Abstract
The biological roles of poly(ADP-ribose) polymers (PAR) and poly(ADP-ribosyl)ation of proteins in the central nervous system are diverse. The homeostasis of PAR orchestrated by poly(ADP-ribose) polymerase-1 (PARP-1) and poly(ADP-ribose) glycohydrolase (PARG) is crucial for cell physiology and pathology. Both enzymes are ubiquitously distributed in neurons and glia; however, they are segregated at the subcellular level. PARP-1 serves as a “nick sensor” for single- or double-stranded breaks in DNA and is involved in long and short patch base-excision repair, while PARG breaks down PAR. The stimulation of PARP-1 and PAR formation can activate proinflammatory transcription factors, including nuclear factor kappa B. However, hyperactivation of PARP-1 can result in depletion of NAD/ATP, and in PAR-dependent mitochondrial pore formation leading to release of apoptosis inducing factor and cell death. The role of PAR as a death signaling molecule in brain ischemia–reperfusion and inflammation as well as the effect of gender and aging is presented in this review. Modulating the PAR level through pharmacological or genetic intervention on PARP-1/PARG activity and gene expression should be a valuable way for neuroprotective strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A variety of reactive oxygen (ROS)/nitrogen species released during recirculation after brain ischemia leads to oxidation of macromolecules including DNA. The central nervous system (CNS) is particularly sensitive to oxidative stress and DNA damage. The cells of adult human brain consume approximately 20% of oxygen utilized by the body, although the brain comprises only 2% of the body weight. The active metabolism in the brain leads to high rate of ROS production [1]. The brain is comprised of both actively dividing (glia) and terminally differentiated cells (neurons), which differ in their capacity to quench various stressors and to repair damage. For example, astrocytes protect neurons against the toxicity of ROS by supplying glutathione precursors to neurons and by pyruvate release [2–5]. Although the CNS is well-equipped for limiting the entry of potentially neurotoxic compounds to the brain via the blood–brain barrier, the high oxygen and glucose demand makes CNS particularly susceptible to perturbations in the delivery of these essential molecules [6]. If the supplies are disrupted for only a brief period of time, the cascade of events that ensue can wreak havoc on cells during reperfusion, including those cells forming the blood–brain barrier [7]. Post-ischemic reperfusion injury is multifactorial with key precipitating events being the release of glutamate, activation of N-methyl-d-aspartate (NMDA) receptors, and production of nitric oxide (NO), which can subsequently react with superoxide anion (O −2 ) to form peroxynitrite (ONOO−) [8]. The injury that accompanies reperfusion is largely caused by generation of reactive oxygen (e.g., O −2 ; \( {}^\bullet {\hbox{OH}} \)) and nitrogen species (e.g., ONOO−; \( {\hbox{N}}{{\hbox{O}}_2}^{ \bullet - } \)), which damage DNA, and ultimately lead to hyperactivation of poly(ADP-ribose) polymerase-1 (PARP-1; EC 2.4.2.30).
PARP-1 is a 116-kDa protein that functions as a DNA nick sensor and nuclear target for various signaling pathways. This protein contains three primary functional domains: an N-terminal DNA-binding domain (46 kDa), including a nuclear localization signal, a central auto modification domain (16 kDa), and a C-terminal catalytic domain (55 kDa). The DNA binding domain contains two zinc-finger motifs that facilitate binding to both single and double-stranded DNA breaks. The functional roles of PARP-1 are largely mediated by its activation after binding to damaged DNA. Upon binding, PARP-1 activity increases rapidly, up to 500 fold, and cleaves β-nicotinamide adenine dinucleotide (β-NAD+) into ADP-ribose and nicotinamide. Linear and branched polymers of ADP-ribose (PAR) are synthesized and covalently attached to more than 40 different acceptor proteins, including histones and PARP-1 itself. The highly negative charge of PAR leads to electrostatic repulsion between DNA and histones. Under normal conditions, PARP-1 acts as a key protein in genome stability by detecting DNA strand breaks and nicks and by being involved in DNA repair machinery [9]. The recent findings of Cohen-Armon [10] presented an alternative mechanism of PARP-1 stimulation through direct interaction with phosphorylated extracellular signal regulated kinase (ERK-2). This pathway is not related to DNA damage and PARP-1 binding to DNA. Moreover, ERK-2 induced PARP-1 activation amplifies ERK signals through transcription factor ELK 1 and its target gene c-fos. These data indicated that PARP-1 through Raf-MEK-ERK-cfos signaling cascade might be involved in growth, differentiation, and proliferations of cells. However, increased activation of PARP-1 can lead to inflammation [11]. PARP-1 activates proinflammatory transcription factors through different mechanisms, including protein–protein interaction, and PAR or poly(ADP-ribosyl)ation. The severe injuries such as ischemia–reperfusion can hyperactivate PARP-1 leading to an energy crisis in the cells [12–18].
PAR/PARP//PARG and Mitochondria—Nucleus Cross Talk
During PAR-mediated cell death, the enhanced consumption of β-NAD+ impairs glycolysis and oxidative metabolism in the mitochondria, leading to ATP depletion [18–20] and to the following key events: (1) PAR-dependent specific mitochondrial permeability transition or pore formation and membrane depolarization, (2) release of apoptosis-inducing factor (AIF), (3) nuclear translocation of AIF, and (4) DNA degradation. However, the exact signals involved in the nuclear-mitochondrial cross talk are unclear. PAR has been shown to act as a signaling molecule that induces cell death following hyperactivation of PARP-1 [21]. Sequential activation of PARP-1, calpains, and BAX is also reported to be essential in AIF-mediated programmed cell death [22]. These authors found that, once activated, PARP-1 mediates mitochondrial AIF release and necrosis, through a mechanism requiring calpains but not caspase and not cathepsins. They observed that single ablation of the BAX but not other proapoptotic protein as for example BAK prevented AIF release and cell death evoked by alkylating DNA damage. This data presented that BAX was indispensable for the highly regulated necrosis. The last study of Wang et al. [23] demonstrated that calpain activation was not required for AIF translocation from mitochondria in PARP-1-dependent cell death.
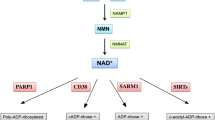
Xu et al. [24] reported that hyperactivation of PARP-1 in mouse embryonic fibroblasts treated with the DNA alkylating agent N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) required c-Jun N-terminal kinase-1 (JNK-1) activation for steps 1, 2, and 3 described in the beginning of this chapter. Based on these findings, Alano and Swanson [25] speculated that JNK-1 activation may play a role in nuclear export of PAR. The in vivo half-life of PAR is less than 1 min. The dynamic process of synthesis and hydrolysis of PAR is performed by PARP and primarily poly(ADP-ribose) glycohydrolase (PARG; EC 3.2.1.143). Catabolism is also performed by ADP-ribosyl protein lyase, although to a much lesser extent, and by three novel proteins possessing PARG activity [26, 27]. PARG cleaves ribose–ribose bonds of both linear and branched portions of PAR, whereas ADP-ribosyl protein lyase cleaves the protein proximal ADP-ribose monomer [28]. Recently, it was demonstrated that PARG was required for the global repair of single-strand DNA breaks and that PARP-1 and PARG accelerated single-strand break repair in concert with one another [29]. The authors further confirmed that XRCC1, a scaffolding protein involved in single-strand break repair, was negatively regulated by PARG, meaning PARG prevented accumulation of XRCC1 at sites of DNA damage and facilitated its disassembly once repair was completed. The tissue distribution of PARG parallels to PARP-1. Both enzymes are detected in glia and neurons. However, differences in the subcellular distribution of these enzymes have been reported. Poitras et al. [30] found that, in the brain, endogenous PARG was associated with the outer mitochondrial membrane and confirmed that the subcellular segregation of PARP-1 and PARG required PARG to translocate to the nucleus upon PARP-1 activation. Recently, Whatcott et al. [31] reported that specific isoform of PARG was targeted to the mitochondrial matrix. According to the authors, the identification of a PARG isoform as a component of the mitochondrial matrix raises several interesting possibilities concerning mechanism of nuclear–mitochondrial cross talk and PAR polymer metabolism within mitochondria. Moreover, they suggested [31] that mitochondrial PARG may play protective role in preventing inappropriate release of AIF or could promote AIF release by generating free ADP-ribose that has been shown to activate membrane calcium channels. The data of Meyer et al. [32] indicated that PARG gene shares a promotor with a gene encoding a protein TIM 23 involved in import of proteins into mitochondria. Poitras et al. [30] described spatial and functional relationships of PARG and PARP-1. It was found that these enzymes, responsible for PAR metabolism and several cell function processes, were evenly distributed throughout the brain. While PARP-1 in neurons is localized exclusively in the nucleus, PARG is mainly found in the mitochondrial fraction under unstimulated conditions together with manganese superoxide dismutase and cytochrome C. However, in contrast to neurons, PARG is presented in different subcellular compartments in other mammalian cells [30]. The co-localization of PARG with cytochrome C in brain has been confirmed using confocal microscopy. A short 63 kDa isoform of PARG was also presented in primary neuronal culture; however, Poitras et al. [30] observed only the full-length isoform in the brain. In other mammalian cells the full-length PARG110 (kDA) is localized in the nucleus, PARG103 and PARG99 in cytoplasm, but PARG60 was found in cytoplasm and mitochondria. Moreover, PARG85 and PARG74, both catalytic active fragments liberated after caspase-3 action, have been detected in cytoplasm [33]. It is important to underline that most of the PARG activity in unstimulated brain cells is localized in mitochondria and cytoplasm. During pathology, when DNA is damaged, PARG is translocated to the nucleus. It was observed that NMDA receptor-dependent PARP-1 activation led to PARG translocation into the nucleus. PARG overexpression reduces PARP-1 dependent NMDA excitotoxicity. Due to potential role of PARG in pathophysiology, the several inhibitors of this enzyme are under investigation [33–35]. The events described in this chapter are presented on Fig. 1.
PAR/ PARP and PARG in mitochondria-nucleus cross talk in brain. The nuclear enzyme PARP-1 use βNAD+ as a substrate. The products of PARP activity are PAR and nicotinamide, which is responsible for the feedback inhibition of PARP. This DNA bound enzyme is also inhibited by auto poly(ADP-ribosyl)ation. PAR is degraded by PARG to ADP-ribose. The overactivation of PARP leads to enhancement of PAR synthesis that during brain ischemia can be release from nucleus and may lead to mitochondria permeability and AIF release and its translocation into nucleus. The excessive PARP activity may also induce translocation of PARG into nucleus. PARP may also evoked BAX and calpain-dependent AIF release. PARG in mitochondrial matrix could be involve in the regulation of PAR level and AIF release
PAR in Brain Ischemia Reperfusion–Injury
Although the role of PAR in cell physiology and pathology has been intensively investigated for the last few years, the molecular mechanism by which PAR is involved in maintenance of genomic stability, regulation of transcription, energy metabolism, and cell death is not well understood. The last study of Ahel et al. [36] indicated the important mechanism for PAR function in chromatin remodeling. A chromatin remodeling enzyme amplified in liver cancer 1 (ALC1) also known as CHD1 interacts with PAR and catalyzes PARP-1 stimulated nucleosome sliding. The details results of this study provide new insights into mechanisms by which PAR regulates DNA repair. However, the last studies were focused on the role of PAR in cell death. Alteration of PAR homeostasis has been indicated recently to be important for cell pathology. The regulation of PAR level and the activity of PARP-1 are crucial in the function of many transcription factors that mediate microglial gene transcription during reperfusion after ischemic insult. Nuclear factor kappa B (NF-κB) and p53 have a specific domain that interacts with PAR [37]. NF-κB requires PARP-1 as a coactivator in systemic inflammatory responses [38]. The recent data demonstrated that PARP-1 modulates the hypoxia inducible factor-1 (HIF-1) in vivo. Immediate post-hypoxic HIF-1 alpha accumulation was significantly higher in the presence of PARP-1 [39]. This data indicated that PARP-1/PAR have an important regulatory role in the in vivo response of brain HIF-1 to hypoxia–reoxygenation. Brain ischemia leads to excessive biosynthesis of PAR polymer that exerts toxicity to neurons and other cells. Degradation of PAR by PARG prevents PAR-induced cell death [21]. The authors demonstrated that NMDA receptor excitotoxicity and NMNG-induced cell death were significantly reduced or abolished by decreasing PAR level or by PARG overexpression. Moreover, in an in vivo model of focal cerebral ischemia significantly reduced infarct volume was observed in mice overexpressing PARG. Analysis of the cell death in brain ischemia pathology induced by overstimulation of PARP-1 indicated that not only β-NAD+/ATP depletion was responsible for cell death, but probably the most important was the action of PAR on transcription factors and mitochondria. The alteration of mitochondrial permeability by PAR and then AIF release and its nuclear translocation seem to be crucial for cell death described as parthanatos [40, 41]. The results of Dawson’s group suggest toxic action of PAR itself [21, 40, 41]. They found that isolated PAR and supernatants containing PAR induced the release of AIF from mitochondria. Andrabi et al. [21] presented that highly complex and long-chain PAR polymers were more toxic in comparison to the shorter PAR molecule. Polymer of 16 ADP-ribose residues elicited a small amount of cell death; however, a polymer of >60 ADP-ribose units induced more than 80% of cell death [21]. The recent study has reported the development of circulating IgG antibodies against PAR in the serum of patients with Alzheimer disease [42]. This phenomenon may also occur in human brain ischemia and may be important for diagnosis and prognosis of ischemic pathology. In human stroke, PARP activation and PAR formation are biphasic. During the early stage of stroke (up to 24 h) the activation of PARP occurs in neuronal cells. In the later stage (3–4 days after stroke), the PARP activity was shown in immune/inflammatory cells [3, 43].
Effect of PARP and PARG Inhibition in Brain Ischemia
Recently, several new regulators of PARP-1 and PARG have been found. Among others: are kinases (including ERK), purines, polyamines, vitamin D3, theophylline, caffeine metabolites, and tetracycline antibiotics [10, 44, 45]. The data of Hamby et al. [46] indicated that inhibitor of PARP-1 (PJ-34) suppressed inflammation and neuronal cell death in CA1 layer of hippocampus when administered 8 h after transient (10 min) forebrain ischemia. Animals treated with PJ-34 showed a significant inhibition of microglia/macrophage activation. These data indicated that administration of PARP-1 inhibitor at delayed time points after transient forebrain ischemia–reperfusion had a large protective effect of neuronal death in CA1 layer of the hippocampus and a significant reduction in inflammatory response in adult rats. The overactivation of PARP promotes inflammation and cell death [47, 48]. Microglia require several hours to become fully activated, and a PARP-1 inhibitor could potentially offer a long therapeutic window. PARP-1 inhibition before global cerebral ischemia preserves the functionality of the blood–brain barrier with reduced edema. PJ-34 decreases the loss of the tight junction protein occludin after an ischemic episode. In addition, neutrophil infiltration of the cortex was lowered after PJ-34 treatment [7]. Our previous data indicated also a protective effect of PARP-1 inhibitor on brain edema. PARP-1 inhibitors protected the brain against swelling, not only of neurons but also of astrocytes and pericytes. 3-AB decreased swelling of mitochondria and Golgi apparatus evoked by ischemia [16, 49]. Alano and coworkers [44] showed that some common antibiotics of the tetracycline class such as doxycycline or minocycline were relatively potent inhibitors of PARP-1. Neuronal β-NAD+ depletion and PAR formation were blocked by 100 nM minocycline [44]. The study presented the neuroprotective effect of minocycline not only in focal but also in global forebrain ischemia. Currently, minocycline is in clinical trials for stroke patients. The anti-inflammatory effect of minocycline is also connected with PARP-1 function as a coactivator of NF-κB. The work of Kauppinen and Swanson [50] demonstrated that PARP-1 interaction with NF-κB promoted microglial metalloproteinase-9 release and neurotoxicity. It was found that many cytokines (TNF-α, MIP-1α, IL-1β, IL-6, IL-8, and INF-γ), inducible nitric oxide synthase (iNOS), and adhesion molecules were marginally induced in the absence of PARP-1 not only in brain where PARP is responsible for over 97% of poly(ADP-ribosyl)ation but also in the other organs [51–54]. Anti-inflammatory and antiapoptotic effects of PARP-1 inhibitors were also described by other groups [45, 47, 55–57]. Pharmacological inhibition of PARP-1 decreased plaque formation in atherosclerosis. Chronic treatment with PJ-34 markedly decreased E-selectin, P-selectin, VCAM-1, and iNOS protein levels [58]. In most studies, the protective effects against stroke or ischemic episodes were observed when PARP-1 inhibitors were used before the beginning of a pathology process. However, papers presented by Strosznajder et al. [16, 49, 59] and Hamby et al. [46] showed that the ameliorating effects of PARP-1 inhibitors were visible even when the inhibitors were applied after an ischemic episode, which is more realistic scenario. Our results with PJ-34 indicated also, that this potent PARP-1 inhibitor significantly augmented the ameliorating effect of preconditioning (2 min nonlethal ischemia, 48 h before the main ischemic episode) on neuronal survival [17]. Our data indicated that 3-AB prevented AIF translocation to nucleus and led to overexpression of Bcl-2 protein level [57]. To better understand the role of AIF in cell death, Harlequin (Hq) mice with about 80% reduction of AIF expression were used in the experiments [60]. The authors showed that Hq mice were resistant to death induced by PAR (parthanatos). These animals had reduced lesions after excitotoxicity and stroke. The other studies confirmed the role of AIF in cell death also propagated by BAX-dependent and BAX-independent or BID-mediated mechanism [61–63]. Our data presented the cytoprotective effect of carvedilol (β-adrenoreceptor antagonist) in brain ischemia–reperfusion injury and suggested that this effect was connected with suppression of PARP activity [64]. Moreover, carvedilol prevented β-NAD+ depletion after ischemic–reperfusion insult [64]. It is known now that PARP-1 inhibition leads not only to neuronal cell protection but also has a positive effect on integrity of the blood–brain barrier and protects brain and endothelial blood vessel cells against inflammation [14, 65, 66]. The data in Table 1 show the effect of PARP-1 inhibitors in animal models of brain ischemia. In contrast, currently available PARG inhibitors display poor specificity and poor blood–brain barrier penetration. The studies on brain ischemia yielded inconsistent results. Wei et al. [35] presented that intranasal administration of PARG inhibitor profoundly decreased ischemic brain injury. Lu et al. [34] demonstrated that an inhibitor of PARG could ameliorate ischemic brain damage and suggested PARG as a new therapeutic target for treatment of ischemic injury. The novel PARG inhibitor GPI 16552 provided significant protection even as a post-ischemic treatment [34]. According to Li et al. [78], PARG and PARP inhibitors display a similar time window when used to treat ischemia. PARG-blocking by gallotannin and nobotannin counteract cell death in the in vitro model induced by pro-oxidative factors including H2O2 and NMDA [79, 80]. However, selective disruption of mouse PARG110 increases the cell damage measured 24 h after middle cerebral artery occlusion [81]. The limitations of PARG inhibitors and knockouts clearly show that further work is needed to clarify the role of this enzyme under pathological conditions.
Effect of Aging and Gender on PARP-1 and PAR Formation
The data presented in this review were obtained using male adult animals. The question arises how PAR metabolism is affected by aging and gender.
PARP-1 activity and PAR formation are significantly modulated by environmental conditions during life span, including accompanying infections and other types of oxidative or genotoxic stress. Until now, little is known on PARP-1 activity and its role in the brain during healthy aging where a lot adaptive/protective processes are activated.
The study of Shibata et al. [82] demonstrated that PARP-1 deficiency caused an increase of point mutations in the brain by aging. These results support the idea that PARP-1 is involved in suppressing imprecise repair of endogenous DNA damage, leading to deletion mutation in brain during aging. Our previous study [83, 84] indicated that, depending on housing conditions (under or without barrier), PARP activity was increased or decreased in the aged hippocampus. Our data showed the significantly higher PARP-1 activity in hippocampus and brain cortex of pathogen-free aged animals comparing to adult animals [84]. The alteration of PARP-1 during aging has different dynamics in particular brain regions. Moreover, PARP-1 activity enhanced by aging in the hippocampus is less or not sensitive for further alteration by additional stress conditions evoked by Fenton reaction or MNNG. These agents significantly stimulated PARP activity in adult hippocampus [84]. In the aged brain, NMDA receptor stimulation had no effect on PARP-1 activity (that has already been increased by aging), while in the adult hippocampus, a significant reaction was observed [85]. In adult male animals, PARP-1 inhibitors exerted several protective effects at cellular and subcellular level [16, 59, 86]. The enhanced activity of PARP-1 in the brain during healthy aging should not be inhibited because of its involvement in DNA repair and genome stability. However, during aging, the immune system is very often disturbed, and inflammation processes through PARP-NF-κB interactions may affect cellular functions leading to cells death. Mabley et al. [87] reported that inhibition of PARP had protective effect by reducing inflammatory mediators and mortality exclusively in male but not in female mice. Moreover, female mice produced less inflammatory mediators compared to the male mice, and they were more resistant to endotoxin-induced mortality when compared to males. To understand the mechanism of this gender specificity and the role of PARP, the effect of ovariectomy was investigated, and it was indicated that this strategy only partially reversed the protection observed in female mice. Pretreatment of male mice with 17-β-estradiol reduced PARP activity and injury evoked by endotoxin (lipopolysaccharide). On the basis of the in vitro study, Mabley et al. [87] suggested that estrogen itself was not responsible for the gender differences but the interaction of estrogen receptor alpha with PARP and complex formation that binds to DNA preventing the recognition of DNA strand breaks by PARP. Moreover, it was found that inhibition of neuronal NO synthase and PARP-1 protected neurons against ischemic damage only in males, while it enhanced ischemic injury in females [88, 89]. Then, Lang and McCullough [90] suggested that divergent cell death pathways are activated after brain ischemic insult in male and female. According to their new concept, the mechanism and outcome of cerebral ischemic injury are influenced strongly by level and availability of sex steroids in the brain. The caspase-mediated neuronal death pathway may occur in female in contrast to male where PAR/AIF signaling is involved in cell death [90]. The further study of Yuan et al. [91] presented that deletion of PARP-1 led to significant decrease in PAR formation and AIF translocation in males and females, but ischemic damage was reduced only in males. Estrogen had no protective effect in this study. Their following studies using AIF-deficient male and female Harlequin mice demonstrated that the neuroprotective effect of this gene deletion occurred only in males. The last data of Liu et al. [92] showed that apoptosis in the female mouse brain reperfused after middle cerebral artery occlusion (MCAO) was dependent on caspase activation. Females treated with the caspase inhibitor (Q-VD-OPh) showed significantly reduced infarct volume and improved neurological scores after MCAO without any significant protection in males. The release of cytochrome C and nuclear caspase-8 level were increased in females after stroke [92]. Moreover, also the effect of minocycline has been found to be sex-dependent [93]. Epidemiological studies indicated that ischemic events occurred with greater frequency in men versus women until advance age. Stroke increases significantly in women after menopause suggesting that sex hormones are mainly responsible in this pathology. Figure 2 presents the signaling pathway evoked by ischemia–reperfusion, which, as consequence, leads to activation of inflammation and to cognitive decline. Gender and age are marked as significant modulatory factors of this pathway.
The role of PARP/PAR in brain ischemia pathology. Effect of age and gender. In brain ischemia pathology, PAR/AIF death pathway occurs mainly in adult male. In adult female, the PARP-1/NF-κB inflammatory pathways are less expressed compared to male. It is suggested that the caspase/cytochrome C signaling is responsible for cell death in adult females. Aging affects the molecular targets as PARP/PAR in nucleus and AIF in mitochondria
General Summary
The data presented in this review underline the novel important role of poly(ADP-ribosyl)ation as a life/death signaling pathway playing a crucial function in cross talk between nucleus and mitochondria in brain ischemic pathology.
Several new regulators of PAR homeostasis have been recently found and described. The presented data indicated that ischemic death pathways were sexually dimorphic, and the pharmacological substances used to reduce PARP activation may have limited benefits to females. Age- and gender-dependent regulations of PARP-1 and PARG may be key factors in the creation of a therapeutic strategy against brain ischemia–reperfusion injury.
References
Dringen R, Gutterer JM, Hirrlinger J (2000) Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem 267(16):4912–4916
Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D (2002) Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev 54(2):271–284
Love S (1999) Oxidative stress in brain ischemia. Brain Pathol 9(1):119–131
Wang XF, Cynader MS (2001) Pyruvate released by astrocytes protects neurons from copper-catalyzed cysteine neurotoxicity. J Neurosci 21(10):3322–3331
Wang JY, Wen LL, Huang YN, Chen YT, Ku MC (2006) Dual effects of antioxidants in neurodegeneration: direct neuroprotection against oxidative stress and indirect protection via suppression of glia-mediated inflammation. Curr Pharm Des 12(27):3521–3533
Tanaka S, Takehashi M, Iida S, Kitajima T, Kamanaka Y, Stedeford T, Banasik M, Ueda K (2005) Mitochondrial impairment induced by poly(ADP-ribose) polymerase-1 activation in cortical neurons after oxygen and glucose deprivation. J Neurochem 95(1):179–190
Lenzsér G, Kis B, Snipes JA, Gáspár T, Sándor P, Komjáti K, Szabó C, Busija DW (2007) Contribution of poly(ADP-ribose) polymerase to postischemic blood–brain barrier damage in rats. J Cereb Blood Flow Metab 27(7):1318–1326
Moncada S, Bolaños JP (2006) Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem 97(6):1676–1689
Dantzer F, Amé JC, Schreiber V, Nakamura J, Ménissier-de Murcia J, de Murcia G (2006) Poly(ADP-ribose) polymerase-1 activation during DNA damage and repair. Methods Enzymol 409:493–510
Cohen-Armon M (2007) PARP-1 activation in the ERK signaling pathway. Trends Pharmacol Sci 28(11):556–560
Kauppinen TM, Suh SW, Berman AE, Hamby AM, Swanson RA (2009) Inhibition of poly(ADP-ribose) polymerase suppresses inflammation and promotes recovery after ischemic injury. J Cereb Blood Flow Metab 29(4):820–829
Ha HC, Snyder SH (1999) Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci USA 96(24):13978–13982
Horvath EM, Szabó C (2007) Poly(ADP-ribose) polymerase as a drug target for cardiovascular disease and cancer: an update. Drug News Perspect 20(3):171–181
Moroni F (2008) Poly(ADP-ribose)polymerase 1 (PARP-1) and postischemic brain damage. Curr Opin Pharmacol 8(1):96–103
Pacher P, Szabó C (2007) Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev 25(3):235–260
Strosznajder R, Gadamski R, Walski M (2005) Inhibition of poly(ADP-ribose) polymerase activity protects hippocampal cells against morphological and ultrastructural alteration evoked by ischemia–reperfusion injury. Folia Neuropathol 43(3):156–165
Strosznajder RP, Jesko H, Banasik M, Gadamski R, Walski M, Gajkowska B, Simonyi A (2007) Poly(ADP-ribose) polymerase inhibitors enhanced the beneficial effects of preconditioning on hippocampal neuronal survival subjected to ischemia reperfusion injury. Neuroscience Meeting San Diego abstract book, poster no. 703.16/V24
Ying W, Alano CC, Garnier P, Swanson RA (2005) NAD+ as a metabolic link between DNA damage and cell death. J Neurosci Res 79(1–2):216–223
Alano CC, Ying W, Swanson RA (2004) Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J Biol Chem 279(18):18895–18902
Ying W, Garnier P, Swanson RA (2003) NAD+ repletion prevents PARP-1-induced glycolytic blockade and cell death in cultured mouse astrocytes. Biochem Biophys Res Commun 308(4):809–813
Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW, Sasaki M, Klaus JA, Otsuka T, Zhang Z, Koehler RC, Hurn PD, Poirier GG, Dawson VL, Dawson TM (2006) Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci USA 103(48):18308–18313
Moubarak RS, Yuste VJ, Artus C, Bouharrour A, Greer PA, Menissier-de Murcia J, Susin SA (2007) Sequential activation of poly(ADP-ribose) polymerase 1, calpains, and Bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Mol Cell Biol 27(13):4844–4862
Wang Y, Kim NS, Li X, Greer PA, Koehler RC, Dawson VL, Dawson TM (2009) Calpain activation is not required for AIF translocation in PARP-1-dependent cell death (parthanatos). J Neurochem 110(2):687–696
Xu Y, Huang S, Liu ZG, Han J (2006) Poly(ADP-ribose) polymerase-1 signaling to mitochondria in necrotic cell death requires RIP1/TRAF2-mediated JNK1 activation. J Biol Chem 281(13):8788–8795
Alano CC, Swanson RA (2006) Players in the PARP-1 cell-death pathway: JNK1 joins the cast. Trends Biochem Sci 31(6):309–311
Mueller-Dieckmann C, Kernstock S, Lisurek M, von Kries JP, Haag F, Weiss MS, Koch-Nolte F (2006) The structure of human ADP-ribosylhydrolase 3 (ARH3) provides insights into the reversibility of protein ADP-ribosylation. Proc Natl Acad Sci USA 103(41):15026–15031
Oka S, Kato J, Moss J (2006) Identification and characterization of a mammalian 39-kDa poly(ADP-ribose) glycohydrolase. J Biol Chem 281(2):705–713
Oka J, Ueda K, Hayaishi O, Komura H, Nakanishi K (1984) ADP-ribosyl protein lyase. Purification, properties, and identification of the product. J Biol Chem 259(2):986–995
Fisher AE, Hochegger H, Takeda S, Caldecott KW (2007) Poly(ADP-ribose) polymerase 1 accelerates single-strand break repair in concert with poly(ADP-ribose) glycohydrolase. Mol Cell Biol 27(15):5597–5605
Poitras MF, Koh DW, Yu SW, Andrabi SA, Mandir AS, Poirier GG, Dawson VL, Dawson TM (2007) Spatial and functional relationship between poly(ADP-ribose) polymerase-1 and poly(ADP-ribose) glycohydrolase in the brain. Neuroscience 148(1):198–211
Whatcott CJ, Meyer-Ficca ML, Meyer RG, Jacobson MK (2009) A specific isoform of poly(ADP-ribose) glycohydrolase is targeted to the mitochondrial matrix by a N-terminal mitochondrial targeting sequence. Exp Cell Res 315(20):3477–3485
Meyer RG, Meyer-Ficca ML, Jacobson EL, Jacobson MK (2003) Human poly(ADP-ribose) glycohydrolase (PARG) gene and the common promoter sequence it shares with inner mitochondrial membrane translocase 23 (TIM23). Gene 314:181–190
Heeres JT, Hergenrother PJ (2007) Poly(ADP-ribose) makes a date with death. Curr Opin Chem Biol 11(6):644–653
Lu XC, Massuda E, Lin Q, Li W, Li JH, Zhang J (2003) Post-treatment with a novel PARG inhibitor reduces infarct in cerebral ischemia in the rat. Brain Res 978(1–2):99–103
Wei G, Wang D, Lu H, Parmentier S, Wang Q, Panter SS, Frey WH 2nd, Ying W (2007) Intranasal administration of a PARG inhibitor profoundly decreases ischemic brain injury. Front Biosci 12:4986–4996
Ahel D, Horejsí Z, Wiechens N, Polo SE, Garcia-Wilson E, Ahel I, Flynn H, Skehel M, West SC, Jackson SP, Owen-Hughes T, Boulton SJ (2009) Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science 325(5945):1240–1243
Pleschke JM, Kleczkowska HE, Strohm M, Althaus FR (2000) Poly(ADP-ribose) binds to specific domains in DNA damage checkpoint proteins. J Biol Chem 275(52):40974–40980
Hassa PO, Hottiger MO (2002) The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-kappaB in inflammatory disorders. Cell Mol Life Sci 59(9):1534–1553
Martínez-Romero R, Cañuelo A, Martínez-Lara E, Javier Oliver F, Cárdenas S, Siles E (2009) Poly(ADP-ribose) polymerase-1 modulation of in vivo response of brain hypoxia-inducible factor-1 to hypoxia/reoxygenation is mediated by nitric oxide and factor inhibiting HIF. J Neurochem 111(1):150–159
Andrabi SA, Dawson TM, Dawson VL (2008) Mitochondrial and nuclear cross talk in cell death: parthanatos. Ann N Y Acad Sci 1147:233–241
David KK, Andrabi SA, Dawson TM, Dawson VL (2009) Parthanatos, a messenger of death. Front Biosci 14:1116–1128
Kanai Y, Akatsu H, Iizuka H, Morimoto C (2007) Could serum antibody to poly(ADP-ribose) and/or histone H1 be marker for senile dementia of Alzheimer type? Ann N Y Acad Sci 1109:338–344
Love S, Barber R, Wilcock GK (2000) Neuronal death in brain infarcts in man. Neuropathol Appl Neurobiol 26(1):55–66
Alano CC, Kauppinen TM, Valls AV, Swanson RA (2006) Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc Natl Acad Sci USA 103(25):9685–9690
Szabo C, Pacher P, Swanson RA (2006) Novel modulators of poly(ADP-ribose) polymerase. Trends Pharmacol Sci 27(12):626–630
Hamby AM, Suh SW, Kauppinen TM, Swanson RA (2007) Use of a poly(ADP-ribose) polymerase inhibitor to suppress inflammation and neuronal death after cerebral ischemia–reperfusion. Stroke 38(Suppl 2):632–636
Ha HC, Hester LD, Snyder SH (2002) Poly(ADP-ribose) polymerase-1 dependence of stress-induced transcription factors and associated gene expression in glia. Proc Natl Acad Sci USA 99(5):3270–3275
Kauppinen TM, Swanson RA (2005) Poly(ADP-ribose) polymerase-1 promotes microglial activation, proliferation, and matrix metalloproteinase-9-mediated neuron death. J Immunol 174(4):2288–2296
Strosznajder RP, Gadamski R, Czapski GA, Jesko H, Strosznajder JB (2003) Poly(ADP-ribose) polymerase during reperfusion after transient forebrain ischemia: its role in brain edema and cell death. J Mol Neurosci 20(1):61–72
Kauppinen TM, Swanson RA (2007) The role of poly(ADP-ribose) polymerase-1 in CNS disease. Neuroscience 145(4):1267–1272
Haddad M, Rhinn H, Bloquel C, Coqueran B, Szabó C, Plotkine M, Scherman D, Margaill I (2006) Anti-inflammatory effects of PJ34, a poly(ADP-ribose) polymerase inhibitor, in transient focal cerebral ischemia in mice. Br J Pharmacol 149(1):23–30
Jijon HB, Churchill T, Malfair D, Wessler A, Jewell LD, Parsons HG, Madsen KL (2000) Inhibition of poly(ADP-ribose) polymerase attenuates inflammation in a model of chronic colitis. Am J Physiol Gastrointest Liver Physiol 279(3):G641–G651
Su CF, Liu DD, Kao SJ, Chen HI (2007) Nicotinamide abrogates acute lung injury caused by ischaemia/reperfusion. Eur Respir J 30(2):199–204
Zingarelli B, Hake PW, O’Connor M, Denenberg A, Kong S, Aronow BJ (2003) Absence of poly(ADP-ribose) polymerase-1 alters nuclear factor-kappa B activation and gene expression of apoptosis regulators after reperfusion injury. Mol Med 9(5–8):143–153
Czapski GA, Cakala M, Gajkowska B, Strosznajder JB (2006) Poly(ADP-ribose) polymerase-1 inhibition protects the brain against systemic inflammation. Neurochem Int 49(8):751–755
Nakajima H, Nagaso H, Kakui N, Ishikawa M, Hiranuma T, Hoshiko S (2004) Critical role of the automodification of poly(ADP-ribose) polymerase-1 in nuclear factor-kappaB-dependent gene expression in primary cultured mouse glial cells. J Biol Chem 279(41):42774–42786
Strosznajder R, Gajkowska B (2006) Effect of 3-aminobenzamide on Bcl-2, Bax and AIF localization in hippocampal neurons altered by ischemia-reperfusion injury. The immunocytochemical study. Acta Neurobiol Exp (Wars) 66(1):15–22
von Lukowicz T, Hassa PO, Lohmann C, Borén J, Braunersreuther V, Mach F, Odermatt B, Gersbach M, Camici GG, Stähli BE, Tanner FC, Hottiger MO, Lüscher TF, Matter CM (2008) PARP1 is required for adhesion molecule expression in atherogenesis. Cardiovasc Res 78(1):158–166
Strosznajder RP, Jesko H, Zambrzycka A (2005) Poly(ADP-ribose) polymerase: the nuclear target in signal transduction and its role in brain ischemia–reperfusion injury. Mol Neurobiol 31(1–3):149–167
Klein JA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, Frankel WN, Bronson RT, Ackerman SL (2002) The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature 419(6905):367–374
Cheung EC, Melanson-Drapeau L, Cregan SP, Vanderluit JL, Ferguson KL, McIntosh WC, Park DS, Bennett SA, Slack RS (2005) Apoptosis-inducing factor is a key factor in neuronal cell death propagated by BAX-dependent and BAX-independent mechanisms. J Neurosci 25(6):1324–1334
Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellecchia M, Blomgren K, Plesnila N (2005) Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J Neurosci 25(44):10262–10272
Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL (2006) Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci USA 103(48):18314–18319
Strosznajder RP, Jesko H, Dziewulska J (2005) Effect of carvedilol on neuronal survival and poly(ADP-ribose) polymerase activity in hippocampus after transient forebrain ischemia. Acta Neurobiol Exp (Wars) 65(2):137–143
Zhang Y, Zhang X, Park TS, Gidday JM (2005) Cerebral endothelial cell apoptosis after ischemia–reperfusion: role of PARP activation and AIF translocation. J Cereb Blood Flow Metab 25(7):868–877
Zhang Y, Park TS, Gidday JM (2007) Hypoxic preconditioning protects human brain endothelium from ischemic apoptosis by Akt-dependent survivin activation. Am J Physiol Heart Circ Physiol 292(6):H2573–H2581
Lee JH, Park SY, Shin HK, Kim CD, Lee WS, Hong KW (2007) Poly(ADP-ribose) polymerase inhibition by cilostazol is implicated in the neuroprotective effect against focal cerebral ischemic infarct in rat. Brain Res 1152:182–190
Takahashi K, Greenberg JH, Jackson P, Maclin K, Zhang J (1997) Neuroprotective effects of inhibiting poly(ADP-ribose) synthetase on focal cerebral ischemia in rats. J Cereb Blood Flow Metab 17(11):1137–1142
Lo EH, Bosque-Hamilton P, Meng W (1998) Inhibition of poly(ADP-ribose) polymerase: reduction of ischemic injury and attenuation of N-methyl-D-aspartate-induced neurotransmitter dysregulation. Stroke 29(4):830–836
Tokime T, Nozaki K, Sugino T, Kikuchi H, Hashimoto N, Ueda K (1998) Enhanced poly(ADP-ribosyl)ation after focal ischemia in rat brain. J Cereb Blood Flow Metab 18(9):991–997
Yap E, Tan WL, Ng I, Ng YK (2008) Combinatorial-approached neuroprotection using pan-caspase inhibitor and poly (ADP-ribose) polymerase (PARP) inhibitor following experimental stroke in rats; is there additional benefit? Brain Res 1195:130–138
Ding Y, Zhou Y, Lai Q, Li J, Gordon V, Diaz FG (2001) Long-term neuroprotective effect of inhibiting poly(ADP-ribose) polymerase in rats with middle cerebral artery occlusion using a behavioral assessment. Brain Res 915(2):210–217
Takahashi K, Greenberg JH (1999) The effect of reperfusion on neuroprotection using an inhibitor of poly(ADP-ribose) polymerase. NeuroReport 10(10):2017–2022
Couturier JY, Ding-Zhou L, Croci N, Plotkine M, Margaill I (2003) 3-Aminobenzamide reduces brain infarction and neutrophil infiltration after transient focal cerebral ischemia in mice. Exp Neurol 184(2):973–980
Iyirhiaro GO, Brust TB, Rashidian J, Galehdar Z, Osman A, Phillips M, Slack RS, Macvicar BA, Park DS (2008) Delayed combinatorial treatment with flavopiridol and minocycline provides longer term protection for neuronal soma but not dendrites following global ischemia. J Neurochem 105(3):703–713
Moroni F, Meli E, Peruginelli F, Chiarugi A, Cozzi A, Picca R, Romagnoli P, Pellicciari R, Pellegrini-Giampietro DE (2001) Poly(ADP-ribose) polymerase inhibitors attenuate necrotic but not apoptotic neuronal death in experimental models of cerebral ischemia. Cell Death Differ 8(9):921–932
Cho KO, Kim SK, Cho YJ, Sung KW, Kim SY (2007) Regional differences in the neuroprotective effect of minocycline in a mouse model of global forebrain ischemia. Life Sci 80(22):2030–2035
Li J, Ferraris DV, Kletzly PW, Li W, Wang E, Xing A et al (2002) Symmetrically disubstituted aromatic compounds and pharmaceutical compositions for inhibiting poly(ADP-ribose) glycohydrolase, and methods for their use. WO, 02057211
Ying W, Swanson RA (2000) The poly(ADP-ribose) glycohydrolase inhibitor gallotannin blocks oxidative astrocyte death. NeuroReport 11:1385–1388
Ying W, Sevigny MB, Chen Y, Swanson RA (2001) Poly(ADP-ribose) glycohydrolase mediates oxidative and excitotoxic neuronal death. Proc Natl Acad Sci USA 98:12227–12232
Cozzi A, Cipriani G, Fossati S, Faraco G, Formentini L, Min W, Cortes U, Wang ZQ, Moroni F, Chiarugi A (2006) Poly(ADP-ribose) accumulation and enhancement of postischemic brain damage in 110-kDa poly(ADP-ribose) glycohydrolase null mice. J Cereb Blood Flow Metab 26(5):684–695
Shibata A, Maeda D, Ogino H, Tsutsumi M, Nohmi T, Nakagama H, Sugimura T, Teraoka H, Masutani M (2009) Role of Parp-1 in suppressing spontaneous deletion mutation in the liver and brain of mice at adolescence and advanced age. Mutat Res 664(1–2):20–27
Strosznajder JB, Jęśko H, Strosznajder RP (2000) Age-related alteration of poly(ADP-ribose) polymerase activity in different parts of the brain. Acta Biochim Pol 47(2):331–337
Strosznajder RP, Jesko H, Adamczyk A (2005) Effect of aging and oxidative/genotoxic stress on poly(ADP-ribose) polymerase-1 activity in rat brain. Acta Biochim Pol 52(4):909–914
Strosznajder JB, Jeśko H, Strosznajder RP (2000) Effect of amyloid beta peptide on poly(ADP-ribose) polymerase activity in adult and aged rat hippocampus. Acta Biochim Pol 47(3):847–854
Mabley JG, Suarez-Pinzon WL, Haskó G, Salzman AL, Rabinovitch A, Kun E, Szabó C (2001) Inhibition of poly (ADP-ribose) synthetase by gene disruption or inhibition with 5-iodo-6-amino-1, 2-benzopyrone protects mice from multiple-low-dose-streptozotocin-induced diabetes. Br J Pharmacol 133(6):909–919
Mabley JG, Horváth EM, Murthy KG, Zsengellér Z, Vaslin A, Benko R, Kollai M, Szabó C (2005) Gender differences in the endotoxin-induced inflammatory and vascular responses: potential role of poly(ADP-ribose) polymerase activation. J Pharmacol Exp Ther 315(2):812–820
Hagberg H, Wilson MA, Matsushita H, Zhu C, Lange M, Gustavsson M, Poitras MF, Dawson TM, Dawson VL, Northington F, Johnston MV (2004) PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem 90(5):1068–1075
McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD (2005) Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab 25(4):502–512
Lang JT, McCullough LD (2008) Pathways to ischemic neuronal cell death: are sex differences relevant? J Transl Med 6:33
Yuan M, Siegel C, Zeng Z, Li J, Liu F, McCullough LD (2009) Sex differences in the response to activation of the poly (ADP-ribose) polymerase pathway after experimental stroke. Exp Neurol 217(1):210–218
Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD (2009) Sex differences in caspase activation after stroke. Stroke 40(5):1842–1848
Li J, McCullough LD (2009) Sex differences in minocycline-induced neuroprotection after experimental stroke. J Cereb Blood Flow Metab 29(4):670–674
Acknowledgments
We express our thanks to Dorota Strosznajder for her involvement and help and to Beata Łuczyńska for typing this manuscript. This work was supported by statutory budget of Mossakowski Medical Research Centre, Polish Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strosznajder, R.P., Czubowicz, K., Jesko, H. et al. Poly(ADP-Ribose) Metabolism in Brain and Its Role in Ischemia Pathology. Mol Neurobiol 41, 187–196 (2010). https://doi.org/10.1007/s12035-010-8124-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-010-8124-6