Abstract
The highly effective g-\(\hbox {C}_{3}\hbox {N}_{4}\) hybridized CdS photocatalysts were synthesized via a successive calcination and hydrothermal process. The as-prepared photocatalysts were characterized by X-ray powder diffraction, transmission electron microscopy and UV–Vis diffuse reflectance spectroscopy. The photocatalytic performance of the \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites was evaluated by the photodegradation of RhB under visible light irradiation. The results showed that photocatalytic ability of the \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites was higher than that of pure \(\hbox {C}_{3}\hbox {N}_{4}\) and CdS. The enhanced photocatalytic activity could be attributed to the high separation efficiency of the photo-excited electron-hole pairs. A possible mechanism of the photocatalytic degradation of RhB on \(\hbox {C}_{3}\hbox {N}_{4}/\)CdS nanocomposites was also proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Artificial semiconductor photocatalysis offers a viable strategy for removal of water pollutants because it represents an easy method to utilize the energy of either artificial illumination or natural sunlight [1,2,3,4]. Recently, it has been confirmed that materials with conjugative \(\pi \) structure (graphene-like carbon and \(\hbox {C}_{60}\), etc.) have high photocatalytic performance, which has attracted an extensive interest in photocatalytic applications [5]. Graphite-like \(\hbox {C}_{3}\hbox {N}_{4}\) exhibits high photocatalytic activity for degradation of organic pollution under visible light irradiation due to its unique optical properties, suitable band gap, high chemical and thermal stability [6, 7]. Moreover, a lot of results concerning the g-\(\hbox {C}_{3}\hbox {N}_{4}\) hybridization have also been reported as effective ways to enhance the catalytic properties of photocatalysts. For instance, Wang et al [8] synthesized \(\hbox {C}_{3}\hbox {N}_{4}/\hbox {Bi}_{2}\hbox {WO}_{6}\) photocatalyst, which showed higher photocatalytic activity than that of pure \(\hbox {Bi}_{2}\hbox {WO}_{6}\) or \(\hbox {C}_{3}\hbox {N}_{4}\) and the enhanced photocatalytic activity could be attributed to the rapid photo-induced charge separation. A novel BiOBr–\(\hbox {C}_{3}\hbox {N}_{4}\) heterojunctions have also been fabricated by depositing BiOBr onto the surface of g-\(\hbox {C}_{3}\hbox {N}_{4}\) and showed preferable photocatalytic activity on RhB decomposition under visible or indoor light [9]. AgX@g-\(\hbox {C}_{3}\hbox {N}_{4}\) (\(X = \hbox {Cl}\), Br and I) phtotocatalysts were recently reported showing excellent visible light-driven photocatalytic activity due to their higher photostability and the efficient separation of photogenerated charge carrier [10]. Although the hybrid \(\hbox {C}_{3}\hbox {N}_{4}\) photocatalysts displayed excellent photocatalytic performance, they did not meet all the needs in practical applications, such as highly efficient decomposition of organic contaminants and the effective utilization of visible light. Therefore, the development of a highly effective visible-light active photocatalyst is still desired.
CdS is an attractive visible-light photocatalyst because of its desired band-gap width (2.4 eV) and oxidation and reduction potentials [11]. However, the photocatalytic efficiency of nano-CdS is severely restricted by photocorrosion under strong illumination and higher recombination rates of photoexcited charge carriers [12]. CdS may also act as important sensitizers, which can sensitize the wide band gap semiconductor and thus improve the photocatalytic activity and stability of the photocatalyst. For example, ZnO/CdS core/shell nanorods were synthesized by a surface-functionalized method and displayed higher photocatalytic activity for the degradation of RhB under simulated solar radiation [13]. Similarly, the \(\hbox {CdS/WO}_{3}\) nanojunction artificial photosynthesis system is successfully constructed, and the rate of \(\hbox {H}_{2}\) evolution on the photocatalysts is five times as high as that of pure CdS [14]. Recently, Huang and others [15] have successfully fabricated the CdS/g-\(\hbox {C}_{3}\hbox {N}_{4 }\) core/shell configuration with high photo-catalytic activity and excellent photostability, which is a very promising candidate for possible practical application. Therefore, it is highly desired that the combination of CdS and \(\hbox {C}_{3}\hbox {N}_{4}\) may be an ideal system to increase the separation efficiency of photogenerated electron-hole pairs and at the same time extend the absorption to visible light. In this work, we reported the synthesis of the \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites through a calcinations–hydrothermal route, which exhibited extended absorption of visible light and enhanced photodegradation efficiency of RhB. Compared with pure \(\hbox {C}_{3}\hbox {N}_{4}\) and CdS, \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites showed a higher photocatalytic activity due to efficient separation of photo-induced electron-hole pairs. The possible mechanism of the enhanced visible light photocatalytic activity of \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites is also discussed in detail.
2 Experimental
2.1 Preparation of \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites
The \(\hbox {C}_{3}\hbox {N}_{4}\) powder was prepared by a facile thermal polycondensation of melamine in air. Typically, 5 g of melamine powder was put into a crucible and then heated to \(520^{\circ }\hbox {C}\) in a muffle furnace with a rate of \(10^{\circ }\hbox {C}\,\hbox {min}^{-1}\) for 4 h. The \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites were prepared by a hydrothermal method. In a typical procedure, appropriate amount of \(\hbox {C}_{3}\hbox {N}_{4}\) powder was initially added into 20 ml of 0.25 M \(\hbox {CdCl}_{2}\cdot 2.5 \hbox {H}_{2}\hbox {O}\) aqueous solution and ultrasonicated for 10 min. Subsequently, 20 ml of 0.25 M \(\hbox {Na}_{2}\hbox {S}\cdot 9\hbox {H}_{2}\hbox {O}\) aqueous solution was added into the above solution and stirred for 30 min. Then, the mixture was transferred into a Teflon-lined stainless-steel autoclave with a capacity of 50 ml, which was heated at \(180^{\circ }\hbox {C}\) for 12 h. Later the product was collected by centrifugation, washed with deionized water and then dried at \(80^{\circ }\hbox {C}\) for 6 h. For comparison, different mass ratios of C3N4/CdS at 5, 10 and 15% were synthesized with the same procedure and denoted as 5% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS, 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS and 15% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS, respectively.
2.2 Characterization
X-ray powder diffraction (XRD) of the as-prepared photocatalysts was investigated on a Thermo ARL SCINTAG X’TRA X-ray diffractometer using Cu-K\(\upalpha \) radiation (\(\lambda = 0.154056~\hbox {nm}\)). High-resolution transmission electron microscopy (HRTEM, Tecnai G2 F30 S-Twin) was employed to observe the morphology and microstructures of as-prepared samples. The optical absorption properties of the samples were investigated via UV–Vis diffuse reflectance spectroscopy (DRS) using a Hitachi U-3010 spectrometer.
2.3 Photocatalytic experiments
The photocatalytic activities of the obtained \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites were evaluated by degrading RhB under visible light. Visible irradiation was obtained from a 300 W Xenon lamp (CEL-HXF300) with a 420 nm cut-off filter. In each experiment, 50 mg of photocatalyst was transferred into 100 ml of RhB (\(C_{0} = 1 \times 10^{-5}~\hbox {M}\)) with constant stirring. The above suspension was stirred in the dark before irradiation in order to reach the adsorption equilibrium between RhB and the catalyst. At each interval, 5 ml of the suspension was withdrawn and centrifuged to remove the photocatalyst. The concentration of RhB was analysed by recording the absorbance at the characteristic band of 553 nm using a UV759S UV-Vis spectrophotometer.
3 Results and discussions
Transmission electron microscopy (TEM) and HRTEM images were taken to analyse the morphology and microstructure of the samples. Figure 1a shows the TEM image of pure CdS. Nanoparticles structure with an average particle size of about 15 nm can be observed on the image suggesting the nanoparticle morphology of CdS. However, pure \(\hbox {C}_{3}\hbox {N}_{4}\) (figure 1b) shows quite different morphology with 2D lamellar structure and smooth surfaces. Moreover, it can be clearly investigated from the TEM image of 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposite (figure 1c) that the CdS nanoparticles were deposited on the surface of \(\hbox {C}_{3}\hbox {N}_{4}\) nanosheets. The HRTEM image of the 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposite is shown in figure 1d. The lattice plane separation of 0.326 nm can be assigned to the (002) crystal plane of \(\hbox {C}_{3}\hbox {N}_{4}\). Furthermore, the measured lattice spacing of 0.203 nm corresponds to the (110) plane of CdS. These results may suggest the formation of nanocomposites between \(\hbox {C}_{3}\hbox {N}_{4}\) and CdS.
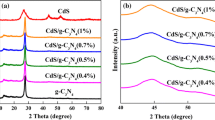
XRD patterns of CdS, \(\hbox {C}_{3}\hbox {N}_{4}\) and 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS are shown in figure 2. It can be observed that the pure CdS is crystallized in a hexagonal Wurtzite structure with lattice parameters \(a = 4.131~{\AA }\) and \(c = 6.735~{\AA }\) (JCPDS no. 41-1049). The \(\hbox {C}_{3}\hbox {N}_{4}\) sample exhibits two pronounced peaks at 27.4 and \(13.1^{\circ }\), which corresponds to the (002) and (100) diffraction planes of the graphite-like \(\hbox {C}_{3}\hbox {N}_{4}\) [16]. The XRD pattern of the 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites is similar to that of the pure CdS. No characteristic peak of \(\hbox {C}_{3}\hbox {N}_{4}\) was examined in the \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites, which might be ascribed to the low \(\hbox {C}_{3}\hbox {N}_{4}\) amount and its relative weaker crystallinity.
The UV–Vis diffuse reflectance spectra of pure CdS, \(\hbox {C}_{3}\hbox {N}_{4}\) and 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS are shown in figure 3a. It can be seen that the pure \(\hbox {C}_{3}\hbox {N}_{4}\) and CdS both have absorbance in the visible-light range with the onset absorption of \(\hbox {C}_{3}\hbox {N}_{4}\) about 460 nm and absorption edge of CdS around 570 nm. The absorbance of 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS is also in the visible-light region with a mixed absorption property of CdS and \(\hbox {C}_{3}\hbox {N}_{4}\) and an obvious decrease of the absorption intensity. The band gaps of \(\hbox {C}_{3}\hbox {N}_{4}\) and CdS can be calculated by the equation \(A({ hv}-E_{\mathrm{g}})^{n/2}=\alpha hv\) [17], where \(A, h, v, E_{\mathrm{g}}\) and \(\alpha \) are constants, the Planck’s constant, the light frequency, the band-gap energy and absorption coefficient, respectively. The value of the exponent n is determined by the type of optical transition of the sample and is defined to be 4 for an indirect transition. The band-gap energy (\(E_{\mathrm{g}}\)) of \(\hbox {C}_{3}\hbox {N}_{4}\) and CdS can be estimated from a plot of \(({ ahv})^{1/2}\) vs. energy (hv). Thus, the \(E_{\mathrm{g}}\) of the \(\hbox {C}_{3}\hbox {N}_{4}\) and CdS are determined to be 2.62 and 2.02 eV, respectively (figure 3b). The conduction band potentials (\(E_{\mathrm{CB}}\)) of a semiconductor can be calculated by the following empirical equation \(E_{\mathrm{CB}}=X{-}E_{\mathrm{c}}{-}0.5E_{\mathrm{g}}\) [18], where X is the absolute electronegativity of the semiconductor, \(E_{\mathrm{c}}\) is the energy of free electrons on the hydrogen scale (about 4.5 eV), and the valence band (\(E_{\mathrm{VB}})\) can be obtained by \(E_{\mathrm{VB}}= \hbox {E}_{\mathrm{CB }}+E_{\mathrm{g}}\). The X values for \(\hbox {C}_{3}\hbox {N}_{4}\) and CdS are 4.73 and 5.19 eV; thus the \(E_{\mathrm{CB}}\) values of \(\hbox {C}_{3}\hbox {N}_{4}\) and CdS are calculated to be \(-1.08\) and \(-0.32~\hbox {eV}\), and the \(E_{\mathrm{VB}}\) value of \(\hbox {C}_{3}\hbox {N}_{4}\) and CdS are estimated to be 1.54 and 1.70 eV, respectively.
The photocatalytic activities of pure CdS, \(\hbox {C}_{3}\hbox {N}_{4}\) and \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites were evaluated using RhB as a target pollutant under visible light irradiation. As shown in figure 4, the photodegradation of RhB over CdS and \(\hbox {C}_{3}\hbox {N}_{4}\) was about 60.9 and 47.4%, respectively, and all \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites showed higher photocatalytic activity than pure CdS and \(\hbox {C}_{3}\hbox {N}_{4}\). The highest photocatalytic activity was obtained over the 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites with about 98% RhB decomposed within 120 min. The enhanced photocatalytic property can be possibly attributed to the efficient heterojunction interface in \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites, which can restrain the recombination of photogenerated electrons and holes. At low CdS content the increase of CdS may result in more heterojunction interface in \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites, which will suppress the recombination of photo-induced electron-hole pairs. However, the photodegradation efficiency may decrease when the CdS content is higher than 10%, which may be due to the reason that the excessive CdS with narrow band-gap could act as the recombination sites of the electron-hole pairs. To determine the photocatalytic activity quantitatively, the reaction kinetics constant k was investigated by fitting the plots of \(\hbox {ln}(C/C_{0})\) vs. irradiation time t according to the pseudo-first-order model \(\hbox {ln}(C/C_{0}) = { kt}\), as shown in figure 5. Where C and \(C_{0}\) are the concentrations of RhB at \(t=t\) and 0, respectively. The reaction constant k for RhB degradation on pure \(\hbox {C}_{3}\hbox {N}_{4}\), CdS, 5% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS, 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS and 15% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS are 0.00557, 0.00754, 0.00918, 0.02364 and \(0.01256~\hbox {min}^{-1}\), respectively. The results demonstrate that 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS exhibits the highest apparent reaction rate constant, indicating greatly enhanced photocatalytic activity on 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS. The stability of the photocatalysts was also investigated by recycling the 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS sample that performed the best photocatalytic activity used to degrade RhB. As shown in figure 6, the photocatalytic activity of RhB decomposition on 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS did not have obvious decrease after three cycles. Thus, the 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites showed good stability for photocatalytic applications during the photocatalytic reaction.
Photocurrent measures are a useful technique to characterize the separation efficiency of the photo-induced charge carriers in a semiconductor, since the photocurrent mainly results from the transfer of free carriers. Figure 7 shows the photocurrent experiment of the 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS composites compared with that of pure CdS and \(\hbox {C}_{3}\hbox {N}_{4}\). All the photocurrents descended and ascended periodically with light-off and light-on at an interval of 10 s, which is recyclable and steady during the several on-off irradiation cycles. It can also be seen that 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS displayed the highest photocurrent than that of CdS and \(\hbox {C}_{3}\hbox {N}_{4}\), and thus had the highest photocatalytic property, which is in accordance with the results from photodegradation experiment.
As is well known, the hydroxyl radicals (\({}^{^{\bullet }}\hbox {OH}\)), photo-induced holes \((\hbox {h}^{+})\) and superoxide anion radicals \(({}^{^{\bullet }}\hbox {O}^{2-})\) are three main species for the photo-degradation of RhB. Therefore, trapping experiments of \({}^{^{\bullet }}\hbox {OH}\), \(\hbox {h}^{+}\) and \({}^{^{\bullet }}\hbox {O}^{2-}\) were examined in an attempt to elucidate the photocatalytic mechanism in the photodegradation process on the 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites. Isopropyl alcohol, benzoquinone and KI were used as \({}^{^{\bullet }}\hbox {OH}\), \({}^{^{\bullet }}\hbox {O}_{2}^{-}\) radicals and \(\hbox {h}^{+}\) scavengers, respectively. As shown in figure 8, the photodegradation efficiency of RhB hardly changed when isopropyl alcohol was added. On the contrary, the degradation rate of RhB decreased significantly upon addition of benzoquinone and KI. The above results indicate that \(\hbox {O}_{2}^{\bullet -}\) radicals and \(\hbox {h}^{+}\) were the main active oxidative species for the photodegradation of RhB on the 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites.
Based on the above-mentioned experimental results and analysis, the mechanism of the enhanced photocatalytic activity of RhB decomposition on the \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites is presented in figure 9. Under visible light irradiation, both CdS and \(\hbox {C}_{3}\hbox {N}_{4}\) in the nanocomposite can be excited to generate photogenerated electron-hole pairs. The photoinduced electrons on the conduction band of \(\hbox {C}_{3}\hbox {N}_{4}\) can move quickly to CdS for the conduction band potential of \(\hbox {C}_{3}\hbox {N}_{4}\) (\(-1.08~\hbox {eV}\)) is more negative than that of CdS (\(-\,0.32~\hbox {eV}\)). In addition, these photogenerated electrons may further react with the adsorbed \(\hbox {O}_{2}\) molecules on the CdS surface to generate \(\hbox {O}_{2}^{\bullet -}\) radicals, because the valence band potential of \(\hbox {C}_{3}\hbox {N}_{4}\) (+ 1.54 eV) is more negative than that of CdS (+ 1.70 eV), the photo-induced holes on the CdS surface can transfer easily to the valence band of \(\hbox {C}_{3}\hbox {N}_{4}\). Therefore, the photo-induced electron-hole pairs are separated efficiently on the nanocomposite interface. This can lead to the \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites that have increased photocatalytic activity. At the same time, the holes and \(\hbox {O}_{2}^{\bullet -}\) radicals on the \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites surface could oxidize the RhB molecules into \(\hbox {H}_{2}\hbox {O}\), \(\hbox {CO}_{2}\) and other intermediates.
4 Conclusion
In summary, a g-\(\hbox {C}_{3}\hbox {N}_{4}\) hybridized CdS nanoparticles photocatalyst was successfully prepared via a successive calcination and hydrothermal process. Enhanced photocatalytic performance of the \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites has been observed. The highest photocatalytic activity was obtained over the 10% \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites with about 98% RhB decomposed within 120 min. The holes and \(\hbox {O}_{2}^{\bullet -}\) radicals generated on the \(\hbox {C}_{3}\hbox {N}_{4}\)/CdS nanocomposites surface played an important role in the photodegradation of RhB.
References
Cao Q W, Cui X, Zheng Y F and Song X C 2016 J. Alloys Compd. 670 12
Yin H, Wang X, Wang L, Nie Q, Zhang Y, Yuan Q et al 2016 J. Alloys Compd. 657 44
Yin H, Wang X, Wang L, Nie Q and Zhao H 2015 J. Alloys Compd. 640 68
Yin H, Wang X, Wang L, Nie Q, Zhang Y and Wu W 2015 Mater. Res. Bull. 72 176
Kumar S, Surendar T, Kumar B, Baruah A and Shanker V 2013 J. Phys. Chem. C 117 26135
Liu W, Wang M, Xu C, Chen S and Fu X 2013 J. Mol. Catal. A 368 9
He Y, Cai J, Li T, Wu Y, Lin H, Zhao L et al 2013 Chem. Eng. J. 721 215
Wang Y, Bai X, Pan C, Heb J and Zhu Y 2012 J. Mater. Chem. 22 11568
Fu J, Tian Y, Chang B, Xi F and Dong X 2012 J. Mater. Chem. 22 21159
Lan Y, Qian X, Zhao C, Zhang Z, Chen X and Li Z 2013 J. Colloid Interface Sci. 395 75
Shi Y, Li H, Wang L, Shen W and Chen H 2012 ACS Appl. Mater. Interfaces 4 4800
Li Q, Guo B, Yu J, Ran J, Zhang B and Yan H 2011 J. Am. Chem. Soc. 133 10878
Khanchandani S, Kundu S, Patra A and Ganguli A K 2012 J. Phys. Chem. C 116 23653
Zhang L J, Li S, Liu B K, Wang D J and Xie T F 2014 ACS Catal. 4 3724
Zhang J Y, Wang Y H, Jin J, Zhang J, Lin Z, Huang F et al 2013 ACS Appl. Mater. Interfaces 5 10317
Matsumoto S, Xie E and Izumi F 1999 Diam. Relat. Mater. 8 1175
Li W T, Zheng Y F, Yin H Y and Song X C 2015 J. Nanopart. Res. 17 271
Cui X, Huang W Z, Zhou H, Yin H Y, Zheng Y F and Song X C 2015 Curr. Nanosci. 11 360
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Q.Y., Qi, Y.L., Zheng, Y.F. et al. Synthesis and enhanced photocatalytic activity of g-\(\hbox {C}_{3}\mathrm{N}_{4}\) hybridized CdS nanoparticles. Bull Mater Sci 40, 1329–1333 (2017). https://doi.org/10.1007/s12034-017-1513-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-017-1513-y