Abstract
In the present work, pristine and cetyl trimethyl ammonium bromide (CTAB)-coated ferric oxide nanoparticles \((\hbox {CTAB@Fe}_{2}\hbox {O}_{3} \hbox { NPs})\) were synthesized and studied as enzyme mimics. The w/w ratio of \(\hbox {Fe}_{2}\hbox {O}_{3}\) to CTAB was varied as 1:1 and 1:2. Transmission electron microscopic analysis revealed that pristine NPs had an average size of 50 nm, whereas the presence of CTAB resulted in the formation of nanorods with length of 130 nm. BET studies confirmed enhancement of surface area on CTAB coating, which was maximum for w/w ratio 1:1. The synthesized pristine NPs and CTAB-coated NPs were evaluated for their peroxidase mimic activity using o-dianisidine dihydrochloride as substrate. Optimum pH, temperature, substrate and NPs concentration for the reaction were 1, \(25^{\circ }{\mathrm{C}}\), \(0.16~\hbox {mg}~\hbox {ml}^{-1}\) and \(1~\hbox {mg}~\hbox {ml}^{-1}\), respectively. Peroxidase mimic activity of \(\hbox {CTAB@Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) (w/w 1:1) was higher than that of pristine NPs. However, further increase in CTAB coating (w/w 1:2) resulted in lowering of peroxidase mimic activity. Kinetic analysis was carried out at optimized conditions; maximum velocity (\(V_{\mathrm{max}})\) and Michaelis constant (\(K_{\mathrm{m}})\) value of \(\hbox {CTAB@Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) at 1:1 w/w ratio were 7.69 mM and \(1.12~\upmu \hbox {mol}~\hbox {s}^{-1}\), respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Artificial enzymes have been attracting the attention of researchers due to several disadvantages in the performance of natural enzymes such as rapid denaturation by environmental changes, which alter their structure and catalytic properties [1]. Preparation, purification and storage of natural enzymes are very difficult, expensive and time-consuming, which limits their widespread applications [2]. Artificial enzymes, on the contrary, have been explored because of their easy preparation, low-cost storage and purification processes [3]. Nanomaterials as enzyme mimics have become a growing area of research. Thus, it is crucial to look for stability, catalytic efficiency and enzyme mimic activity from the practical point of view. They have already gained several applications in the fields such as immunoassays, therapy, stem cell growth, pollutant removal and cancer diagnostics [4]. To date, several artificial enzymes have been reported to mimic natural analogues, including hydrolase, aldolase, nitrile hydratase, ligase, lipase, peroxidase and superoxide dismutase [5, 6].
A variety of nanostructures, including carbon materials [7,8,9], nanocomposites [10, 11], noble metals [12, 13] and metal oxides [14,15,16], have displayed peroxidase-like activity. Of late, magnetic nanoparticles (NPs) have become rapidly growing and most exciting areas for studying peroxidase mimic activity. \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) are reported to exhibit enhanced peroxidase mimic activity as compared with natural horseradish peroxidase (HRP) [17]. These NPs have the ability of scavenging or generating reactive oxygen species that can be used to mimic the peroxidase activity of natural ones. Similar to HRP, \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) catalyse the oxidation of various substrates such as 3,3,5,5,-tetramethylbenzidine (TMB), o-phenylenediamine (OPD), 3,3-diaminobenzidine (DAB) and o-dianisidine dihydrochloride. All substrates undergo differential colour change when catalytically oxidized on exposure to \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\). To date, a number of peroxidase mimic NPs, including maghemite, magnetite and enzymes containing \(\hbox {Fe}^{2+}\) and \(\hbox {Fe}^{3+}\) in their reaction centre, have been explored [18,19,20,21].
Activity of \(\hbox {Fe}_{2}\hbox {O}_{3}\) gets altered on coating. Citrate-capped \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) \((\hbox {N}_{\mathrm{cit}})\) showed higher turnover value as compared with caboxymethyl dextrane \((\hbox {K}_{\mathrm{CMD}})\)-coated NPs with both TMB and ABTS [2,\(2'\)-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)] as substrate. A possible reason for the low activity of K\(_{\mathrm{CMD}}\) was its thicker coating that resulted in the steric effects. On the other hand, glycerine-coated ferric oxide NPs \((\hbox {N}_{\mathrm{gly}})\) displayed higher \(K_{\mathrm{cat}}\) value than polylysine-coated ferric oxide NPs \((\hbox {N}_{\mathrm{PLL}})\) towards TMB but displayed low \(K_{\mathrm{cat}}\) value towards ABTS. \(\hbox {N}_{\mathrm{PLL}}\) have a slightly higher positive charge present on their surface than \(\hbox {N}_{\mathrm{gly}}\), which affects the activity [22].
Several other NPs have been reported, such as \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) coated with both high molecular mass and low molecular mass dextran [23,24,25,26], iron NPs coated with carboxyl dextran [27,28,29], silica-coated super-paramagnetic iron oxide NPs [30, 31] and non-stoichiometric super-paramagnetic \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) coated with polyglucose orbital carboxymethyl ether [32]. Cetyl trimethyl ammonium bromide (CTAB) surfactant is mostly used in the preparation of NPs due to its property of controlling the shape and size of the particles [33, 34]. To the best of our knowledge, peroxidase mimic activity of CTAB-coated \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) has not been reported. In the present work, cationic CTAB was chosen as surfactant for \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) coating because it can interact with the negatively charged iron oxide NPs formed during co-precipitation. Effect of changing CTAB coating on the peroxidase mimic activity of \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) has also been studied using o-dianisidine dihydrochloride as substrate.
2 Experimental
2.1 Synthesis and characterization
All chemicals used, i.e., hydrogen peroxide \((\hbox {H}_{2}\hbox {O}_{2}-30\%), \hbox {NH}_{4}\hbox {OH}\) (wt. 25%), CTAB \((\hbox {C}_{19}\hbox {H}_{42}\hbox {BrN})\), ferrous ammonium sulphate \([(\hbox {NH}_{4})_{2}\hbox {Fe}(\hbox {SO}_{4})_{2}\cdot \hbox {6H}_{2}\hbox {O}]\), ammonium ferric sulphate \([\hbox {NH}_{4}\hbox {Fe}(\hbox {SO}_{4})_{2}\cdot \hbox {6H}_{2}\hbox {O}]\), o-dianisidine dihydrochloride \([\hbox {C}_{14}\mathrm{H}_{18}\hbox {Cl}_{2}\hbox {N}_{2}\hbox {O}_{2}]\) were of analytical reagent grade. Pristine and CTAB-coated \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) (with varying w/w ratio of \(\hbox {Fe}_{2}\hbox {O}_{3}:\hbox {CTAB}\), i.e., 1:1 and 1:2) were synthesized by co-precipitation method; these NPs were designated as F-1, F-2 and F-3, respectively. To synthesize pristine \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\), 0.022 mol of \([\hbox {NH}_{4}\hbox {Fe}(\hbox {SO}_{4})_{2}\cdot 6\hbox {H}_{2}\hbox {O}]\) and 0.0146 mol of \(\hbox {(NH}_{4})_{2}\hbox {Fe}(\hbox {SO}_{4})_{2} \cdot 6\hbox {H}_{2}\hbox {O}\) were dissolved in 50 ml de-ionized water with continuous stirring [35]. In order to form precipitates, 5 ml ammonium hydroxide was added to this solution dropwise with vigorous stirring until the pH of the mixture reached up to 9. This solution was further stirred for 45 min with continuous heating at \(85^{\circ }\mathrm{C}\). Precipitates formed were separated by centrifugation. Filtrate was washed with ethanol followed by de-ionized water several times until the pH of the filtrate became 7. Filtrate was dried in an oven at \(100^{\circ }\mathrm{C}\) for 8 h. Similar method was employed for the synthesis of \(\hbox {CTAB@Fe}_{2}\hbox {O}_{3}\hbox { NPs}\). During synthesis of \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\), CTAB was added in w/w ratio of 1:1 and 1:2 in the reaction mixture and CTAB-coated \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) were labelled as F-2 and F-3, respectively.
The Scherrer relationship was applied to find the average particle size:
where \(\lambda \) represents wavelength of X-ray (1.54 nm), \(\beta \) represents full-width at half-maximum (FWHM) and \(\theta \) is diffraction angle \((17.866^{\circ })\). Details of techniques employed, viz., FT-IR, X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), VSM and BET analysis are described in the previous publication [36].
2.2 Kinetic analysis
Enzyme mimic activity of synthesized NPs was evaluated by the method of Shannon et al [37]; pH of the reaction mixture was varied from 1 to 10 using 0.1 N HCl and 0.1 N NaOH to ascertain optimum pH. Effect of catalyst dose, substrate concentration, temperature and contact time was studied to optimize the reaction conditions. Kinetic experiments were carried out under optimized conditions. The solution was analysed at 430 nm using a UV–vis spectrophotometer in time course mode for 3 min, with a 30-s time interval. The Lineweaver–Burk plot was employed for the calculation of Michaelis–Menten constant as follows:
Here, \(\nu \) is initial velocity, \(V_{\mathrm{max}}\) and [S] represent the maximum velocity of the reaction and substrate concentration, respectively, whereas \(K_{\mathrm{m}}\) represents the Michaelis–Menten constant, which is an indicator of enzyme affinity for its substrate. Smaller the value of \(K_{\mathrm{m}}\), greater the affinity between enzyme and substrate.
3 Characterization
3.1 Structural analysis
The crystalline structure of F-1, F-2 and F-3 was identified by the XRD technique. Diffraction peaks in XRD (figure 1a) pattern of \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) were observed at \(2\theta =20, 30.39, 35.73, 43.42, 53.81, 57.39\) and \(63.01^{\circ }\) with the inter-planar spacing of 0.29, 0.25, 0.20, 0.17, 0.16 and 0.16 nm, which were assigned to (111), (104), (110), (202), (116), (018) and (214) planes, respectively. These data matched with the values reported in literature for \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) [38]. In F-2 and F-3, the peaks were broadened and increase in FWHM was observed, which confirmed decrease in average particle size as compared with bare \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\). These results suggested that the inter-planar spacing increased, which led to the decrease in particle size.
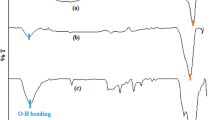
The binding of CTAB to the \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) was investigated by FT-IR spectra. Figure 1b presents the IR spectra of pristine and CTAB-coated \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\). In pristine \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\), the bands at 1582 and \(3172~\hbox {cm}^{-1}\) are attributed to O–H bending and stretching vibrations, respectively, which is due to the water molecules adsorbed onto the surface of bare NPs. Absorption bands in the region of 415–618 \(\hbox {cm}^{-1}\) are assigned to Fe–O stretching vibrations [39]. The absorption bands at 445 \(\hbox {cm}^{-1}\) in bare NPs and 416, 487 \(\hbox {cm}^{-1}\) in the surfactant-coated NPs are due to Fe–O bond vibrations in octahedral sites. The bands at 589 \(\hbox {cm}^{-1}\) in bare NPs and at 586, 618 \(\hbox {cm}^{-1}\) in CTAB-coated NPs correspond to Fe–O bond vibrations in the tetrahedral sites. In case of bare and CTAB-coated Fe\(_{2}\)O\(_{3}\) NPs, the bands at 3172, 3103 and 3177 \(\hbox {cm}^{-1}\) correspond to O–H stretching vibrations. Furthermore, the bands at 1124, 1215 and 1226 \(\hbox {cm}^{-1}\) in CTAB-coated NPs correspond to C–N bond stretching. The bands at 1359 and 1423 \(\hbox {cm}^{-1}\) are due to the residual ammonia used at the time of NPs preparation.
Furthermore, the bands at 1665 and \(1618~\hbox {cm}^{-1}\) correspond to N–H bending vibrations, and the bands at \(3177~\hbox {cm}^{-1}\) in coated NPs correspond to stretching vibrations of O–H bonds. The bands at 968, 891, 971 and \(854~\hbox {cm}^{-1}\) in both types of CTAB-coated NPs are due to the presence of tertiary amine. The bands over 3000–3600 \(\hbox {cm}^{-1}\) are attributed to reduced amount of water present in the samples. The bands at \(795~\hbox {cm}^{-1}\) correspond to C–H bending of long alkane chain present in CTAB. Furthermore, additional bands in the range of 2800–3000 \(\hbox {cm}^{-1}\) in coated NPs are attributed to the stretching vibrations of C–H bonds of the saturated alkane [40]. In addition, a small shift in peaks was seen on coating \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) with CATB, which is due to the change in environment of bare NPs.
3.2 Morphological studies
SEM and TEM were used to study the surface morphology and particle size distribution of synthesized pristine and CTAB-coated NPs. SEM micrographs of pristine NPs (figure 2a) clearly depict their porous surface, whereas CTAB-coated NPs, F-2 and F-3 (figure 2b and c), have somewhat more porous structure as compared with F-1 due to the immobilization of CTAB onto the surface of \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\). TEM images (figure 3) reveal shape and morphology of NPs. F-1 appeared to be spherical and somewhat agglomerated due to the strong dipole–dipole magnetic interactions between NPs. However, rods were formed when \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) were synthesized in the presence of CTAB due to the adsorption of CTAB on the surface of NPs, thus preventing their agglomeration due to the repulsion between the similar charged ions of CTAB. Particle size of \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) also decreased on coating CTAB.
A similar reason for the rod formation was given by Chen et al [41] in their study when \(\hbox {Fe}_{3}\hbox {O}_{4}\hbox { NPs}\) were treated with \(\hbox {FeCl}_{3}\) solution. \(\hbox {Fe}^{3+}\) ions were absorbed on the surface of NPs due to the common ion effect. This scheme was co-ordinated with our approach for the CTAB-coated \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\). Another approach towards the formation of rod-shaped NPs was reported by Zhao and Nan [40]. The stability arises from the fact that a certain amount of space was occupied by the polymers coated on the surface of NPs. The space was compressed when the NPs came too close, thus triggering the formation of rod-shaped NPs.
Nitrogen adsoprtion–desorption studies of pristine and CTAB-coated \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) were carried using BET analysis to characterize the surface properties of synthesized NPs. Figure 4 displays nitrogen sorption isotherms and pore size distribution curves. IUPAC classification designates these isotherms as type IV, which represents mesoporous structure with comparatively broad hysteresis loop belonging to H1 type. The meso-porosity of the F-2 increased with increase in the pore volume as compared with bare ones but decreased on further increase in coating. The BET surface area of F-2 and F-3 NPs was 69.174 and \(49.072~\hbox {m}^{2} \hbox {g}^{-1}\), respectively (table 1). Lower surface area of F-1 was due to the strong Van der Walls forces of interaction among particles, which led to their agglomeration.
3.3 Magnetic studies
Hysteresis loop depicted the effect of change in magnetic field on magnetization and magnetic flux of synthesized NPs. Variation in magnetic character of the NPs on coating with CTAB was also seen from the loop (figure 5) and the magnetic parameters of the synthesized NPs (table 2). The saturation magnetization (\(M_\mathrm{s})\) of F-2 and F-3 NPs was decreased due to coating of non-magnetic CTAB on the surface of \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\). Low coercivity values of these NPs also categorized them as soft ferromagnetic material. Narrow hysteresis loops along with low coercivity range of NPs from 12.98 to 54.05 O\(_\mathrm{e }\) show that these NPs can be easily demagnetized [42].
4 Enzyme mimic activity of pristine and CTAB-coated \(\hbox {Fe}_{2}\hbox {O}_{3} \hbox { NPs}\)
The peroxidase-like activity of synthesized NPs with o-dianisidine dihydrochloride as substrate was studied in the presence of \(\hbox {H}_{2}\hbox {O}_{2}\). o-dianisidine dihydrochloride possessed opposite charge character than that of synthesized NPs, thus increasing its ability to interact with NPs [40]. Pristine and CTAB-coated \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) could catalyse the oxidation of o-dianisidine dihydrochloride by \(\hbox {H}_{2}\hbox {O}_{2}\), producing a dark brown colour. The colour of the \(\hbox {CTAB@Fe}_{2}\hbox {O}_{3}\hbox { NPs}\)–\(\hbox {H}_{2}\hbox {O}_{2}\)–substrate system showed more absorbance than that of the \(\hbox {H}_{2}\hbox {O}_{2}\)–substrate system, whereas \(\hbox {CTAB@Fe}_{2}\hbox {O}_{3}\hbox { NPs}\)–substrate system did not produce any colour (as shown in the inset of figure 6). It indicates that \(\hbox {CTAB@Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) possess peroxidase-like activity in the presence of \(\hbox {H}_{2}\hbox {O}_{2}\). Also, activity gets enhanced on increasing the substrate concentration because more substrate molecules starts interacting with \(\hbox {H}_{2}\hbox {O}_{2}\), thus producing more oxidized product.
The probable mechanism for peroxidase-like activity of \(\hbox {CTAB@Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) (1:1) is shown in scheme 1. These NPs catalysed the decomposition of \(\hbox {H}_{2}\hbox {O}_{2}\) to produce OH radicals, which furthur oxidized o-dianisidine dihydrochloride, producing dark brown oxidized product.
4.1 Effect of temperature, contact time and pH on peroxidase mimic activity
The effect of temperature, contact time and pH on peroxidase mimic activity in the reaction mixture was evaluated. Peroxidase mimic activity of NPs was observed by varying the pH of the reaction mixture from 1 to 10 (figure 7a). The synthesized NPs showed the highest activity at pH 1 and activity decreased on further increasing pH of the reaction mixture. At low pH, the presence of H\(^{+}\) facilitates the decomposition of \(\hbox {H}_{2}\hbox {O}_{2}\), thus increasing the rate of reaction. The variation in temperature from 10 to \(45^{\circ }\mathrm{C}\) resulted in increase in the rate of reaction up to \(25^{\circ }\mathrm{C}\) and later reaction rate showed a decreasing trend, indicating that \(25^{\circ }\mathrm{C}\) was the optimum temperature for peroxidase mimic activity of NPs. The decrease in activity at high temperature can be explained on the basis of reaction mechanism as given in scheme 1. The first step involves the adsorption of \(\hbox {H}_{2}\hbox {O}_{2}\) on the surface of NPs followed by the cleavage of \(\hbox {H}_{2}\hbox {O}_{2}\) to liberate OH radicals. At high temperature the adsorptive interactions between \(\hbox {H}_{2}\hbox {O}_{2}\) and NPs slowed down, leading to decrease in activity [43].
Similarly, concentration of substrate and NPs was optimized by varying their content. Optimum concentration of the substrate was \(0.16~\hbox {mg}~\hbox {ml}^{-1}\) and that for F-2 NPs was \(1.0~\hbox {mg}~\hbox {ml}^{-1}\) using 0.1 N HCl as solvent (figure 8a). The absorbance of the oxidized product increased with increase in the concentration of F-2 NPs till \(1.0~\hbox {mg}~\hbox {ml}^{-1}\) and afterwards it decreased. This was attributed to the aggregation of these particles, which decreased the surface/volume ratio and hence decreased the enzyme mimic activity. Under optimized conditions, peroxidase mimic activities of F-1, F-2 and F-3 were compared. F-2 displayed the highest peroxidase mimic activity followed by F-1 and F-3 (figure 8b). These results suggested that at lower CTAB concentration, activity was increased due to smaller particle size and lesser agglomeration of NPs, which resulted in greater interaction with substrate. However, at higher CTAB concentration, decrease in peroxidase mimic activity was observed due to steric hindrance by CTAB molecules. The lower surface area of F-3 NPs as compared with F-2 NPs (as confirmed by BET analysis) decreased the number of active sites, thus lowering its interaction with the substrate, and diminished its enzyme mimic activity.
Higher peroxidase mimic activity for the \(\hbox {CTAB@Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) was due to the coating, which increased surface area of \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) [44]. Thus, more number of substrate molecules attached to the surface of catalyst due to increase in active sites, which increased turn over number, thus resulting in increased rate of reaction. Another reason for the activity of F-2 NPs was the presence of CTAB, which prevented particles agglomeration, due to which surface of the particles remained free for the substrate to interact. \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) with higher CTAB concentration (1:2) led to decrease in activity due to steric hindrance, which decreased the surface area and thus number of active sites.
4.2 Kinetic analysis
Kinetic analysis was performed for the NPs, showing maximum peroxidase mimic activity, i.e., for F-2 NPs. From the Lineweaver–Burk plot (figure 6), the calculated \(K_{\mathrm{m}}\) and \(V_{\mathrm{max}}\) values of F-2 NPs are 7.69 mM and \(1.12~\upmu \hbox {mol}~\hbox {s}^{-1}\), respectively. Savitsky et al [45] also studied the kinetic analysis of o-dianisidine oxidation using free and antibody complexes of iron(lll) coproporphyrin as peroxidase mimic. The \(K_{\mathrm{m}}\) obtained for various complexes are in the range 0.53–5.9 \(\times \, 10^{6}\) M, whereas in the present study the \(K_{\mathrm{m}}\) value is much lower, which confirms higher affinity of \(\hbox {CTAB@Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) for the substrate. Lower value of \(K_{\mathrm{m}}\) is an essential parameter for potential activity of enzyme mimics. Thus, kinetic studies authenticated the promising peroxidase mimic activity of \(\hbox {CTAB@Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) having w/w ratio 1:1.
5 Conclusion
We have synthesized pristine and CTAB-coated ferric oxide NPs \((\hbox {CTAB@Fe}_{2}\hbox {O}_{3}\hbox { NPs}\)) by co-precipitation method and compared their peroxidase mimic activity. Results showed that \(\hbox {Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) synthesized in the presence of CTAB having w/w ratio of 1:1 displayed higher peroxidase mimic activity, which was correlated with the higher surface area of the NPs. Further increase in CTAB concentration resulted in decrease in activity due to steric hindrance by the CTAB molecules adhering on the surface of NPs. \(\hbox {CTAB@Fe}_{2}\hbox {O}_{3}\hbox { NPs}\) as peroxidase mimic showed promising properties, viz., easy preparation, storage, reusability, stability, dispersibility and easy separation, as compared with natural enzymes. They can be explored for varied applications as an economically viable substitute to peroxidase enzyme in various practical bio-medical applications such as biosensors for the detection of hydrogen peroxide in various commodities and glucose level in blood.
References
Shoji E and Freund M S 2001 J. Am. Chem. Soc. 123 3383
Breslow R 1995 Acc. Chem. Res. 28 146
Wei H and Wang E 2013 Chem. Soc. Rev. 42 6060
Colombo M, Carregal R S, Casula M F, Gutierrez L, Morales M P, Bohm I B et al 2012 Chem. Soc. Rev. 41 4306
Chen W, Chen J, Feng Y B, Hong L, Chen Q Y, Wu L F et al 2012 Analyst 137 1706
Chen Z, Yin J J, Zhou Y T, Zhang Y, Song L, Song M et al 2012 ACS Nano 6 4001
Cui R, Han Z and Zhu J J 2011 Chem. Eur. J. 17 9377
Song Y, Qu K, Zhao C, Ren J and Qu X 2010 Adv. Mater. 22 2206
Zheng A X, Cong Z X, Wang J R, Li J, Yang H H and Chen G N 2013 Biosens. Bioelectron. 49 519
Bi S, Zhao T, Jia X and He P 2014 Biosens. Bioelectron. 57 110
Xing Z, Tian J, Asiri A M, Qusti A H, Al Youbi A O and Sun X 2014 Biosens. Bioelectron. 52 452
Jiang H, Chen Z, Cao H and Huang Y 2012 Analyst 137 5560
Jv Y, Li B and Cao R 2010 Chem. Commun. 46 8017
Andre R, Natalio F, Humanes M, Leppin J, Heinze K, Wever R et al 2011 Adv. Funct. Mater. 21 501
Chen W, Chen J, Liu A L, Wang L M, Li G W and Lin X H 2011 Chem. Cat. Chem. 3 1151
Mu J, Wang Y, Zhao M and Zhang L 2012 Chem. Commun. 48 2540
Gao L Z, Zhuang J, Nie L, Zhang J B, Zhang Y, Gu N et al 2007 Nat. Nanotechnol. 2 577
Henriksen A, Smith A T and Gajhede M 1999 J. Biol. Chem. 274 35005
Kvaratskhelia M, Winkel C and Thorneley R N F 1997 Plant Physiol. 114 1237
Kariya K, Lee E, Hirouchi M, Hosokawa M and Sayo H 1987 Biochim. Biophys. Acta 911 95
Hartert M M, Bourgeois E, Grülke S, Dupont G D, Caudron I, Deby C et al 1998 Can. J. Vet. Res. 62 127
Yu F, Huang Y, Cole A J and Yang V C 2009 Biomaterials 30 4716
Clement O, Siauve N, Cuenod C A and Frija G 1998 Top. Magn. Reson. Imaging 9 167
Johnson L, Pinder S E and Douek M 2013 Histopathology 62 481
Kernstine K H, Stanford W and Mullan B F 1999 Ann. Thorac. Surg. 68 1022
Harisinghani M G, Saini S, Hahn P F, Weissleder R and Mueller P R 1998 Acad. Radiol. 5 167
Reimer P and Balzer T 2003 Eur. Radiol. 13 1266
Vogl T J, Hammersting l R and Schwarz W 1996 Invest. Radiol. 31 696
Reimer P, Rummeny E J and Daldrup H E 1995 Radiology 195 489
Bonnemain B 1998 J. Drug Target. 6 167
Campbell J L, Arora J, Cowell S F, Garg A, Eu P, Bhargava S K et al 2011 PLoS One 6 21857
Bullivant J P, Zhao S, Willenberg B J, Kozissnik B, Batich C D and Dobson J 2013 Int. J. Mol. Sci. 14 17501
Zhang Y, Xu D, Li W, Yu J and Chen Y 2012 J. Nanomater. 2012 1
Qiu Y, Liu Y, Wang L, Xu L, Bai R, Ji Y et al 2010 Biomaterials 31 7606
Deng J H, Zhang X R, Zeng G M, Gong J L, Niu Q Y and Liang J 2013 Chem. Eng. J. 226 189
Grewal J K and Kaur M 2017 Ceram. Int. 43 16611
Shannon L M, Kay E and Lew J Y 1966 J. Biol. Chem. 241 2166
Uzunov I and Aleksandrova A 2002 Chem. Inorg. 62 195
Hoan N T V, Thu N T A, Duc H V, Cuong N D, Khieu D Q and Vo V 2016 J. Chem. 2016 1
Zhao B and Nan Z 2011 Nanoscale Res. Lett. 6 1
Chen A, Wang H, Zhao B and Li X 2003 Synth. Met. 139 411
Ubhi M K, Kaur M, Singh D and Granchee J M 2017 Pure Appl. Chem. 11 247
Johnson B B 1990 Environ. Sci. Technol. 24 112
Cai S, Jia X, Han Q, Yan X, Yang R and Wang C 2017 Nano Res. 10 2056
Savitsky A P, Nelen M I, Yatsmirsky A K, Demcheva M V, Ponomarev G V and Nikov I V 1994 Appl. Biochem. Biotechnol. 47 317
Acknowledgements
We are grateful to EMN Lab of Punjab Agricultural University, Ludhiana, for SEM and TEM recordings.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garg, D., Kaur, M., Sharma, S. et al. Effect of CTAB coating on structural, magnetic and peroxidase mimic activity of ferric oxide nanoparticles. Bull Mater Sci 41, 134 (2018). https://doi.org/10.1007/s12034-018-1650-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-018-1650-y