Abstract
The preparation of crystalline antimony sulphide (Sb2S3) by chemical route at room temperature was reported in this paper. The structural, morphological and optical properties of as-synthesized sample were systematically investigated. X-ray diffraction (XRD) analysis confirms the orthorhombic crystal phase for prepared Sb2S3. Scanning electron microscope (SEM) images show uniform, dense spherical morphology having diameter around 200–220 nm. Energy band gap calculated from optical absorption spectra was observed around 2.17 eV. Contact angle measurement confirms the hydrophilic nature of the deposited film. The photoluminescence analysis shows low green luminescence as well as Stoke’s shift for as-prepared Sb2S3. The nanostructured solar cell is fabricated for energy harvesting purpose with Sb2S3-sensitized SnO2 photoanode and polysulphide electrolyte. The solar cell with FTO/SnO2/Sb2S3 photoanode shows V OC ∼ 240 mV, J sc ∼ 0.640 mA cm−2 and FF ∼ 35%. The working mechanism and energy level diagram of Sb2S3/SnO2 system have been discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent times, the ability of nanostructured solar cell with dye sensitized metal oxide as photoanode has received attention of researchers owing to their low cost, simple fabrication technique and feasibility.[1] Although the dye-sensitized solar cells (DSSCs) have considerable conversion efficiency, the complex structure of dyes, their availability and stability put certain constrains on their industrial applications.[2] Therefore, nowadays semiconductor materials with narrow band gap are attractive alternative to the light-harvesting dye molecules because of their tunable electrical, optical and structural properties.[3,4]The semiconductor with unique properties like tunable band gap over a wide range to match the solar spectrum, good photostability, broad excitation spectra, high extinction coefficient and multiple exciton generation capability makes them potentially suitable for photovoltaic’s applications.[3]Some previous investigations in this concern have been carried out using CdS,[4–6]CdSe,[7–10]Ag 2S,[11]In 2S3,[12] PbS,[13]Sb 2S3[14] semiconductor nanoparticles. Among these Sb 2S3 has attracted attention of the researchers owing to its narrow band gap of about 1.7 eV which can allow extension of the absorption band toward the near infrared (NIR) part of the solar spectrum.[14] Sensitization with Sb 2S3 nanoparticles also helps in multiple exciton generation and enhancement in the charge separation process.[15] Many reports are available on the deposition of Sb 2S3 thin films by chemical route,[16–18] while synthesis of crystalline Sb 2S3 at room temperature is seldom reported.[19] Although Sb 2S3 has more compatible properties for photovoltaic applications, very few reports are available on TiO 2/Sb 2S3 configuration.[20–22] However, so far SnO 2/Sb 2S3 system has not been studied for energy harvesting purpose.
With this inspiration, in the present study, we report for the first time, the preparation of crystalline Sb 2S3 by chemical bath deposition at room temperature. Deposited Sb 2S3 is used for the fabrication of Sb 2S3 sensitized SnO 2 photoelectrode via the doctor blade method to explore the system for nanostructured solar cell application. The working mechanism and energy level diagram of Sb 2S3/SnO 2 system have been discussed.
2 Experimental
2.1 Synthesis of Sb2S3, preparation of SnO2 photoelectrode and fabrication of solar cell
Chemical bath deposition (CBD) has been followed for the synthesis of nanospheres of Sb 2S3. Depositions were conducted at room temperature for 1 h using a solution mixture of antimony chloride and sodium thiosulfate (Na 2S2 O 3) as a precursor source of Sb 3+ and S 2− ions, respectively.[19] Ethylene diamine tetraacetic acid (EDTA) was used as complexing agent.
To make SnO 2 paste, 0.5 g of SnO 2 powder was mixed with ethanol, acetic acid, ethylene glycol and α-terpineol in mortar and pestle for 40 min, then SnO 2 film was prepared on fluorine-doped tin oxide (FTO) glass by the doctor blade method.[23] After drying, all samples were annealed at 450 ∘C for 1 h. Further the SnO 2 films were immersed into Sb 2S3 colloidal solution for 1 h to adsorb Sb 2S3 nanoparticles onto the SnO 2 photoelectrode surface.
The solar cell was assembled with Sb2S3 sensitized SnO 2 photoanode, polysulphide as electrolyte and carbon coated FTO as a counter electrode. For fabrication of solar cell, few drops of polysulphide electrolyte solution were added between Sb 2S3/SnO 2 photoelectrode and counter electrode (carbon-coated FTO). Finally, they were clamped together facing conducting surfaces inwards.
The structural analyses were carried out by RigakuDmax-2400 (Cu K α=0.154 nm) X-ray diffractometer. The optical absorbance was recorded on JASCO V-670 spectrophotometer and the emission spectrum was recorded by using Perkin Elmex LS55 photoluminescence spectroscopy technique with He–Ne LASER line (325 nm) as excitation source at room temperature. The morphology of the deposited films was studied by JEOL-JSM 6360 scanning electron microscope (SEM). J–V measurements were conducted in the dark and under an illumination of 50 mW cm −2 to study the performance of fabricated solar cell.
3 Results and discussion
3.1 Structural analysis
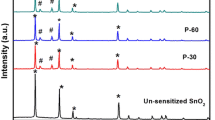
Phase and purity of prepared Sb 2S3 nanospheres were confirmed from X-ray diffraction (XRD) analysis as shown in figure 1a. The XRD pattern shows defined peaks around 2 𝜃=28.85∘, 32.13 ∘ and 45.75 ∘, indexed to the diffraction from the (321), (221) and (441) planes, respectively. Almost single larger intensity peak with smaller full-width at half-maxima (FWHM) was observed, indicating highly oriented and crystalline nature of the prepared Sb 2S3 nanospheres. Defined peaks match with the standard peaks from JCPDS file no. 42-1393, readily confirm the orthorhombic phase for Sb 2S3. Many reports are available on the synthesis of polycrystalline Sb 2S3 deposited at various bath temperatures (low as well as high).[19,24] The elemental analysis of prepared Sb 2S3 was carried out by energy-dispersive spectroscopy (EDS). Figure 1b gives the average atomic percentage of Sb:S as 53 .46, revealing the typical stochiometric formation of the antimony sulphide at room temperature.
The average crystallite size of the as-prepared Sb 2Se3 was predicted by the Scherer formula,[25]
where λ (= 1.54 Å) is the wavelength of the X-rays, β the full-width in radians at half-maximum of diffraction peaks and 𝜃 Bragg’s angle of the X-ray pattern at maximum intensity. The average crystallite size estimated is in the range of 30–60 nm in crystalline orientations of (321), (221) and (441) planes.
3.2 Contact angle measurement
The water contact angle measurement was performed for the films of antimony sulphide, in order to get information about the surface wetting capacity of the deposited material. The water droplet was poured with the help of syringe onto the surface of as-deposited films of Sb 2S3 and the images of the drop were captured as shown in figure 2. It has been observed that the angle of contact for films of antimony sulphide with water around 50 ∘. The acute angle of contact reveals the hydrophilic nature of the prepared Sb 2S3 nanospheres.
3.3 Morphological analysis of Sb2S3, SnO2 and SnO2/Sb2S3 thin films
The typical SEM images of as-deposited Sb 2S3 in figure 3a show the well-distributed spherical morphology. The study carried out by Lokhande et al[19] also showed the spherical morphology for antimony sulphide, deposited at very low temperature (6 ± 2 ∘C). While, Han et al[26] observed olivary microcrystallines of Sb 2S3 synthesized via a hydrothermal procedure at high temperature about 180 ∘C. An observation from figure 3b and c reveals that after Sb 2S3 sensitization, the morphology of SnO 2 photoelectrode changes from porous nanoparticles network to the well-developed flakes like structure. The observed changes in the surface morphology is attributed to the growth of Sb 2S3 on SnO 2.
3.4 Optical analysis of Sb2S3, SnO2 and SnO2/Sb2S3 thin films
The wavelength dependence of optical absorption spectra of as-prepared Sb 2S3 nanospheres was analysed in the wavelength range of 300–850 nm and the band gap was calculated. Figure 4a shows the plot of (absorbance) 2 vs. h υ for Sb 2S3 nanospheres. By extrapolating the lines obtained to (absorbance) 2=0 on energy axis direct band gap of Sb 2S3 was observed as 2.17 eV for the deposited Sb 2S3 film. Such a value agrees well with the value 2.26 eV for crystalline Sb 2S3 reported in Rajpure et al,[16] but it is somewhat higher than band gap energy of 1.62 eV reported by Desai and Lokhande.[17]
Figure 4b shows wavelength-dependent absorption spectra for Sb 2S3, SnO 2 and SnO 2/Sb 2S3. The SnO 2 film exhibits absorption around wavelength less than 340 nm, quite suitable as window material, seldom studied for semiconductor sensitized-based solar cell,[27] as compared with TiO 2.[20–22]Sb 2S3 shows prominent peak of absorption at wavelength about 350 nm and has very promising absorbance extended into far visible region of electromagnetic waves. This makes it very appropriate absorber material for photovoltaic cells.[28]
Optical absorption spectra shown in figure 4b suggest that deposition of a Sb 2S3 helps to enhance the photoresponse of the SnO 2 film by extending absorption up to 613 nm for SnO 2/Sb 2S3 system. The enhancement in the absorbance of the SnO 2/Sb 2S3 system in the visible region from UV-region confirms the growth of the Sb 2S3 on the SnO 2 particles.
3.5 Photoluminescence (PL) study of Sb2S3
Figure 5 shows emission spectra for chemically deposited Sb 2S3 measured at room temperature with excitation wavelength at 350 nm gives four distinct peaks around 472, 532, 610 and 716 nm. The first peak centred around 472, this blue emission can be attributed to deep trap states or defects in material resulted during experimental conditions.[29] Second peak about of 532 nm, corresponds to the green emission, which is in agreement with the previous study by Kulyk et al.[30] Third peak at 610 nm is the PL peak for deposited Sb 2S3 and agrees well with the observations made by Xu et al[31] for Sb 2S3. Fourth emission peak observed approximately at 717 nm, which is at higher wavelength than that of absorption peak approximately at wavelength of 430 nm. This observed red shift in position of emission peak can be attributed to Stoke’s shift, which may be due to the localized centres in band structure.[32]
3.6 Photovoltaic analysis
Figure 6a presents the schematic of charge generation-transfer process for Sb 2S3-sensitized SnO 2 photoelectrode-based solar cell. Illumination of the conducting electrode causes excitation of Sb 2S3, excited electron will enter in the conduction band (E c) of Sb 2S3 and hole will remain in the valence band (E v). The possible charge generation-transfer processes on the basis of band positions of SnO 2, Sb 2S3 and electrolyte has been shown in figure 6b. From the energy level alignment of SnO 2 and Sb 2S3, it is observed that the E c of Sb 2S3 is negative enough to inject electron into SnO 2, a wide band semiconductor, which has the conduction band edge minimum more positive as compare to TiO 2 (at −4.5 eV for SnO 2; −4.2 eV for TiO 2).[33,34]Hence the excited Sb 2S3 can easily inject electrons from its E c quickly into the E c of SnO 2, as compare to TiO 2. The flakes like morphology of SnO 2 helps the received electrons to percolate through SnO 2 to the conducting FTO. Finally, it travels through the external load and complete the circuit by entering the system via counter electrode. The function of redox species is to quickly reduce back the excited Sb 2S3 to its ground state and to set the system for next excitation. Thereafter, the redox species takes electrons from counter electrode for further action and the electron cycle repeats.
3.7 Cell performance analysis
Figure 7 represents J–V characteristics for solar cell fabricated using Sb 2S3-sensitized SnO 2 photoelectrode having effective area of 0.25 cm 2 in the dark and under an illumination of 50 mW cm −2 showed V oc∼240 mV and J sc∼0.640 mA cm −2 and FF ∼35%.
Study of J–V characteristics reveals that, Sb 2S3 a good absorber due to its optimum energy band gap, helps photoelectrochemical active SnO 2 so that it can accept electrons from the adjacent semiconductor layer of Sb 2S3 nanoparticles followed by efficient charge separation. This helps in the enhancement of photocurrent density by 20% for SnO 2/Sb 2S3 system in comparison with SnO 2/CdSe system which shows J sc ∼ 25–30 μA cm−2 as reported by Nasr et al.[28] The low value of fill factor (FF ∼ 35%) attributes to the poor electron transfer at electrolyte–counter electrode interface. In order to improve FF it is essential to optimize electrolyte and counter electrode system for SnO 2/Sb 2S3 photoanode-based solar cells.
4 Conclusion
In this paper, it has been reported that a template free, simple chemical route for the preparation of crystalline Sb 2S3 nanospheres at room temperature can be a substitute for controlled and high temperature synthesis techniques. Prepared Sb 2S3 has orthorhombic phase. Optical absorbance observed in the visible far IR-region, makes it suitable as absorber material in solar cell applications. Noticeable enhancement in absorption of visible light was observed after deposition of Sb 2S3 over SnO 2, confirming the deposition of Sb 2S3 onto SnO 2. The charge generation and transfer processes discussed in detail for SnO 2/Sb 2S3-based solar cell suggest SnO 2 as an alternative to TiO 2 as well as ZnO. The photovoltaic performance for solar cell comprising SnO 2/Sb 2S3 nanostructure shows V oc∼240 mV and J sc ∼0.640 mA cm −2 and FF ∼ 35%.
References
Rokesh K, Pandikumar A and Jothi Venkatachalam K 2013 Mater. Sci. Forum 771 1
Jinchu I, Sreekala C and Sreelatha K 2013 Mater. Sci. Forum 771 39
Nozik A 2008 J. Chem. Phys. Lett. 457 3
Sun W, Yu Y, Pan H, Gao X F, Chen Q and Peng L M 2008 J. Am. Chem. Soc. 130 1124
Zhu G, Su F F, Lv T, Pan L K and Sun Z 2010 Nanoscale Res. Lett. 5 1749
Wang C B, Jiang Z F, Wei L, Chen Y X, Jiao J, Eastman M and Liu H 2012 Nano Energy 1 440
Zhang Q X, Guo X Z, Huang X M, Huang S Q, Li D M, Luo Y H, Shen Q, Toyoda T and Meng Q B 2011 Phys. Chem. Chem. Phys. 13 4659
Luan C Y, Aleksandar V, Andrei S S, Xu X Q, Wang H E, Chen X, Xu J, Zhang W J, Lee C S, Andrey L R and Juan A Z 2011 Nanoscale Res. Lett. 6 340
Chen Y X, Wei L, Zhang G H and Jiao J 2012 Nanoscale Res. Lett. 7 516
Chen J, Lei W and Deng W Q 2011 Nanoscale 3 674
Chen C, Xie Y, Ali G, Yoo S H and Cho S O 2011 Nanoscale Res. Lett. 6 462
Kieven D, Dittrich T, Belaidi A, Tornow J, Schwarzburg K, Allsop N and Lux-Steiner M 2008 Appl. Phys. Lett. 92 153107
Wang L D, Zhao D X, Su Z S and Shen D Z 2012 Nanoscale Res. Lett. 7 106
Maiti N, Im S H, Lim C S and Seok S I 2012 Dalton Trans. 41 11569
Li Y, Wei L, Zhang R, Chen Y, Mei L and Jiao J 2013 Nanoscale Res. Lett. 8 89
Rajpure K Y and Bhosale C H 2000 J. Phys. Chem. Solids 61 561
Desai J D and Lokhande C D 1994 Thin Solid Films 237 29
Pawar S H, Tamhankar S P, Bhosale P N and Upllane M D 1983 Ind. J. Pure Appl. Phys. 21 665
Lokhande C D, Sankpal B R, Mane R S, Pathan H M, Muller M, Giersig M and Ganesan V 2002 Appl. Surf. Sci. 193 1
Yafit I, Olivia N, Miles P and Gary H 2009 J. Phys. Chem. C 113 4254
Moon S J, Itzhaik Y, Yum J H, Zakeeruddin S M, Hodes G and Gratzel M 2010 J. Phys. Chem. Lett. 1 1524
Im S H, Kim H J, Rhee J H, Lim C S and Seok S I 2011 Energy Environ. Sci. 4 2799
Arote S A, Ingale R V, Tabhane V and Pathan H M 2014 J. Renew. Sustain. Energy 6 013132
Meherzi H M, Nasr T B, Kamoun N and Dachraoui M 2010 Physica B 405 3101
Azaroff L V 1968 Elements of X-ray crystallography (New York: McGraw-Hill) p. 552
Han Q, Lu J, Yang X, Lu L and Wang X 2008 Cryt. Growth Des. 8 395
Messina S, Nair M T S and Nair P K 2008 J. Phys. D: Appl. Phys. 41 095112
Nasr C, Kamat P and Hotchandani S 1996 J. Electroanal. Chem. 420 201
Alemi A, Hanifehpour Y and Joo S W 2011 J. Nanomater. 2011 414798
Kulyk B, Kapustianyk V, Krupka O and Sahraoui B 2011 J. Phys.: Conf. Ser. 289 012003
Xu Y, Ren Z, Cao G, Ren W, Deng K and Zhong Y 2009 Cryst. Res. Technol. 44 851
Fujita T, Kurita K, Takiyama K and Oda T 1988 J. Lumin. 39 175
Rhee J H, Chung C and Diau E W-G 2013 NPG Asia Mater. 5 e68
Patrick C E and Giustino F 2011 Adv. Funct. Mater. 21 4663
Acknowledgements
HMP is thankful to Science and Engineering Research Board, Department of Science and Technology, New Delhi (Fast track Scheme for Young Scientists) and Departmental Research Development Program, Savitribai Phule Pune University, for financial support. SAA is also thankful to University Grant Commission, New Delhi for Faculty Improvement Program fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
KULKARNI, A.N., AROTE, S.A., PATHAN, H.M. et al. Room temperature synthesis of crystalline Sb2S3 for SnO2 photoanode-based solar cell application. Bull Mater Sci 38, 493–498 (2015). https://doi.org/10.1007/s12034-014-0836-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-014-0836-1