Abstract
1-D ZnO nanorods and PPy/1-D ZnO nanocomposites were prepared by the surfactant-assisted precipitation and in situ polymerization method, respectively. The synthesized nanorods and nanocomposites were characterized by UV–Vis spectrophotometer, Fourier transform-infrared spectroscopy (FTIR), X-ray diffraction (XRD) and field emission scanning electron microscope (FE-SEM), which gave the evidence of 1-D ZnO nanorods, polymerization of pyrrole monomer and strong interaction between PPy and 1-D ZnO nanorods, respectively. Photocatalytic activity of 1-D ZnO nanorods was conducted by 33 level full-factorial design to evaluate the effect of three independent process variables viz., dye concentration (crystal violet), catalyst concentration (1-D ZnO nanorods) and the reaction time on the preferred response: photodegradation efficiency (%). The PPy/1-D ZnO nanocomposites were used for the sensing of NH3, LPG, CO2 and H2S gases, respectively, at room temperature. It was observed that PPy/1-D ZnO nanocomposites with different 1-D ZnO nanorod weight ratios (15 and 25%) had better selectivity and sensitivity towards NH3 at room temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The synthesis of zinc oxide (ZnO) nanostructures such as nanoparticles, nanowires, nanorods, etc., has gained increasing research importance due to their unique properties and potential applications in various fields, such as gas sensing, solar cell, microelectronics, pharmaceutics, photovoltaic and catalysis [1–9]. In the past two decades, ZnO nanostructures have prominent attribute because of its stability, wide band gap energy of 3.37 eV and large excitation binding energy of 60 meV at room temperature [10–13]. In addition, ZnO nanostructures offer a larger surface area to volume ratio than nanoparticulate thin films, owing to this characteristic advantage, it plays an important role in photodegradation [14]. However, this property has made ZnO as one of the most appealing photocatalyst semiconductor material for the degradation of organic pollutants in water under UV source. As a family of catalyst materials, ZnO nanostructures have been successfully prepared by variety of synthetic techniques. Among these techniques, the well-known precipitation method [15] is more useful and cost effective method. Kumar et al[16] prepared ZnO nanorods by a precipitation method with zinc sulphate and sodium hydroxide as starting materials followed by calcination. In this study, we use precipitation method for the synthesis of 1-D ZnO nanorod. The shape and size controlled 1-D ZnO nanorod synthesis is quite difficult via a precipitation method, because the growth rate is too fast for the precursor to control the size. To overcome this difficulty, CTAB used as a structure directing surfactant [17].
The output of textile or dyestuff industries consists of coloured wastewater and dyes have more impact on the environment [18]. Among the wide range of dyes, crystal violet is a triphenylmethane dye which is highly toxic and carcinogenic [18,19]. Many techniques involving reverse osmosis, flocculation, chemical oxidation and reduction, electrochemical treatment and photocatalysis have been investigated for the treatment of crystal violet (CV) wastewater [20]. Of these, semiconductor-assisted photocatalysis has a prominent attribute because it is very effective to treat pollutants in both gas and liquid phases [21].
In this era with increased presence of pollutant gases in the atmosphere, it has become very essential to detect hazardous gases in standpoint of human safety measures. ZnO is a strong candidate for monitoring lethal gases because of its high electrochemical stability, good resistivity and excellent sensitivity towards gas pollutants [22]. Still, high operating temperature (350–450∘C) of ZnO limits its real-time application. Conducting polymers (CPs) also show attractive gas sensing properties. Polypyrrole (PPy) is environmentally stable, can be easily prepared by chemical or electrochemical oxidative polymerization and have higher conductivity than other CPs [23]. However, metal oxide conducting polymer composites have been found to indicate smart sensing properties. PPy is a p-type material, while ZnO is n-type of material by tailoring this inorganic–organic composite, it is possible to make tunable conductivity sensors for better selectivity [24]. Some well-known materials for NH3 gas sensing are ZnO, modified-ZnO [25], polyaniline [26], polypyrrole [27], Au and MoO3-modified WO3[28,29], Pt and SiO2-doped SnO2[30], etc. Various ammonia sensors reported, basically work at higher temperature such as 350∘C, but it is inconvenient to attain such high temperature while sensing. So, the sensors operable at room temperature are needed. The electrolytic techniques using diaphragm electrodes are generally used for the detection of ammonia. However, this method is expensive and does not have sufficient response and selectivity for ammonia [31]. Another technique utilizes Pd-metal oxide semiconductor MOS device. This device is sensitive to ammonia, but it suffers from selectivity. So, semiconducting oxide sensors are considered here. Few sensor models are also available for detecting ammonia gas. They are: Figaro gas sensor model-TGS 824 (detection range 300 ppm) and Sierra gas monitor model-CM 99-447 (electrochemical type, detection range 200 ppm).
Despite of high sensitivity, selectivity and long-term stability, the main drawback of such sensors is that they operate at high working temperature (>300∘C). Also, the use of metal additives likes Pt, Pd, Au, Ag, etc. in base material like ZnO for the modification, increases the cost of the sensors. Therefore, the applicability of these sensors remains limited. Hence, the sensors operable at room temperature with the low-cost metal additives must be developed for large applicability. In the present work, we have used 1-D ZnO nanorod for photocatalytic degradation of crystal violet and its nanocomposite with polypyrrole to develop the room temperature ammonia sensors.
2 Materials and methods
2.1 Materials
Zinc sulphate heptahydrate (ZnSO4⋅7H2O, Merck), Iron chloride (FeCl3, Merck) and sodium hydroxide (NaOH, S.D. Fine Chem. Ltd) were used as received. Cetyltrimethylammonium bromide (CTAB, Spectrochem) was used as the stabilizing agent, pyrrole monomer (Sigma-Aldrich) was used for the synthesis of PPy/ZnO nanocomposite. All other chemicals were of analytical grade and used as received. Double-distilled water was used throughout the experiments.
2.2 Synthesis of 1-D ZnO nanorods
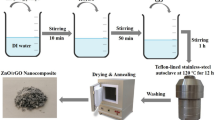
1-D ZnO nanorods were synthesized by the surfactant-assisted precipitation method followed by calcination. In brief, ZnSO4⋅7H2O and NaOH were taken as the precursors of the synthesis process. Both precursors were dissolved separately in double-distilled water to obtain molar ratio (1 : 1 to 1 : 4) of solutions. ZnSO4⋅7H2O solution (50 ml) was added drop by drop and vigorously stirred in NaOH and CTAB solution. The addition rate of ZnSO4⋅7H2O solution was maintained using a syringe pump (Infusor, Universal Medical Instruments, India). The white precipitate product obtained, filtered, washed and dried at 70∘C in an oven for 2 h. Finally, the resultant product was calcined at 200∘C for 4 h [32,33].
2.3 Synthesis of PPy/1-D ZnO nanocomposite
PPy/1-D ZnO nanocomposite was synthesized by in situ polymerization [34] of pyrrole monomer in the presence of 1-D ZnO nanorods. 1-D ZnO was dispersed in 50 ml of 0.35 M FeCl3 solution for 10 min using bath sonicator. Then, 1 ml of pyrrole monomer added drop by drop into the solution under stirring. Vigorous stirring of the mixture for 3 h resulted in a black coloured solution, which was filtered and washed repeatedly with double-distilled water. The solid product obtained was dried in an air oven for 4 h.
2.4 Characterization
Degradation performance of CV dye was examined by UV–Vis spectrophotometer (HITACHI U-2900, Tokyo, Japan). The surface morphology of the products was examined by scanning electron microscopy (FESEM-S 4800, Hitachi, Japan) at a working distance of 8.6–8.8 mm and accelerating voltage of 15.0 kV. The components of materials were measured by a FTIR spectrophotometer (FTIR-8400; Shimadzu, Asia Pacific Pvt. Ltd, Singapore) by potassium bromide pellet method. For that, sample (1 mg) was mixed with KBr (40 mg) and compressed into a disc in a manual press. Spectra were recorded in the wavelength region of 4000–400 cm−1. The physical nature of the samples was examined by an X-ray diffractometer (Bruker, D8 Advanced, Germany) with CuK α radiation (\(\lambda = 1.5406~\acute {\mathrm {\AA }}\)). The anode X-ray tube was operated at 40 kV and 40 mA. The samples were analysed over the angle range 0–80∘ on the 2 𝜃 scale at a step size of 0.03 s−1.
2.5 Photodegradation experiments
The photodegradation of CV dye was performed under UV light source. The aqueous suspension of CV was prepared by adding a known quantity of ZnO nanorod powder into a solution containing a suitable concentration of CV. Then, the suspension was magnetically stirred in the dark for 1 h to allow an adsorption–desorption equilibrium between CV and the surface of the 1-D ZnO nanorod catalyst. Irradiation was carried out in a quartz cell located at 10 cm away from the source. At known time interval, the decomposed dye sample was withdrawn, centrifuged and then filtered. The quantitative analysis of CV was carried out using UV–Vis spectrophotometer (Hitachi U 2900, Tokyo, Japan) by measuring absorbance at 590 nm [35]. The photodegradation efficiency of CV dye was calculated according to the following equation,
Various factors were evaluated in the degradation of CV dye using 33 level full-factorial design. The selection of 3 level factorial design is suitable for investigating the quadratic response surface model based on the second-order polynomial equation [36,37]. The independent variables selected were the concentration of dye (A, ppm), concentration of catalyst (B, g l−1) and reaction time (C, min), which were varied at three levels (−1, 0, + 1). Table 1 shows 33 experimental design with 3 independent variables (A, B, C) at 3 different levels (−1, 0, + 1) was used to study the effects on the dependent variable (Y1). The photodegradation efficiency (%, Y1) was selected as a dependent factor. The statistical experimental data were analysed using the Design-ExpertⓇ Software (Vers.-8.0.7.1, Stat-Ease Inc., Minneapolis).
2.6 Preparation of thick films
In this study, a thick film of PPy/1-D ZnO nanocomposite was used as a gas sensing module. The thixotropic pastes [38–41]were formulated by mixing the resulting fine powders with a solution of ethyl cellulose (a temporary binder) in a mixture of organic solvents such as butyl cellulose, butyl carbitol acetate and turpineol. The ratio of inorganic to organic part was kept at 75 : 25 in formulating the pastes. The thixotropic pastes were screen printed onto glass substrates in desired patterns. The films prepared were fired at 90∘C for 2 h. Thus thick films of PPy/1-D ZnO nanocomposite were prepared. Silver contacts were made by vacuum evaporation for electrical and gas sensing measurements.
2.7 Gas sensor system
Gas sensing was performed by static gas sensing system, which comprises of 15 L glass gas chamber containing an electric heating plate, a thermocouple and a temperature controller. Required temperature conditions for sensing were maintained by heater resting on the base plate. The temperature was tuned by means of varying current through heater with adjustable ON and OFF time interval. Electrical connections and gas inlet were provided through ports at the base plate. The necessary gas concentration in the glass dome was achieved by injecting a required quantity of gas by means of a syringe. The current change was recorded by using a digital pico-ammeter keeping constant voltage. The sensing measurements were performed by exposing the thick films of nanocomposite to gases in a closed glass dome (15 L) to mimic atmospheric condition. The electrical conductivity of a thick film in air and in the presence of gas was used to evaluate the sensor response as [38],
where G a is the conductance in the air and G g the conductance upon exposure to test gas. Response time (RST) was defined as the time necessary to attain the 90% of the maximum increase in conductance subsequent to exposure of the sensing film to a gas, whereas recovery time (RCT) as the time needed to regain 90% of the maximum conductance in the air.
3 Results and discussion
3.1 UV–Vis absorption
The UV–Vis absorption spectra of synthesized 1-D ZnO nanorods and crystal violet are illustrated in figure 1. The precursor of ZnO synthesis shows the absorption spectra at 285.65 nm of zinc sulphate heptahydrate in the reported literature [42], after complete precipitation reaction with NaOH, it shows the absorption maxima at 361.5 nm of synthesized 1-D ZnO nanorods shown in figure 1. Similarly, crystal violet showed the absorption maxima at 584.1 nm. The photocatalytic property involves the electron transition from the valence band to the conduction band resultant to the direct band gap of 1-D ZnO nanorods. The band gap energy of the 1-D ZnO nanords was determined by the following equation [43],
where h = 4.135 ∗ 10−15 eVs, c = 2.99 ∗ 108 m s−1 and λ = 361.5 nm for 1-D ZnO nanorod synthesized by precipitation method. Applying above equation, the band gap energy of the synthesized 1-D ZnO nanorods was found to be 3.42 eV.
3.2 Field emission-scanning electron microscopy analysis
Field emission-scanning electron microscopy (FE-SEM) was performed to examine the surface characteristics of 1-D ZnO nanorods and PPy/1-D ZnO nanocomposite. Figure 2a shows uniform surface morphology of the 1-D ZnO nanorod with an average diameter of 30–50 nm. The synthesized 1-D ZnO nanorods were found to be isolated and free flowing in nature. The morphology of 1-D ZnO nanorods was changed after polymerization of pyrrole as shown in figure 2b. It was clear from the image that the 1-D ZnO nanorods were uniformly coated by polypyrrole. This revealed that the 1-D ZnO nanorods interact with PPy. The large area and uniform morphology of PPy/1-D ZnO nanocomposite are appropriate for gas sensing application [44].
3.3 Fourier transform infrared spectroscopy analysis
The Fourier transform infrared spectroscopy (FT-IR) spectra of 1-D ZnO nanorod and PPy/1-D ZnO nanocomposite material are shown in figure 3. The IR spectrum of ZnO nanorods (figure 3a) showed the principle peaks at 3364.63 and 1553.71 cm−1 and are due to the bending and stretching vibration of a hydroxyl group (–OH). The peak situated at 485.11 cm−1 corresponds to the stretching vibration of Zn–O bond. The peaks at 1478 and 1558 cm−1 are assigned to stretching mode of C =C and C–C in pyrrole ring are disappeared [45] and wide peak appears at 1536.35 cm−1 in the PPy/1-D ZnO nanocomposite (figure 3b). The broadening and change in peak position indicate that there is a strong chemical interaction between PPy and 1-D ZnO nanorod.
3.4 X-ray diffraction
The XRD spectra of 1-D ZnO nanorod and PPy/1-D ZnO nanocomposite are shown in figure 4. From figure 4a, the XRD pattern of ZnO nanorods shows the characteristic peaks at scattering angles (2𝜃) of 30.78, 34.29, 35.34, 46.72, 54.74, 62.09 and 66.20∘ are correspond to the diffractions from (100), (002), (101), (102), (110), (103) and (112) crystal planes, respectively [21]. The sharp diffraction peaks implies the crystalline nature and no other peaks of impurity are observed in pure ZnO hexagonal (wurzite) structure. The average crystallite size of 1-D ZnO nanorods is calculated by using Scherrer equation was found to be 20.13 nm. The XRD pattern of PPy/1-D ZnO nanocomposite is shown in figure 4b. This pattern exhibits a broad peak at a lower diffraction angle 2𝜃 = 23.9, which implies their amorphous nature. This broadening of peak can be ascribed to the scattering of the PPy around 1-D ZnO nanorods [45].
3.5 Mechanism of photodegradation of crystal violet
In general, the UV light absorbed capacity of 1-D ZnO nanorods is more than any other nanostructures. This causes the production of more electrons–hole (e−/h+) pairs of conduction and valence band. This electron–hole pair plays an important role in the redox reaction. The highly oxidizing valence band holes react with H2O to produce the hydroxyl radicals and strongly reducing conduction band electrons produce the O\(_{2}^{\mathrm {-}}\) molecules [46,47]. The hydroxyl radicals formed on the ZnO surface are strong oxidizing agents, which lead to the photodegradation of crystal violet. The photodegration reaction steps of crystal violet at the 1-D ZnO nanorod surface is described as follows [32],






3.6 Statistical analysis of photodegradation efficiency (%)
33 full-factorial experimental designs were used to evaluate the effect of independent variables on the dependent variables. Concentration of dye (A), concentration of catalyst (B) and reaction time (C) were selected as independent variables, while photodegradation efficiency (%, Y1) was selected as a dependent variable. The effect of independent variables on dependent variable was investigated by deriving polynomial equation and contour plots. The photodegradation efficiency from different experimental runs is reported in table 2. Diagnostics case statistics for various response variables are shown in table 3. The polynomial equation obtained for (Y1),
A positive value in the above equation represents the synergistic effect of the independent variable, while a negative value represents the inverse relationship (antagonistic effect) [48,49]. −17.28 represents the antagonistic effect of independent factor A (concentration of dye) on photodegradation efficiency (%, Y1). While + 1.26 represents the synergistic effect of independent factor C (reaction time) on photodegradation efficiency (%, Y1). The suggested model for the Y1 was found to be quadratic as per design expert software with R 2=0.9764, which is shown in table 4. The P-value for the Y1 model was found to be <0.0001, which clearly indicates the model is statistically significant (P<0.05 indicates that model terms are significant). The results showed that the photodegradation efficiency of CV dye was affected by the independent variables. Table 5 shows the ANOVA of model for Y1. The effect of A (P<0.0023), B (P=0.0003), C (P=0.0001) and C 2 (P=0.0001) were found to be statistically significant. The interaction terms AB in Y1 show how photodegradation efficiency changes when 2 variables are simultaneously changed. The model F-value for Y1 was 101.07, which implies that the model was significant. To analyse the effect of independent variables on the dependent variable, a 2D contour plot was constructed for the photodegradation efficiency of CV dye. From the contour plot (figure 5), it was observed that the photodegradation efficiency of CV dye was increased from lower level to a higher level of dye concentration (A) and reaction time (C). The same type of results was observed in the case of catalyst concentration (B) and reaction time (C) from contour plot (figure 6). From contour plot (figure 7), it could be concluded that when the concentration of the catalyst (B) was kept constant and the concentration of dye (A) was increased, the photodegradation efficiency of CV dye decreases. This might be due to an increase in concentration of dye which decreases the path length of photons that arrive in the solution, therefore only few photons reached the catalyst surface, which decreases the production rate of hydroxyl and superoxide radical formation [50]. Also, when the concentration of dye (A) was kept constant and concentration of catalyst was increased, the photodegradation efficiency of crystal violet increases. This may be due to an increase in concentration of catalyst, increases the active sites available on photocatalyst surface for the reaction, which successively increases the production rate of hydroxyl and superoxide radical formations [32,50]. The degradation performance of the nanomaterials was studied by various scientists and its performance in terms of % degradation and time interval was compared with the present study, shown in table 6.
3.7 Gas sensing mechanism
The mechanism for gas detection in nanomaterials is based on reactions that occur at the active sites of the sensor surface, resulting in a change in the concentration of adsorbed oxygen (O−) ions. Oxygen ions adsorb onto the material’s surface, capture electrons from the bulk, building a potential barrier of depth δ that decreases the channel width Lc of passage of electrons through a nanoparticle of diameter D and hence, decrease in electron movement and the conductivity occurs [51].
If the particle size is reduced up to nanometer level (<100 nm), the channel width would be almost blocked and the initial resistance of the sensor would be infinitely high in air ambient. On exposure, the target gas would consume oxygen atoms to oxidize, liberating electrons behind it on the surface. Desorption of oxygen from the surface of the sensor would decrease the potential barrier height, which broadens the channel width for migration of electrons. Thus, nanoscaled materials offer the grain sizes where the depletion layer has about the same dimensions as the particle radii. The charge transfer is, therefore, predominantly grain-controlled. When reactive gases combine with this oxygen, the barrier height is reduced, broadening the passage of electrons and hence, conductivity increases. This change in conductivity is directly related to the amount of a specific gas present in the environment, resulting in a quantitative determination of gas presence and concentration. To maximize the opportunities for surface reactions, a high ratio of surface area to volume is needed. As an inverse relationship exists between surface area and particle size, nanoscale materials, which exhibit very high surface area,
are highly desirable.
3.8 Gas sensing measurements
3.8.1 Selectivity
Figure 8 shows gas selectivity performance of PPy/1-D ZnO nanocomposites at room temperature. It can be seen that PPy/ZnO-15 is highly selective towards NH3 gas. In addition, the decrease in selectivity to ammonia was observed to increase the mass% of 1-D ZnO nanocomposite.
3.8.2 Effect of operating temperature
For the gas sensing study, we prepare two nanocomposite PPy/ZnO-15 and PPy/ZnO-25, where 15 and 25 are the mass percentages of 1-D ZnO nanorods in the nanocomposite. The response of PPy/1-D ZnO nanocomposite thick films to 1000 ppm of NH3, as a function of operating temperature, is shown in figure 9. It is observed from the figure that the gas response is highest at room temperature and decreases with operating temperature. The sample, PPy/ZnO-15 showed the highest response to 1000 ppm NH3 at room temperature (32∘C). The response to ammonia could be attributed to the surface active sites to sense ammonia gas.
3.8.3 Effect of gas concentration (ppm)
The change in response of PPy/ZnO thick film with variation in gas concentration is shown in figure 10a and b. The highest response recorded were 36.1 and 29.19% for 1000 ppm of NH3 at room temperature for PPy/ZnO-15 and PPy/ZnO-25, respectively. It was observed that the increase in ZnO doping results in a decrease in the response of sensing film. Response of sensing film to NH3 was found to be decreased with increase in temperature. However, interestingly, recovery time was found to be decreased at elevated temperature, although at 90∘C only ∼89% of original conductance was recovered.
3.8.4 Response–recovery profile of the sensor
Recovery time of PPy/ZnO-15 sensing film was 2, 6 and 10 min for 300, 600 and 1000 ppm of NH3, respectively, at 50∘C after introduction of clean air. The reproducing capability of nanocomposite was investigated by exposing it to 300 ppm at 50∘C and then blowing air repeatedly for three times. It was observed from figure 11 that the PPy/ZnO-15 nanocomposite showed excellent recovery. The gas sensing performance of the nanocomposites/nanomaterials was studied by various scientists and its performance in terms of sensitivity, response and recovery time was compared with present study as shown in table 7.
4 Conclusion
1-D ZnO nanorods were successfully synthesized by surfactant-assisted precipitation method using ZnSO4 ⋅7H2O as precursor, which serve as photocatalyst for the degradation of crystal violet dye. 33 full-factorial experimental design was used to evaluate the effect of independent variables on the photodegradation efficiency (%). The photocatalytic activity of synthesized 1-D ZnO nanorods shows very rapid degradation of crystal violet dye with the degradation rate of 97.13% was achieved by run F19 using 5 ppm concentration of dye, 1 g l−1 concentration of catalyst and reaction time interval of 120 min. This might be due to an increase in concentration of catalyst, increases the active sites available on photocatalyst surface for the reaction. However, the catalyst concentration less than 1 g l−1 restricts the degradation rate of crystal violet at 5 ppm of dye concentration. The present result suggests that the 33 full-factorial experimental design was reliable for optimizing the degradation rate of crystal violet by the 1-D ZnO nanorods. On the other hand, PPy/1-D ZnO nanocomposite was successfully prepared by in situ polymerization of pyrrole monomer. The synthesized PPy/ZnO-15 nanocomposite showed high sensitivity and selectivity towards NH3 (36.1%) at room temperature. During study, it was found that increase in concentration of 1-D ZnO in nanocomposite resulted in a decrease in sensitivity as well as recovery time. Owing to high sensitivity and good recovery time at room temperature, the proposed nanocomposite holds great promises in the gas sensor field.
References
Tian F, Zhao Y, Liu Y, Cao H, Zhao J and Lu X 2014 Mater. Lett. 119 8
Okada T, Kawashima K and Nakata Y 2006 Thin Solid Films 506 274
Rao C, Kulkarni G, Thomas P and Edwards P 2000 Chem. Soc. Rev. 29 275
Deng Z, Chen M, Gu G and Wu L 2008 J. Phys. Chem. B 112 16
Yang S and Park C 2008 Nanotechnology 19 4
Krishnakumar T, Jayaprakash R, Pinna N, Singh V, Mehta B and Phani A 2008 Mater. Lett. 63 242
Li M, Bala H, Lv X, Ma X, Sun F and Tang L 2007 Mater. Lett. 61 690
Patzake G, Krumeich F and Nesper R 2002 Angew. Chem. Int. Ed. 419 2446
Cao B and Cai W 2008 J. Phys. Chem. C 112 680
Kundu S 2014 Colloids Surface A 446 199
Nithiyanantham U and Kundu S 2014 Ind. Eng. Chem. Res. 53 13667
Shen W, Li Z, Wang H, Liu Y, Guo Q and Zhang Y 2008 J. Hazard Mater. 152 172
Ba-Abbad M M, Kadhum A A H, Mohamad A B, Takriff M S and Sopian K 2013 Chemosphere 91 1604
Baruah S, Jaisai M, Imani R, Nazhad M and Dutta J 2010 Sci., Technol. Adv. Mater. 11 7
Hong R Y, Li J H, Chen L L, Liu D Q, Li H Z, Zheng Y and Ding J 2009 Powder Technol. 189 426
Yadav B C, Srivastava R and Kumar A 2007 Int. J. Nanotechnol. App. 1 1
Jothi N S, Gunaseelan P and Sagayaraj P 2012 Arch. Appl. Sci. Res. 4 1698
Chen Z, Wang T, Jin X, Chen Z, Megharaj M and Naidu N 2013 J. Colloid Interf. Sci. 398 59
Ovejero G, Rodríguez A, Vallet A, Willerich S and García J 2012 Appl. Catal. B 111 586
Chen C C, Liao H J, Cheng C Y, Yen C Y and Chung Y C 2007 Biotechnol. Lett. 29 391
Kanmoni V G, Daniel S and Raj G A 2012 Reac., Kinet. Mech. Cat. 106 325
Baruwati B, Kumar D K and Manorama S V 2006 Sens. Actuators B 119 676
Karin P K 2002 Crit. Rev. Anal. Chem. 32 121
Saxena V, Aswal D, Kaur M, Koiry S, Gupta S, Yakhmi J et al 2007, Appl. Phys. Lett. 90 1077
Patil D R and Patil L A 2006 Sens. Transducers 70 661
Chabukswar V V, Pethkar S and Athawale A A 2001 Sens. Actuators B 77 657
Lahdesmaki G, Lewenstam A and Ivaska A 1996 Talanta 43 125
Wang X, Miura N and Yamazoe N 2000 Sens. Actuators B 66 74
Xu C N, Miura N, Ishida Y, Matuda K and Yamazoe N 2000 Sens. Actuators B 65 163
Wang Y, Wu X, Su Q, Lee Y and Zhou Z 2001 Solid State Electron. 45 347
Moseley P T and Williams D E 1990 Sens. Actuators B 1 113
Selvam N C S, Narayanan S, Kennedy L J and Vijaya J J 2013 J. Environ. Sci. 25 2157
Kumar S S, Venkateswarlu P, Rao V R and Rao G N 2013 Int. Nano Lett. 3 1
Batool A, Kanwal F, Imran M, Jamil T and Siddiqi S A 2012 Synth. Met. 161 2753
Ameen S, Akhtar M S, Nazim M and ShikShin H 2013 Mater. Lett. 96 228
Roy P and Shahiwala A 2009 Eur. J. Pharm. Sci. 37 363
Vaghasiya H, Kumar A and Sawant K 2013 Eur. J. Pharm. Sci. 49 311
Patil D R, Patil L A and Patil P P 2007 Sens. Actuators B 126 368
Hankare P P, Sanadi K R, Garadkar K M, Patil D R and Mulla I R 2013 J. Alloys Compd. 553 383
Gawas U B, Verenkar V M S and Patil D R 2011 Sens. Transducers 134 45
Patil R P, Nikam P N, Delekar S D, Patil D R and Hankare P P 2014 Sens. Lett. 12 01
Kanagadurai R, Durairajan R, Sankar R, Sivanesan G, Elangovan S P and Jayavel R 2009 E.-J. Chem. 6 871
Sharma D, Sharma S, Kaith B, Rajput S and Kaur M 2011 Appl. Surf. Sci. 257 9661
Chougule M A, Sen S and Patil V B 2012 Synth. Met. 162 1598
Barkade S S, Pinjari D V, Singh A K, Gogate P R, Naik J B, Sonawane S H et al 2013, Ind. Eng. Chem. Res. 52 7704
Chena C C, Fan H J, Jang C Y, Jan J L, Lin H D and Lua C H 2006 J. Photoch. Photobiol.: A 184 147
Khezrianjoo S and Revanasiddappa H D 2013 J. Catal. Article ID 582058
Gannu R, Yamsani V V, Yamsani S K, Palem C R, Yamsani M R 2009 AAPS Pharm. Sci. Tech. 10 505
Nayak A K, Pal D, Pradhan J and Hasnain M S 2013 Int. J. Biol. Macromol. 54 144
Byrappa K, Subramani A K, Ananda S, Rai K M L, Dinesh R and Yoshimura M 2006 Bull. Mater. Sci. 29 433
Patil D R 2007 Studies on ZnO based gas sensors Ph.D Thesis
Velmurugan R and Swaminathan M 2011 Sol. Energ. Mat. Sol. C 95 942
Senthilkumaar S and Porkodi K 2005 J. Colloid. Interf. Sci. 288 184
Chougule M A, Dalvi D S, Mali S, Patil P S, Moholkar A V, Agawane G L et al 2012, Measurement 45 1989
Dighavkar C 2013 Arch. Appl. Sci. Res. 5 96
Joshi A, Gangal S A and Gupta S K 2011 Sens. Actuators B 156 938
Acknowledgement
We are grateful to Technical Education Quality Improvement Programme (TEQIP-II) for providing financial assistance to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
PATIL, P., GAIKWAD, G., PATIL, D.R. et al. Synthesis of 1-D ZnO nanorods and polypyrrole/1-D ZnO nanocomposites for photocatalysis and gas sensor applications. Bull Mater Sci 39, 655–665 (2016). https://doi.org/10.1007/s12034-016-1208-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-016-1208-9