Abstract
Lead-free piezoelectric ceramics (1 − x)[0.95(K0.5Na0.5)NbO3–0.05LiSbO3]–xBiFe0.8Co0.2O3(KNN–LS–xBFC) were prepared by a conventional sintering technique. The effect of BFC content on the structure, piezoelectric and electrical properties of KNN–LS ceramics was investigated. The results reveal that the BFC is effective in promoting the sinterability and the electrical properties of the ceramics sintering at low temperature of 1030°C. The ceramics show a single perovskite structure, in which the tetragonal phase decreases while the orthorhombic phase increases with the increase of x. The more the BFC content is, the smaller and homogeneous grains were formed. With the increase of x, the d 33 and the k p increase to a maximum value and then slightly decrease, but the Q m increases continuously. As BFC content increases, the Curie temperature T c and remnant polarization P r decrease, but the diffusivity of phase transition in KNN–LS ceramics will intensify and the coercive field E c fluctuate between 1.16 and 1.51 kV mm −1. The samples with x = 0.004 exhibit optimum electrical properties at room temperature (d 33 =268 pC N−1, k p = 52%, ε r = 1366, tan δ = 2.11%, T c = 325°C, P r = 20.4 μC cm−2, E c = 1.16 kV mm−1).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
As important functional material, piezoelectric ceramics are mainly used in sensors, actuators and transducers. From many decades, lead-base piezoceramics play a dominant role in industrial production of piezoelectric applications owing to their excellent electrical properties [1,2]. However, PbO is a toxic oxide, which lead to environmental pollution and seriously threaten humans health [3,4]. More and more countries have restricted the use of lead-base ceramics by law [5,6]. Therefore, investigation in environment-friendly ceramics with excellent piezoelectric properties to replace the lead-base piezoceramics becomes an emergent work. Till now, the extensive investigation for lead-free piezoelectric ceramics mainly focus on three systems: Na 0.5Bi 0.5TiO 3-based materials, BaTiO 3-based ceramics and K (1−x)Na x NbO 3-based ceramics [3–8].
Among the different alternatives, K 0.5Na 0.5NbO 3-based (KNN) ceramics are considered as one of the promising substitutes for lead-base piezoceramics, due to their high Curie temperature and good piezoelectric properties [8,9]. However, dense and well-sintered pure KNN-base ceramics are difficult to obtain by conventional sintering method. It has been reported that BiScO 3[10], BiFeO 3[11], BiCoO 3[12] are effective in improving the density and electrical properties of (KNa)NbO 3 ceramics. In preliminary works, we observed that the partial Co (20%) suitable for Fe is helpful to improve the properties of (K 0.5Na 0.5)NbO 3–LiSbO 3–BiFe 1−x Co x O 3 ceramics [13].
In this work, BiFe 0.8Co 0.2 O 3 (BFC) was chosen as a sintering aid to add into 0.95(K 0.5Na 0.5NbO 3)–0.05LiSbO 3 (KNN–LS) basic composition, and the effect of BFC doping on the structure, piezoelectric and electrical properties of samples is investigated.
2 Experimental
A series of (1 −x)[0.95(K 0.5Na 0.5)NbO 3–0.05LiSbO 3]–x BiFe 0.8Co 0.2 O 3 (KNN–LS–xBFC) (x = 0.000, 0.002, 0.004, 0.006, 0.008) samples were prepared by the conventional solid-state reaction method using analytical-grade metal oxides and carbonates powders: Na 2 CO 3 (99.5%), K 2 CO 3 (99.5%), Li 2 CO 3 (99.8%), Sb 2 O 3 (99.5%), Nb 2 O 5 (99.5%), Bi 2 O 3 (99.9%), Fe 2 O 3 (99.9%) and Co 2 O 3 (99.5%). The stoichiometric powders were mixed by ball-milling in alcohol for 24 h, then, the mixed powders were calcined at 880 ∘C for 6 h. The calcined mixture was ball-milled again for 12 h, then dried, sifted and mixed with 5 wt% poly vinyl alcohol (PVA) solution. The obtained powders were pressed into pellet disk of 15 mm diameter and 1.2–1.5 mm thickness. After burning off PVA, pellets were sintered at 1030 ∘C for 3 h. Silver paste electrodes were formed on top and bottom surfaces of the samples after firing at 600 ∘C for 10 min. For electrical measurements, the samples were poled at 80 ∘C in a silicon oil bath at 3.5 kV mm −1 for 15 min.
Phase purity and crystal structure of sintered ceramics were characterized using X-ray diffraction (XRD) (D8-2-Advanced, Bruker Inc. Germany) with CuK α radiation. The microstructure was observed by a scanning electron microscope (SEM) (JSM-5610LV). The piezoelectric constant (d 33) was measured using a quasi-static piezoelectric meter (ZJ-3A). The planar electromechanical coupling coefficient (k p), mechanical quality factor (Q m), dielectric constant (ε r) and dielectric loss (tan δ) were measured by impedance analyzer (Agilent4294A). Ferroelectric hysteresis (P–E) loops were measured at room temperature using a ferroelectric tester (Radiant Precision Workstation, USA).
3 Results and discussion
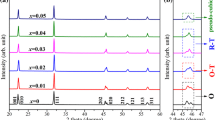
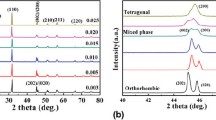
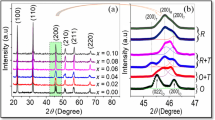
Figure 1a shows the X-ray diffraction (XRD) patterns of KNN–LS–xBFC ceramics with various x sintered at 1030 ∘C. It is seen that all the specimens show a pure perovskite phase at room temperature, and no secondary phase is observed in the investigated range. From figure 1a, it is observed that the specimens with x≤0.002 show a typical structure of tetragonal phase, in agreement with JCPDS card no. 71-0945 for KNN, but the double peaks at 22 and 45 ∘ are gradually weakened and amalgamated into a single peak with the increase of BFC content, which indicated that the phase structure changes from tetragonal phase to orthorhombic phase, in agreement with JCPDS card no. 71-2171 for KNN. The XRD pattern around x=0.002–0.008 shows mixed phases This result indicates that the BFC has completely diffused into the KNN lattice to form a new solid solution in the investigated range and the transition point for the structural change is confirmed to be around x= 0.002 −0.008. Figure 1b shows the magnified XRD of KNN–LS–xBFC ceramics in the range of 44–47 ∘. It also found that the (200) and (020) peaks positions shift slightly towards higher angles with x≤0.004, but while x>0.004, the diffraction peaks positions shift towards lower angles. This may be due to the Co 2+ ions enter into A- and B-sites of the perovskite structure and lead to a variation of lattice parameter. The smaller ionic radius of Co 2+ (0.72 Å) substituted A-site ion (Na +: 0.97 Å) while x≤0.004, the lattice parameter decreases; when BFC content further increases, the Co 2+ begin to diffuse into B-site ions (Nb 5+: 0.69 Å), and the lattice parameter increases.
SEM micrographs of the microstructure of KNN–LS–xBFC ceramics with various BFC contents sintered at 1030 ∘C are shown in figure 2a–d. From figure 2, it can be seen that the microstructures of BFC doped KNN–LS ceramics are dense, and the crystalline grains show cubic shape, but the grain size becomes smaller and homogeneous with increase in the BFC content, which indicated that the addition of BFC is effective to crystalline refinement. Some larger grains and holes have been observed in KNN–LS without doping BFC shown in figure 2a, but the size of grain and number of holes decrease with increasing the BFC content as shown in figure 2b. However, no abnormal larger grains are observed in the samples when x≥0.004, as shown in figure 2c–d, the grains are relatively homogeneous and the size of grain decreases.
Figure 3 shows the piezoelectric properties of KNN–LS–xBFC ceramics with various BFC contents. From figure 3a, it can be seen that the piezoelectric constant d 33 and planar electromechanical coupling coefficient k p exhibit the similar transformational trend with increase in the BFC contents, i.e., d 33 and k p increases with the increase of x reaches a maximum value of d 33=276 pC N −1 when x=0.002 and k p=52% when x=0.004, respectively, and then slightly decreases. Similar phenomenon was also observed in non-stoichiometric NKNT ceramics reported by Lee [14], and the increase of k p is attributed to the increase of density and uniformity of the grain size. In addition, the value of mechanical quality factor Q m for the ceramics initially increases slightly with increase of the BFC contents when x<0.002, following a sharp increase from 31 (x=0.002) to 56 (x=0.004), then again to a slow increase, indicating that the addition of BFC is effective to increase the Q m. The main reason for the phenomenon is attributed to the ion substitution. When BFC was doped into KNN–LS ceramics, most of the Co 2+ substituted for Nb 5+ to produce the oxygen vacancies, which result in a pinning effect on the domain walls, and as the amount of BFC is increased, the oxygen vacancies are also increased. The result indicates that the doping of BFC cause ‘hard doping’ effect in the KNN–LS–xBFC ceramics. Through contrast, it is easy to find that the value of d 33 (276 pC N −1) for KNN–LS–xBFC ceramics with x=0.004 is higher than that of 231 pC N −1 for KNN–LS–BF ceramics sintering at 1100 ∘C [15], but in the k p and Q m only tiny changes occur.
Figure 4 shows the variation in dielectric properties ε r and dielectric loss tan δ values of the KNN–LS–xBFC ceramics measured at room temperature. It can be seen that the dielectric constant ε r increase with the increase of BFC contents x from 0 to 0.008, but the dielectric loss tan δ decreases to a lowest value of 1.95% with the increase of BFC contents x from 0 to 0.002, then increase with the further increase of BFC contents x. It is considered that the variety of dielectric constants and dielectric loss may be ascribe to the change of grain size and density of the ceramic. Similar to the d 33, the electrical properties (ε r=1284−1366, tan δ=1.95− 2.11%) of KNN–LS–xBFC ceramics with x=0.002−0.004 are also better than that (ε r=1041, tan δ=3.15%) of KNN–LS–BF ceramics [15], these results indicated that the suitable amounts of BFC can improve the electrical properties of KNN–LS–xBFC ceramics.
Figure 5 shows the temperature dependence of the dielectric constant ε r for KNN–LS–xBFC ceramic measured at 1 kHz. From figure 5, it can be seen that all samples of KNN–LS–xBFC ceramics exist as a single peak in the curve, corresponding to the Curie temperature (T c) of tetragonal–cubic ferroelectric phase transition, and the orthorhombic–tetragonal phase transition temperatures are all lower than room temperature. From the illustration, it also can be seen that the T c peak shift continually towards lower temperature with the increase of BFC content x, indicating that the T c decreases with the increase of BFC content x. When BFC content x increased from 0 to 0.008, the T c decreased from 340 to 305 ∘C. Besides, it can be observed that the T c peak broaden with the increase of BFC content x, which indicated that the introduction of BFC would conduce the diffusivity of phase transition in KNN–LS ceramics.
Figure 6 shows the P–E hysteresis loops of the KNN–LS–xBFC ceramics. All the samples show a hysteresis loop of P–E when an electric field of 3.5 kV mm −1 is applied. From figure 6, it can be seen that the shapes of P–E loop for samples with various BFC contents x are different. The P–E loops for KNN–LS–xBFC ceramics with x≤0.004 are more saturated, suggesting better ferroelectric properties. Variations of the remnant polarization (P r) and the coercive field (E c) of the KNN–LS–xBFC ceramics are showed in figure 7. As can be seen, P r decreases from 22.59 to 12.75 μC cm −2 as BFC content x increase from 0 to 0.008, but the E c of the ceramics initially increases to a maximum value of 1.51 kV mm −1 with the increase of BFC content before x=0.002, then decrease sharply to the minimum value of 1.16 kV mm −1 when x=0.004, finally increases again when x>0.004. The decrease in P r is due to the reduced grain amount in tetragonal phase. As for the variety of E c, further work needs to be carried out to understand the reasons behind it.
4 Conclusions
BFC can be completely diffused into the KNN lattice to form a new solid solution in the investigated range, and good piezoelectric properties of the ceramics can be obtained with proper BFC content at low sintering temperature of 1030 ∘C. The ceramics contain a single perovskite structure with tetragonal phase and orthorhombic phase, but the tetragonal phase decreases while the orthorhombic phase increases with the increase of x. The grain size KNN–LS–xBFC ceramics becomes smaller and homogeneous with increase in the BFC content, which indicated that the addition of BFC is an effective method to crystalline refinement. With the increase of BFC content x, the Curie temperature T c decreases, but the T c peak broadens, which indicated that the introduction of BFC would conduce the diffusivity of phase transition in KNN–LS ceramics. The d 33 and k p increase with the increase of x to a maximum value of d 33=276 pC N −1 when x=0.002 and k p=52% when x=0.004, respectively, and then slightly decreases, but the Q m increases continuously. The P–E loops for KNN–LS–xBFC ceramics with x≤0.004 are more saturated, suggesting better ferroelectric properties. With the increase of BFC content x, remnant polarization P r decreases continually, but the coercive field E c fluctuate between 1.16 and 1.51 kV mm −1. For the compositions of x=0.004, the samples exhibit optimum piezoelectric and ferroelectric properties at room temperature.
References
Cross L E 1987 Ferroelectrics 76 241
Haertling G H 1999 J. Am. Ceram. Soc. 82 797
Ji W J, Chen Y B, Zhang S T, Yang B, Zhao X N and Wang Q J 2012 Ceram. Int. 38 1683
Zhou C, Feteira A, Shan X, Yang H, Zhou Q, Cheng J, Li W and Wang H 2012 Appl. Phys. Lett. 101 032901
Liu C Y, Liu X Y, Jiang M H and Ma J F 2010 J. Alloy Compd. 503 209
Qiao S, Wu J G, Wu B, Zhang B Y, Xiao D Q and Zhu J G 2012 Ceram. Int. 38 4845
Hao J G, Xu Z J, Chu R Q, Zhang Y J, Chen Q, Li W, Fu P et al 2010, J. Lectron. Mater. 39 347
Zhang C, Chen Z and Ji W 2011 J. Alloy Compd. 509 2425
Gao Y, Zhang J L, Qing Y L, Tan Y Q, Zhang Z and Hao X P 2011 J. Am. Ceram. Soc. 94 2968
Marcos F R, Romero J J, Navarro-Rojero M G and Fernandez J F 2009 J. Eur. Ceram. Soc. 29 3045
Azough F, Wegrzyn M, Freer R, Sharma S and Hall D 2011 J. Eur. Ceram. Soc. 31 569
Park M H and Yoo J Y 2012 J. Electron. Mater. 41 3095
Zhao X, Wang H, Yuan C, Xu J, Cui Y and Ma J 2013 J. Mater. Sci.: Mater. Electron. 24 1480
Lee K S and Yoo J H 2012 J. Curr. Appl. Phys. 12 798
Jiang M H, Liu X Y and Chen G H 2009 Scripta Mater. 60 909
Acknowledgement
We wish to acknowledge the financial support of the Guangxi Nature Science Foundations, grant no. 2010G XNSFD013007.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
WANG, H., ZHAO, X., XU, J. et al. Structure and properties of (1−x)[(K0.5Na0.5)NbO3–LiSbO3]– xBiFe0.8Co0.2O3 lead-free piezoelectric ceramics. Bull Mater Sci 39, 743–747 (2016). https://doi.org/10.1007/s12034-016-1191-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-016-1191-1