnt signaling [51]. However, these simple and appealing models have been challenged by a study of a conditional mouse mutant of Pkd1 [52] that revealed that abnormal flow sensing, through loss of either the cilium or the mechanosensing components, is not sufficient to cause disease pathology. Furthermore, the subcellular localization of the PC1 receptor is not limited to primary cilia, and has been described at cell–cell contacts and focal adhesions [53, 54] suggesting that PC1 has additional, nonciliary functions. For example, PC1 regulates renal epithelial cell morphology through actin cytoskeleton rearrangements, including actin-based cell migration, and controlling adhesion between cells through the formation of stabilized, actin-associated, adherens junctions [55]. Furthermore, the actin cytoskeleton rearrangements induced by PC1 were dependent on phosphoinositide 3-kinase (PI3K) signaling [55], a key pathway in mediating adhesive cell–cell recognition as well as epithelial cell differentiation and polarity [56].
Canonical Wnt Signaling and Non-Canonical (Planar cell Polarity) Wnt Signaling
Wnt signaling regulates a varied set of developmental processes [57], with regulation of the pathway mediated by proteasomal degradation of β-catenin and phosphorylation of Disheveled by the kinase CK1δ. The best characterized components of the Wnt pathway are Frizzled receptors, a family of serpentine receptors [58]. Several experiments suggest that loss of cilia may lead to inappropriate activation of canonical Wnt signaling, for example the observation of high levels of cytoplasmic and nuclear β-catenin in postnatal kidneys [59] and pancreas [60] of animals that are unable to form primary cilia due to mutations in Kif3a, encoding a kinesin motor that mediates anterograde IFT. These findings suggest β-catenin degradation is a process that requires ciliary signaling. In addition, members of the β-catenin destruction complex, Gsk3β [61] and APC [62] are localized to the cilia. Moreover, it has been suggested that the basal body is an important regulator of Wnt signal interpretation through selective proteolysis [8]. In accordance with these findings, primary cilia restrict the activity of the canonical Wnt signaling pathway in the mouse embryo, primary fibroblasts and embryonic stem cells [62]. Similarly, non-canonical or planar cell polarity (PCP) Wnt signaling pathways have also been linked to cilia [18, 29, 63]. Loss of MKS gene expression in a number of model systems correlates with both cilia loss and hyperactivation of the PCP pathway such as increased actin stress fibers, aberrant phosphorylation of Disheveled and mislocalization of the small GTPase RhoA [18, 63]. Vangl2 (homologue of Drosophila van gogh-like 2), a protein mutated in the mouse loop-tail (Lp) model of severe NTDs [64], is a core component of the PCP pathway and is enriched in the basal bodies and cilia. The Vangl2 gene has also been shown to interact genetically with BBS genes, with perturbation of PCP signaling as a probable pathogenic mechanism in this ciliopathy [65]. The study also implicates PCP in the early process of neurulation during neurodevelopment, since Lp homozygous embryos show a completely open neural tube in the hindbrain and spinal region, a condition similar to the severe craniorachischisis defect in humans.
Sonic Hedgehog Signaling
Patterning of the mammalian neural tube is an early developmental event that is primarily dependent on Shh to specify the formation of the motor neurons and sensory neurons from the floor and roof plates of the neural tube, respectively (reviewed in [66]). In the neural tube of the developing embryo, neuronal precursor cell populations and the cell fate of the embryonic tissues are specified by the morphogen Shh. Shh is secreted by notochordal cells, diffuses dorsally into the surrounding cells of the neural tube, and then stimulates the ventral cells of the neural tube to form the floor plate cells. In turn, these cells secrete more Shh which diffuses to form a dorso-ventral concentration gradient. Both the concentration and length of Shh exposure result in specific fields of expression for the transcription factor complements that define neural cell types [67]. Crucially, the specification of ventral cell populations (the floorplate and V3 interneurons) in the neural tube is dependent on the expression of higher concentrations of Shh for a longer time, whereas lower levels specify motorneurons and additional interneuron fates (reviewed in [68]).
Recent evidence has implicated the primary cilium as a mediator of mammalian Shh signaling (reviewed in [47, 68]). In mammals, the Shh pathway is initiated by binding of hedgehog ligands to the transmembrane receptor Patched-1 (Ptch) in the cilia membrane. In the absence of ligand, Ptch represses the activity of the G protein-coupled receptor Smoothened (Smo), but allows Smo to translocate into the cilium upon ligand binding [69]. This alleviates Smo repression and results in pathway activation. The trafficking of Smo into the primary cilium appears to be essential for activation of the Shh pathway, since a mutant form of Smo that cannot localize to the cilium does fully mediate Shh signaling [69]. The important connection between anterograde IFT in cilia and Shh signaling emerged from a mouse mutagenesis screen for neural tube closure and patterning defects that identified the wimple (wim) and flexo (fxo) mutants [6]. These had mutations in the ciliary IFT genes Ift172 and Ift88 [6] that mediate anterograde IFT. Embryos with mutations in these genes did not have correct specification of ventral cell types in the developing neural tube and had reduced expression of Ptch1, both of which suggest a direct inhibition of Shh signaling (reviewed in [70]). In contrast, another IFT mutant, alien (aln), with mutations in Ift139/Ttc21b that normally mediates retrograde IFT, had dorsal expansion of ventral cell types in the neural tube [71]. The differences in neural tube patterning and Shh response may therefore depend on whether anterograde or retrograde IFT is affected. These studies revealed the requirement of ciliogenesis, or proteins involved in ciliogenesis, for Shh signaling in mammals. However, the situation is likely to be more complex, since preaxial polydactyly was also observed in the Ift88 fxo mutants, which is suggestive of hyper-responsive Shh signaling in some developmental zones [6].
Subsequent studies attributed the Shh defect observed in IFT mutant mice to aberrant processing of the Gli3 transcription factor [72]. Proteolytic processing of Gli3 occurs in proteosomes, that can themselves also localize to the basal body of cilia [73]. Furthermore, many other regulators of the Shh pathway, such as Suppressor of Fused (SuFu) and the Gli transcription factors (Gli1, Gli2, and Gli3) localize to cilia [34, 74]. Conversely, basal body proteins are thought to play a role in Shh signaling (Fig. 1c), including the Ftm/Rpgrip1l protein [75]. In humans, mutations in RPGRIP1L cause MKS and other severe ciliopathies (see below). However, the functional role of RPGRIP1L and other basal body or ciliary proteins such as MKS1 and meckelin in Shh signaling remain unclear.
Other Signaling Pathways
Primary cilia have also been implicated in the mammalian target of rapamycin (mTOR) signaling pathway which is a major effector of cell growth and proliferation through the regulation of protein synthesis [76]. The pathway is mediated by mTOR, a serine/threonine protein kinase that is the catalytic subunit of two molecular complexes, mTOR Complex 1 and 2 (mTORC1 and 2) [77]. A role for this pathway during normal ciliary signaling is suggested by the finding that the cytoplasmic tail of PC1, one of the ciliary protein mutated in ADPKD, regulates the inhibition of mTOR through an interaction with tuberin [78]. Furthermore, mTOR is inappropriately activated in the epithelial lining of renal cysts in animal models of ciliopathies [78–80], and rapamycin treatment of these animals has been shown to be highly effective in reducing cystogenesis [80, 81]. Growth control by PDGF also appears to be dependent on primary cilia. In a mechanism reminiscent of Smo activation, the PDGFRα receptor is translocated into the primary cilium to activate the Mek1/2-Erk1/2 pathway [7]. Mek1/2 is itself phosphorylated within the cilium and at the basal body [7]. Finally, an unexpected link between PDGF growth factor and PI3K signaling at the primary cilium has recently been revealed through the identification of mutations in the phosphatidylinositol (3,4,5)-triphosphate (PtdIns(3,4,5)P3) 5-phosphatase INPP5E/Inpp5e as a cause of JS and ciliopathies in humans and mice [82, 83]. Activation of the ciliary PI3K signaling pathway appeared to be essential for maintaining ciliary stability, which may prevent premature entry into the cell cycle.
Molecular Genetics and Cell Biology of Meckel–Gruber Syndrome Genes and Proteins
Whilst the function and importance of motile cilia has long been recognized, the role of primary cilia in mammalian development has been established only recently. The first, seminal observation was the identification of an insertion mutation in the mouse homologue of the Ift88 gene (known as Tg737, encoding the protein Polaris) which gave rise to a ciliopathy phenotype resembling human autosomal recessive polycystic kidney disease and that was first described in the orpk (Oak Ridge Polycystic Kidney disease) mouse [84]. Subsequent work by Pazour and colleagues demonstrated that the phenotype of homozygous Ift88 orpk mutants had bilateral polycystic kidneys, abnormal outer segment development and retinal degeneration, and resulted in premature death within a few weeks of birth [85]. The delineation of the ciliopathy phenotype and positional cloning efforts led to the discovery that the ciliopathy BBS is caused by mutations in genes that encode proteins localized to the cilium/basal body or are implicated in ciliogenesis [86]. With the subsequent remarkable progress in recent years in the identification of additional BBS genes (reviewed in [87]) further insights into the important roles of the cilium/basal body have been possible. In particular, it is now clear that the genetic architecture of a supposedly simple, monogenic condition is unexpectedly complex, with the epistatic effect of modifier alleles contributing to the pleiotropy seen in BBS.
Defects in the structure of function of primary and motile cilia have now been implicated as causative for a range of clinical features that include primary cilary dyskinesia, hydrocephalus, polycystic kidney disease (PKD) and some forms of Leber congenital amaurosis [1]. These typical ciliopathy phenotypes have led to possibilities for therapeutic interventions. For example, the mTOR pathway is inappropriately activated in PKD [80], and mTOR inhibitors such as rapamycin (Sirolimus) and the related Everolimus are potential therapeutics now in Phase II and III clinical trials (http://www.pkdcure.org/).
Genetic Basis of Phenotypic Variation in Severe Ciliopathies
The description of “typical ciliopathy” features for MKS is somewhat less clear, since the MKS phenotype is broad and variable, with multi-systemic involvement and extensive pleiotropy even for mutations in individual MKS genes [16–18]. The situation for MKS is undoubtedly complicated by the probable involvement of defects in both the Shh and Wnt signaling pathways as important factors in disease pathogenesis [18, 63, 88]. This has prompted the suggestion that pleiotropy in MKS and other ciliopathies cannot be explained from simple allelism in a single gene, and that modifier alleles contribute to the phenotypic variation [89, 90]. Furthermore, mutations in the MKS genes MKS1, TMEM67/MKS3, and CEP290 can either cause BBS or may have an epistatic effect on mutations in known BBS-associated genes [29]. Conversely, mutations in the BBS2, BBS4, BBS6, and NPHP3 genes have also been associated with MKS-like phenotypes [28, 91], although not the full MKS phenotype with the classic triad of clinical features.
MKS also has extensive genetic heterogeneity. To date, mutations in six genes have been identified as causative for MKS (Table 1), which in total account for approximately 75% of cases. All MKS genes encode proteins that localize to either the cilium or the basal body (Fig. 1a and b), and are likely to be involved in the process of ciliogenesis. A further two loci have been mapped, but the causative gene has yet to be identified (unpublished data), and there are undoubtedly additional loci still to be identified. In the next section, we discuss the six known MKS genes in further detail and review the accrued evidence for the function of the encoded proteins.
MKS1, a Novel Basal Body Protein
The first MKS locus, MKS1, locus was mapped to chromosome 17q in endogamous Finnish kindreds [92]. Pathogenic mutations were identified in FLJ20345/MKS1 a previously uncharacterized gene of 14-Kb [93]. The Finnmajor (IVS15-7_35 del) mutation was identified as the Finnish founder mutation, in which 29 bp are deleted from intron 15 resulting in skipping of exon 16, and subsequent frameshift and premature termination of the MKS1 protein [93]. Further splice site, frameshift, and truncating mutations in MKS1 have been identified as causes of MKS [5], but there is no apparent genotype–phenotype correlation. Missense mutations in MKS1 do not appear to causative for MKS, but may exert an epistatic effect on recessive BBS mutations [29] and may be associated with multiple episodes of seizures, a phenotype that is not associated with either BBS or MKS. MKS1 is widely expressed, with high levels in the brain, liver, kidney and developing digits [93]. The encoded protein MKS1 contains a highly conserved B9 domain, of unknown function. MKS1 localizes at the basal body (Fig. 1c) and is required for ciliogenesis in a kidney epithelial cell-line model, as demonstrated by transient knockdown with siRNAs [94]. The same study also demonstrated that MKS1 is required for correct branching during kidney tubulogenesis and either the apical organization or correct determination of apical–basal polarity in epithelial cells [94]. During ciliogenesis, this caused a defect in basal body positioning and/or docking at the apical cell surface, and was a cellular defect also observed following transient knockdown of MKS3/TMEM67.
Two other mammalian proteins are known to contain the MKS1 B9 domain: B9D1 and B9D2 (also known as MKS-related1 [mksr1] and 2 [mksr2], respectively, in C. elegans). In C. elegans, disruption of any of the B9 proteins causes a defect in ciliogenesis, and genetic interactions have been shown for all mks/mksr double mutant combinations [95]. Both B9 proteins and MKS1 are thought to localize to the basal body/transition zone of sensory cilia and their correct localization is dependent on their mutual interaction [95]. Both B9D1 and B9D2 are therefore excellent functional candidate for MKS or related ciliopathies. However, screening of these genes in large MKS and JS patient cohorts have not revealed any putative pathogenic mutations (data unpublished).
TMEM216, a Novel Tetraspan Protein and Possible Co-Receptor
The second locus, MKS2, was mapped to chromosome 11q13 [96]. Recent work has identified mutations in TMEM216, encoding a novel 17-kDa tetraspan protein (Fig. 1d), as the causative MKS2 gene [18]. Predominantly splice and premature termination mutations were found in MKS patients, whereas missense mutations were shown to be causative for JS. In addition to encephalocele and meningoencephalocele, bile duct proliferation and cystic kidneys, MKS fetuses with TMEM216 mutations frequently displayed cleft palate, polydactyly and bowing of the long bones [18].
TMEM216 was shown to localize to the basal body, ciliary membrane and other microtubule structures in mouse and human cell lines and tissue. Transient knockdown of Tmem216 in kidney epithelial cells resulted in reduced ciliogenesis and failure of basal body docking at the apical cell surface. Previous studies have shown that basal body docking is prevented by the interruption of actin cytoskeleton remodeling [97], and is dependent on both RhoA activation and regulation by the core PCP protein Disheveled [98]. Consistent with this model, TMEM216-mutated fetal fibroblasts displayed prominent actin stress fibers in the cyoskeleton, and showed evidence of increased activation of the small GTPase RhoA, indicative of disrupted non-canonical (PCP) Wnt signaling [99, 100]. TMEM216-GFP also co immunoprecipitated with meckelin, RhoA and Disheveled-1, further supporting a role of TMEM216 in Wnt signaling. Morpholino knockdown of tmem216 in zebrafish embryos gave rise to morphant phenotypes associated with PCP defects, such as shortened body axis, broad notochords and misshaped somites. Tetraspan proteins can act as membrane scaffolds to promote multimerization of receptors and signaling complexes[101], and it is therefore proposed that TMEM216 acts to bring together various components of the PCP pathway including meckelin, to allow efficient Wnt signal transduction [18].
TMEM67/Meckelin, a Frizzled-like Receptor
MKS3 was identified by autozygosity mapping on chromosome 8q24 [102], and the causative gene established to be TMEM67, encoding a predicted transmembrane domain protein since named meckelin [103]. Meckelin shares topological homology with the Frizzled (Fzd) family of Wnt receptors [104, 105]. TMEM67/MKS3 mutations include nonsense, splice site, frameshift, inframe deletions and missense [5], and although there is no apparent genotype–phenotype correlation, almost all of the known mutations occur in exons 1 to 18 of the gene which encode the extracellular and transmembrane domains of meckelin (Fig. 1d).
Meckelin has been shown to localize to the apical and ciliary membrane of epithelial cells, and has also been reported at basal bodies and basal/basolateral actin cables [63]. siRNA-mediated knockdown of Tmem67/Mks3 expression in kidney epithelial cells and TMEM67-mutated fetal fibroblasts had prominent actin stress fibers and hyperactivation of RhoA [63]. Knockdown also prevented migration of the basal body to the apical cell surface, thereby also preventing ciliogenesis [94]. In contrast, in patient kidney tissue and following stable knockdown with shRNA longer, multi-ciliated and multi-centrosomal cellular phenotypes have been reported [106], which suggests a regulatory role for meckelin during ciliogenesis that is dependent on either differentiation or the cell cycle. MKS1 and meckelin could therefore regulate cilia length and appropriate cilia number through control of centrosome duplication, although it is unclear how the proposed signaling role of meckelin may mediate this process.
Biochemical assays have shown that meckelin interacts with MKS1 [94], subsequently confirmed by a genetic interaction between mks-1 and mks-3 in a study of C. elegans mutants [107]. Meckelin has also been shown to interact with TMEM216 [18], suggesting the existence of a Wnt signaling receptor-co-receptor complex, although interactions with other mediators of Wnt signaling such as the Disheveled proteins have yet to be confirmed. The ligands for meckelin, by analogy with Frizzled receptors, are likely to be Wnt proteins, with the non-canonical PCP effector Wnt5A a likely candidate on the basis of function. Zebrafish morphant experiments suggest a genetic interaction between mks3 and wnt5a [29], but evidence for a direct biochemical interaction is lacking. Yeast-2-hybrid assays with the coiled-coil domain of meckelin have allowed further understanding of the mechanism through which meckelin acts to mediate basal body migration. Nesprin-2 was identified as a potential interactant and confirmed by biochemical assays [63]. Nesprin-2 is a nuclear envelope protein with an actin binding domain that mediates actin-mediated positioning of organelles, so it is proposed that a meckelin-nesprin-2 interaction facilitates correct docking of the basal body at the apical surface through actin cytoskeleton remodeling [63].
CEP290, a Centrosomal Protein with Many Coiled-Coil Domains
Mutations in CEP290 have been identified as a fourth cause of MKS (MKS4) [16]. Interestingly, mutations in CEP290 had been identified previously as a cause of a range of ciliopathies, varying in severity from JS [108], nephronophthisis and Senior–Løken syndrome (NPHP/SLS) [108] to Leber congenital amaurosis (LCA) [109]. CEP290-mutated fetuses had the classic MKS triad of features (brain malformation, cystic kidneys and bile duct proliferation of liver), caused by predominantly nonsense mutations. The most common CNS malformations were occipital encephalocele, prosencephalic dysgenesis and rhombic roof dysgenesis [16]. The extreme pleiotropy for CEP290 mutations have also helped to further elucidate the allelism and phenotype continuum between JS and MKS. The identification of CEP290 mutations in patients with a cerebro-reno-digital syndrome further demonstrates that MKS and JS can be variable expressions of the same ciliopathy [16]. The co-existence of JS and MKS within sibships suggests that there is little genotype–phenotype correlation and is strong evidence for the existence of modifier alleles.
CEP290 encodes a large protein of 290 kDa, with several predicted domains including many repeats of the coiled-coil domain that mediates protein–protein interactions [110]. CEP290 was initially characterized as a centrosomal protein: it localizes to the centrosomes of mitotic cells [111] and to the basal bodies in post-mitotic cells [108]. Biochemical assays show that CEP290 interacts with several microtubule-associated proteins (including centrin, gamma-tubulin, KIF3a, RPGR, and RPGRIP1) [111]. Transient knockdown of CEP290 also disrupts the cytoplasmic microtubule network [112]. CEP290 is required for ciliogenesis, and may mediate the ciliary targeting of Rab8, a small GTPase shown to interact with the “BBSome” BBS protein complex to promote ciliogenesis [112]. CEP290 has also been suggested to be a molecular linker between the transition zone microtubules of the cilium and the plasma membrane to ensure basal body docking at the apical cell surface and subsequent ciliogenesis [31]. The rd16 mouse model of early-onset retinal degeneration is the only current model with a mutation in Cep290 [111]. This mutation is an inframe deletion, and is likely to be hypomorphic since rd16 homozygotes only manifest an eye phenotype. The development of a new model with a knockout or truncating mutation is therefore important since it will provide valuable insights into the severe ciliopathies and pleiotropy caused by CEP290 null mutations.
RPGRIP1L
A genome-wide linkage scan identified a fifth locus, MKS5, for MKS on chromosome 16q in two MKS families and two JS families with extraneurological features. The minimal interval contained KIAA1005, an excellent candidate gene, because of a previously described interaction with ciliary protein nephrocystin-4 [113]. The gene was subsequently renamed as RPGRIP1L, due to the close identity to RPGRIP1. Mutations in RPGRIP1L cause both MKS and JS, with some evidence of a genotype–phenotype correlation: MKS mutations are nonsense or frameshift, whereas JS patients have at least one missense mutation [17]. MKS fetuses with RPGRIP1L mutations had enlarged cystic kidneys, bile duct proliferation and severe brain abnormalities that included encephalocele and anencephaly. Other unusual features include microphthalmia, medial orofacial clefting, and other craniofacial anomalies such as maxillary hypoplasia and micrognathia [17]. A recent study has also implicated a common allele in the RPGRIP1L protein, p.A229T, as a modifier of retinal degeneration in ciliopathies [89]. The minor allele T229 was over-represented in BBS and Senior–Løken syndrome patient cohorts, for which retinal phenotype is obligatory, whereas it was absent in nephronophthisis patients that lack any retinal features.
The RPGRIP1L protein consists of an RPGR-interacting domain (RID, homologous to the RID of RPGRIP1), five coiled-coil domains and two calcium-binding C2 domains (Fig. 1c). The latter mediate the interaction with nephrocystin-4 [113]. The C2 domain consists of 120 amino acids that are involved in calcium-dependent binding of intracellular proteins, inositol polyphosphates and phospholipids during membrane targeting [114]. Colocalisation of RPGRIP1L with nephrocystin-4 and -6 occurred at and around the basal body, although RPGRIP1L can also be found along the ciliary axoneme and throughout the cytoplasm [17]. A possible role for RPGRIP1L may be to mediate IFT and more general cellular microtubule transport, although direct evidence for such a role is lacking. Defective IFT may explain the Shh signaling defects in the Ft mouse (mutant for Rpgrip1l; see below), although a further, perhaps complementary, possibility is that loss of the ciliary signaling compartment in this model has an indirect effect on Shh reposiveness.
CC2D2A, a Potential Mediator of Intracellular Ca2+ Signaling
The sixth locus, MKS6, for MKS on chromosome 4p15 was identified through the careful analysis of homozygous haplotypes in Finnish endogamous kindreds to determine regions of linkage disequilibrium. A common Finnish founder mutation was found in CC2D2A, the c.1762 C > T substitution, that creates a new donor splice site leading to a defective transcript. Affected fetuses showed typical MKS features including occipital encephalocele [114]. Other features included genital abnormalities, ocular coloboma, cleft palate, bowing of the long bones, and situs defects such as accessory spleens and dextrocardia [114, 115]. Truncating mutations result in MKS, whereas damaging missense mutations cause JS [115]. CC2D2A contains a single calcium-binding C2 domain and two coiled-coil domains, similar in domain repertoire to RPGRIP1L. A single C2 domain is usually a feature of signal transduction enzymes [114] such as protein kinase C, and since CC2D2A is also a potential calmodulin binding partner [116] this implicates CC2D2A in mediating an intracellular Ca2+ signaling response, although the molecular mechanism is still to be elucidated. Further work to elucidate this mechanism will provide important insights into the normal mechanisms of development that are disrupted in MKS, and further work on mouse models will be particularly informative.
Insights into Neurodevelopment from Mammalian Models of MKS
In the following section, we summarize some of the existing and emerging data on mammalian models of MKS, and describe some of the insights into neurodevelopment and patterning of the neural tube.
The krc Model of MKS1
A mouse model of human MKS1 mutations was identified in the kerouac (krc) ENU-induced mouse mutant [88]. This model provides a good representation of MKS with high penetrance of key features such as enlarged cystic kidneys, hepatic developmental defects and exencephaly [88]. Occipital encephalacele was not observed in Mks1 krc, but anencephaly (a failure of neural tube closure) and splitting of the superoccipital bone seem to reiterate the occipital anomalies seen in humans [88]. The specification of neuronal cell types in the neural tube of Mks1 krc mutant E10.5 embryos were consistent with defects in Shh signaling. Mutants had a broader domain of low-level Shh signaling, in comparison to wild-type litter mates. However, high-level Shh signaling was lost, which prevented the specification of ventral cell fates and reduced the number of floor plate cells and V3 interneuron progenitors [88]. In addition, this was accompanied by a ventral expansion of motor neurons at cervical, forelimb and hindlimb levels. Both observations are consistent with a reduction in high-level Shh signaling, but the molecular mechanism by which MKS1 mediates the Shh signal is unknown.
A conditional knockout for the Mks1 orthologue B9d2 exists in the stumpy mouse model, which has hydrocephalus and polycystic kidneys in mice lacking B9d2 [117]. The cerebral ventricle dilation in these mice was suggested to be secondary to the altered flow of cerebrospinal fluid due to the dysmorphic or absent cilia on ependymal cells lining the ventricles [117].
Mouse and Rat Models of MKS3
The bpck (bilateral polycystic kidney disease) mouse has a spontaneous 295 kb deletion that includes Tmem67. The predominant feature of this viable MKS model is severe renal cystic dysplasia causing rapidly progressive PKD and death by 3 weeks [118]. Although some homozygous mutants also developed hydrocephalus, as with other MKS mouse models, there were no other reported features comparable with human TMEM67-mutated patients [118]. The Wpk (Wistar polycystic kidney) rat has a similar viable phenotype caused by a probable hypomorphic missense mutation in Tmem67 [103]. Tmem67 Wpk Homozygotes have rapidly progressive PKD, hydrocephaly, hypoplasia to agenesis of the corpus callosum, and retinal degeneration [106].
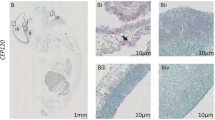
We have recently developed a targeted knockout model of Tmem67, which appears to reiterate many of the features of human MKS, including the neurodevelopmental defects. Homozygotes die after birth at the first postnatal day (P0), but at early stages of development E11.5 and E12.5 embryos have a variable range of cranial and spinal neural tube defects (NTDs). In wild-type embryos the neural folds of the midbrain fuse completely and become covered with a well-developed layer of the head mesoderm (Fig. 2a), while in homozygous knockout embryos the neural folds remain separate, their ends are thickened, the head mesoderm overlaying the defect is very poorly developed and it is formed of a thin, loose layer of mesodermal tissues (Fig. 2b). Homozygotes also manifest midbrain exencephaly and thoraco-lumbar neural tube defects (Fig. 2d) in some E11.5 and E12.5 embryos, respectively. In addition, other diagnostic features of MKS manifest in this model, including renal cysts and hepatic ductal plate proliferation defects. These findings indicate that Tmem67/meckelin is essential for normal neurulation, as well as kidney and biliary system development, although the exact molecular function of the receptor in these processes remains unknown.
Cranial and spinal neural tube defects in Tmem67 −/− knockout mouse E11.5 and E12.5 embryos. a and b Hematoxylin and eosin stained transverse brain sections at the level of the third ventricle (3V). Wild-type+/+ a brain sections show normally fused neural folds overlaid by a well-developed layer of the head mesenchyme. b A corresponding section in a Tmem67 −/− knockout E11.5 embryo, clearly showing a neural tube defect (NTD, indicated by the brace) at the level of the mesencephalon (MS) with widely separated neural folds (NF). c and d Median sagittal sections through whole wild-type+/+ (c) and Tmem67-/- knockout (d) E12.5 embryos. In the wild-type section (c) the vertebral bodies (VT) and spinal neural tube (NT) are well developed and covered with a well-developed adherent layer of surface ectoderm (SE). In the Tmem67 −/− section (d), the spinal neural tube defect (NTD) in the thoraco-lumbar region is indicated by the brace, and the surface ectoderm (SE) covering the defect is raised and abnormal. The vertebral bodies (VB) are also defective
The Fused Toes (Ft) Model of MKS5/RPGRIP1L
The Fused toes (Ft) mouse is a result of a deleted region syntenic to part of the human MKS5 locus, which includes the Rpgrip1l/RPGRIP1L genes. Homozygous Ft mutant mice are not viable, and die between embryonic day (E) 10.5 and E14.5 due to a complex heart malformation [119]. They exhibited major craniofacial and forebrain abnormalities, polydactyly and syndactyly. Ft/Ft mutant mice lacked Shh gene expression in the floor plate and the majority of the spinal cord by E11.5, although Shh was expressed in the most anterior and posterior parts of the neural tube and dorsal neural tube patterning was not disturbed. This suggests that Shh is inappropriately inactivated, so that although floor plate induction is initiated in these mutants, it is not then sustained which gives rise to floor plate and midline defects. As expected, the expression of the transcription factor Foxa2 at the floor plate was reduced, and the correct specification of all ventral neural subtypes was lost. This mouse model is therefore likely to provide important insights into the mechanism of neural tube defects and neurogenesis defects in ciliopathies [75].
Conclusions
It is now clear that primary cilia have a fundamental role as a specialized compartment for extracellular signal transduction that control cell fate, the patterning of the tissues and morphogenesis. Meckel–Gruber syndrome, the most severe ciliopathy, provides a fascinating insight into these processes during neurulation and patterning of the neural tube during early embryogenesis. In MKS, it is clear that Wnt signaling is disrupted, with hyperactivation of the PCP pathway [18, 63] and defects in convergent-extension cell movements during embryogenesis [29]. One predication, that needs to be experimentally verified, is that defects in the PCP pathway will probably indirectly impact on the canonical Wnt signaling. Previous work suggests that primary cilia restrict the activity of the canonical Wnt signaling pathway [62], so loss of cilia due to an MKS gene mutation is likely to increase the activity of the canonical pathway through an indirect mechanism. However, it is unclear if this mechanism would be restricted to the loss of expression of TMEM67/meckelin, encoding a Frizzled-like receptor [103], and TMEM216, a probable co-receptor [18].
RPGRIP1L, in particular, is implicated in mediating Shh signaling, with the Ft mouse model suggesting inappropriate inactivation of this pathway [75]. Anterograde IFT appears to be essential for Shh pathway activation [6], either directly through translocation of Smo to the cilium [69] or indirectly by ensuring the assembly of the cilium during ciliogenesis. One possible role for RPGRIP1L is therefore in mediating anterograde IFT. However, RPGRIP1L also contains two calcium-binding C2 domains that are often found in membrane trafficking proteins such as the synaptotagmins [120]. Synaptotagmins are proposed to be calcium sensors that mediate early synaptic vesicle docking to the presynaptic membrane and regulate exocytosis of neurotransmitter release and hormone secretion [120]. An analogous process of vesicle trafficking and targeting at the basal body may therefore be mediated by RPGRIP1L, perhaps in concert with other proteins that either participate in vesicular transport such as Rab8 [44, 112] or SNARE proteins that regulate vesicle fusion at a target compartment. The functional role of the MKS1 protein is also still unclear, and there are no obvious hints from the highly conserved but unique B9 domain. MKS1 interacts at both the genetic and biochemical level with TMEM67/meckelin [94, 107] and localizes to the basal body [94], so it may be a key component in the transduction of Wnt signaling by this organelle. Basal body integrity appears to be essential for the proteasomal targeting of signaling proteins, such as β-catenin [8] or Disheveled [50] for degradation, and a reasonable hypothesis is that MKS1 regulates proteasomal processing of these proteins during ciliary signaling. Furthermore, the defects in Shh signaling observed in the krc mouse could also be explained, at least in part, by a proteasomal processing defect, since disruption of both the basal body and cilium results in impaired processing of Gli2/3 [34, 70].
If the different MKS proteins have such disparate functional roles, why does their loss or mutation lead to the same clinical phenotype of a severe ciliopathy? We would argue that severe ciliopathies may be more accurately described as “inborn errors of embryonic development”, and that any disruption of the individual components that mediate ciliary signaling will result in a ciliopathy phenotype. What is perhaps puzzling is why the clinical phenotype is not even more severe, but the explanation may be simply that not all cilia mediate the same signaling processes, and that these events are both time and tissue specific during embryogenesis. However, even allelism in a single gene does not satisfactorily explain the remarkable pleiotropy seen for Meckel–Gruber syndrome, for mutations in individual MKS genes even within single families [16–18]. If MKS proteins are indeed important mediators of embryonic signaling, then a reasonable hypothesis is therefore that modifier alleles in the anterograde IFT genes could contribute to the MKS phenotype through an epistatic mechanism. Hypomorphic alleles in anterograde IFT genes would be expected to have a particular impact on Shh signaling, and therefore on the manifestation of clinical features such as polydactyly and midline defects. In the future, it will therefore be important to correlate these features with sequence variants in IFT genes. This may further elucidate the genetic basis of phenotypic variability in a mendelian condition and hence the molecular basis of pleiotropic ciliopathies. We envisage that future exciting research in the field of ciliopathies will focus on identifying additional modifier loci using next generation sequencing technologies and animal models to suggest genetic interactions. The insights gained from human disease and the development of MKS animal models should elucidate the fundamental biology of signaling pathways during neurulation, neurogenesis, and other developmental processes and the still undetermined functional roles of primary cilia in these events.
References
Badano JL et al (2006) The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet 7:125–148
Adams M et al (2008) Recent advances in the molecular pathology, cell biology and genetics of ciliopathies. J Med Genet 45(5):257–267
Simpson JL et al (1991) Genetic heterogeneity in neural tube defects. Ann Génét 34(3–4):279–286
Doherty D et al (2005) Prenatal diagnosis in pregnancies at risk for Joubert syndrome by ultrasound and MRI. Prenat Diagn 25(6):442–447
Khaddour R et al (2007) Spectrum of MKS1 and MKS3 mutations in Meckel syndrome: a genotype–phenotype correlation. Mutation in brief #960. Online. Hum Mutat 28(5):523–524
Huangfu D et al (2003) Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426(6962):83–87
Schneider L et al (2005) PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol 15(20):1861–1866
Gerdes JM et al (2007) Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet 39(11):1350–1360
Krahe C (1684) Description of a monstrous child, born Friday the 29th of February 1684 at a village called Heisagger, distant about 4 English miles from Hattersleban, a town in South-Jutland, under the King of Denmark’s dominion. Phil Trans Roy Soc 14:599–600
Kompanje EJ (2003) Features described and illustrated in 1684 suggesting Meckel–Gruber syndrome. Pediatr Dev Pathol 6(6):595–598
Meckel JF (1822) Beschreibung zweier, durch sehr ahnliche Bildungsabweichhungen entstellter Geschwister. Dtsch Arch Physiol 7:99–172
Gruber GB (1934) Beitrage zur Frage ‘gekoppelter’ Missbildungen. (Akrocephalo-Syndaktylie und Dysencephalia). Beitr Pathol Anat 93:459–476
Howe JJ, Opitz JM (1969) The Meckel syndrome (dysencephalia splancnocystica, the Gruber syndrome). Birth Defects OAS 5:167–179
Fraser FC, Lytwyn A (1981) Spectrum of anomalies in the Meckel syndrome, or: “Maybe there is a malformation syndrome with at least one constant anomaly”. Am J Med Genet 9(1):67–73
Wright C et al (1994) Meckel syndrome: what are the minimum diagnostic criteria? J Med Genet 31(6):482–485
Baala L et al (2007) Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet 81(1):170–179
Delous M et al (2007) The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet 39(7):875–881
Valente EM et al (2010) Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat Genet 42(7):619–625
Salonen R, Norio R (1984) The Meckel syndrome in Finland: epidemiologic and genetic aspects. Am J Med Genet 18(4):691–698
Shen-Schwarz S, Dave H (1988) Meckel syndrome with polysplenia: case report and review of the literature. Am J Med Genet 31(2):349–355
Hori A et al (1980) CNS dysplasia in dysencephalia splanchnocystica (Gruber’s syndrome). A case report. Acta Neuropathol 51(2):93–97
Ahdab-Barmada M, Claassen D (1990) A distinctive triad of malformations of the central nervous system in the Meckel–Gruber syndrome. J Neuropathol Exp Neurol 49(6):610–620
Herriot R, Hallam LA, Gray ES (1991) Dandy–Walker malformation in the Meckel syndrome. Am J Med Genet 39(2):207–210
Baala L et al (2007) The Meckel–Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am J Hum Genet 80(1):186–194
Maria BL et al (1999) Molar tooth sign in Joubert syndrome: clinical, radiologic, and pathologic significance. J Child Neurol 14(6):368–376
Holmes LB, Driscoll SG, Atkins L (1976) Etiologic heterogeneity of neural-tube defects. N Engl J Med 294(7):365–369
Seller MJ (1978) Meckel syndrome and the prenatal diagnosis of neural tube defects. J Med Genet 15(6):462–465
Karmous-Benailly H et al (2005) Antenatal presentation of Bardet–Biedl syndrome may mimic Meckel syndrome. Am J Hum Genet 76(3):493–504
Leitch CC et al (2008) Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet–Biedl syndrome. Nat Genet 40(4):443–448
Pazour GJ, Bloodgood RA (2008) Targeting proteins to the ciliary membrane. Curr Top Dev Biol 85:115–149
Craige B et al (2010) CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol 190(5):927–940
Simons M, Mlodzik M (2008) Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet 42:517–540
Eggenschwiler JT, Anderson KV (2007) Cilia and developmental signaling. Annu Rev Cell Dev Biol 23:345–373
Haycraft CJ et al (2005) Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 1(4):e53
Shah AS et al (2009) Motile cilia of human airway epithelia are chemosensory. Science 325(5944):1131–1134
Hirokawa N et al (2006) Nodal flow and the generation of left–right asymmetry. Cell 125(1):33–45
Basu B, Brueckner M (2008) Cilia multifunctional organelles at the center of vertebrate left–right asymmetry. Curr Top Dev Biol 85:151–174
Marszalek JR, Goldstein LS (2000) Understanding the functions of kinesin-II. Biochim Biophys Acta 1496(1):142–150
Rosenbaum JL, Witman GB (2002) Intraflagellar transport. Nat Rev Mol Cell Biol 3(11):813–825
Hirokawa N (1998) Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279(5350):519–526
Binder LI, Dentler WL, Rosenbaum JL (1975) Assembly of chick brain tubulin onto flagellar microtubules from Chlamydomonas and sea urchin sperm. Proc Natl Acad Sci USA 72(3):1122–1126
Huang B, Rifkin MR, Luck DJ (1977) Temperature-sensitive mutations affecting flagellar assembly and function in Chlamydomonas reinhardtii. J Cell Biol 72(1):67–85
Pazour GJ, Wilkerson CG, Witman GB (1998) A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT). J Cell Biol 141(4):979–992
Nachury MV et al (2007) A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129(6):1201–1213
Gillingham AK, Munro S (2007) The small G proteins of the Arf family and their regulators. Annu Rev Cell Dev Biol 23:579–611
Lancaster MA, Gleeson JG (2009) The primary cilium as a cellular signaling center: lessons from disease. Curr Opin Genet Dev 19(3):220–229
Berbari NF et al (2009) The primary cilium as a complex signaling center. Curr Biol 19(13):R526–R535
Praetorius HA, Spring KR (2001) Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184(1):71–79
Nauli SM et al (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33(2):129–137
Simons M et al (2005) Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 37(5):537–543
Benzing T, Simons M, Walz G (2007) Wnt signaling in polycystic kidney disease. J Am Soc Nephrol 18(5):1389–1398
Garcia-Gonzalez MA et al (2007) Genetic interaction studies link autosomal dominant and recessive polycystic kidney disease in a common pathway. Hum Mol Genet 16(16):1940–1950
Huan Y, van Adelsberg J (1999) Polycystin-1, the PKD1 gene product, is in a complex containing E-cadherin and the catenins. J Clin Invest 104(10):1459–1468
Roitbak T et al (2004) A polycystin-1 multiprotein complex is disrupted in polycystic kidney disease cells. Mol Biol Cell 15(3):1334–1346
Boca M et al (2007) Polycystin-1 induces cell migration by regulating phosphatidylinositol 3-kinase-dependent cytoskeletal rearrangements and GSK3beta-dependent cell cell mechanical adhesion. Mol Biol Cell 18(10):4050–4061
Rivard N (2009) Phosphatidylinositol 3-kinase: a key regulator in adherens junction formation and function. Front Biosci 14:510–522
Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810
Bovolenta P et al (2008) Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci 121(Pt 6):737–746
Lin F et al (2003) Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA 100(9):5286–5291
Cano DA et al (2004) Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development 131(14):3457–3467
Wilson NF, Lefebvre PA (2004) Regulation of flagellar assembly by glycogen synthase kinase 3 in Chlamydomonas reinhardtii. Eukaryot Cell 3(5):1307–1319
Corbit KC et al (2008) Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol 10(1):70–76
Dawe HR et al (2009) Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton. J Cell Sci 122(Pt 15):2716–2726
Kibar Z et al (2001) Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet 28(3):251–255
Ross AJ et al (2005) Disruption of Bardet–Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet 37(10):1135–1140
Goodrich LV, Scott MP (1998) Hedgehog and patched in neural development and disease. Neuron 21(6):1243–1257
Dessaud E, McMahon AP, Briscoe J (2008) Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development 135(15):2489–2503
Wong SY, Reiter JF (2008) The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol 85:225–260
Corbit KC et al (2005) Vertebrate Smoothened functions at the primary cilium. Nature 437(7061):1018–1021
Huangfu D, Anderson KV (2005) Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA 102(32):11325–11330
Tran PV et al (2008) THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet 40(4):403–410
Liu A, Wang B, Niswander LA (2005) Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 132(13):3103–3111
Wigley WC et al (1999) Dynamic association of proteasomal machinery with the centrosome. J Cell Biol 145(3):481–490
Wen X et al (2010) Kinetics of hedgehog-dependent full-length Gli3 accumulation in primary cilia and subsequent degradation. Mol Cell Biol 30(8):1910–1922
Vierkotten J et al (2007) Ftm is a novel basal body protein of cilia involved in Shh signalling. Development 134(14):2569–2577
Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18(16):1926–1945
Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124(3):471–484
Shillingford JM et al (2006) The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA 103(14):5466–5471
Brown JH et al (2005) Missense mutation in sterile alpha motif of novel protein SamCystin is associated with polycystic kidney disease in (cy/+) rat. J Am Soc Nephrol 16(12):3517–3526
Tao Y et al (2005) Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol 16(1):46–51
Wahl PR et al (2006) Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol Dial Transplant 21(3):598–604
Bielas SL et al (2009) Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet 41(9):1032–1036
Jacoby M et al (2009) INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet 41(9):1027–1031
Moyer JH et al (1994) Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science 264(5163):1329–1333
Pazour GJ et al (2002) The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol 157(1):103–113
Ansley SJ et al (2003) Basal body dysfunction is a likely cause of pleiotropic Bardet–Biedl syndrome. Nature 425(6958):628–633
Zaghloul NA, Katsanis N (2009) Mechanistic insights into Bardet–Biedl syndrome, a model ciliopathy. J Clin Invest 119(3):428–437
Weatherbee SD, Niswander LA, Anderson KV (2009) A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and Hedgehog signaling. Hum Mol Genet 18(23):4565–4575
Khanna H et al (2009) A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet 41(6):739–745
Louie CM et al (2010) AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat Genet 42(2):175–180
Bergmann C et al (2008) Loss of nephrocystin-3 function can cause embryonic lethality, Meckel–Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet 82(4):959–970
Paavola P et al (1995) The locus for Meckel syndrome with multiple congenital anomalies maps to chromosome 17q21-q24. Nat Genet 11(2):213–215
Kyttala M et al (2006) MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat Genet 38(2):155–157
Dawe HR et al (2007) The Meckel–Gruber Syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Hum Mol Genet 16(2):173–186
Bialas NJ et al (2009) Functional interactions between the ciliopathy-associated Meckel syndrome 1 (MKS1) protein and two novel MKS1-related (MKSR) proteins. J Cell Sci 122(Pt 5):611–624
Roume J et al (1998) A gene for Meckel syndrome maps to chromosome 11q13. Am J Hum Genet 63(4):1095–1101
Pan J et al (2007) RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. J Cell Sci 120(Pt 11):1868–1876
Park TJ et al (2008) Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet 40(7):871–879
Veeman MT, Axelrod JD, Moon RT (2003) A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 5(3):367–377
Winter CG et al (2001) Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105(1):81–91
Junge HJ et al (2009) TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell 139(2):299–311
Morgan NV et al (2002) A novel locus for Meckel–Gruber syndrome, MKS3, maps to chromosome 8q24. Hum Genet 111(4–5):456–461
Smith UM et al (2006) The transmembrane protein meckelin (MKS3) is mutated in Meckel–Gruber syndrome and the wpk rat. Nat Genet 38(2):191–196
Xu YK, Nusse R (1998) The Frizzled CRD domain is conserved in diverse proteins including several receptor tyrosine kinases. Curr Biol 8(12):R405–R406
Cadigan KM, Nusse R (1997) Wnt signaling: a common theme in animal development. Genes Dev 11(24):3286–3305
Tammachote R et al (2009) Ciliary and centrosomal defects associated with mutation and depletion of the Meckel syndrome genes MKS1 and MKS3. Hum Mol Genet 18(17):3311–3323
Williams CL, Masyukova SV, Yoder BK (2010) Normal ciliogenesis requires synergy between the cystic kidney disease genes MKS-3 and NPHP-4. J Am Soc Nephrol 21(5):782–793
Sayer JA et al (2006) The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet 38(6):674–681
den Hollander AI et al (2006) Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet 79(3):556–561
Moradi P et al (2010) Focus on molecules: Centrosomal protein 290 (CEP290). Exp Eye Res. doi:10.1016/j.exer.2010.05.009
Chang B et al (2006) In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet 15(11):1847–1857
Kim J, Krishnaswami SR, Gleeson JG (2008) CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet 17(23):3796–3805
Roepman R et al (2005) Interaction of nephrocystin-4 and RPGRIP1 is disrupted by nephronophthisis or Leber congenital amaurosis-associated mutations. Proc Natl Acad Sci USA 102(51):18520–18525
Tallila J et al (2008) Identification of CC2D2A as a Meckel syndrome gene adds an important piece to the ciliopathy puzzle. Am J Hum Genet 82(6):1361–1367
Mougou-Zerelli S et al (2009) CC2D2A mutations in Meckel and Joubert syndromes indicate a genotype–phenotype correlation. Hum Mutat 30(11):1574–1582
Shen X et al (2005) Scanning the human proteome for calmodulin-binding proteins. Proc Natl Acad Sci USA 102(17):5969–5974
Town T et al (2008) The stumpy gene is required for mammalian ciliogenesis. Proc Natl Acad Sci USA 105(8):2853–2858
Cook SA et al (2009) A mouse model for Meckel syndrome type 3. J Am Soc Nephrol 20(4):753–764
Anselme I et al (2007) Defects in brain patterning and head morphogenesis in the mouse mutant Fused toes. Dev Biol 304(1):208–220
Chapman ER (2002) Synaptotagmin: a Ca(2+) sensor that triggers exocytosis? Nat Rev Mol Cell Biol 3(7):498–508
Acknowledgments
We acknowledge funding from the Medical Research Council (project grant G0700073; CAJ), an Egyptian Government Scholarship (ZA) and the Sir Jules Thorn Charitable Trust (09/JTA). We thank Tamara Caspary, Jeremy Reiter, and Joe Gleeson for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Logan, C.V., Abdel-Hamed, Z. & Johnson, C.A. Molecular Genetics and Pathogenic Mechanisms for the Severe Ciliopathies: Insights into Neurodevelopment and Pathogenesis of Neural Tube Defects. Mol Neurobiol 43, 12–26 (2011). https://doi.org/10.1007/s12035-010-8154-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-010-8154-0