Abstract
Cadmium sulfide (CdS) nanowires (NWs) were prepared by the solvothermal method using ethylenediamine as a solvent. Two sets of CdS NWs were synthesized at 160 and 200 °C for various reaction durations (3⋅5, 7, and 24 h). Scanning/tunneling electron microscopy was used to examine the surface morphology of the grown NWs. Their dimensions are found to depend on the reaction temperature and duration. The CdS NWs grown at 200 °C for all durations are longer than those prepared at 160 °C, with diameters ranging from 15 to 40 nm. A three-armed structure is exhibited by all the samples. The grown CdS NWs display a hexagonal wurtzite phase and grows along the \(\mathbf {\left \langle {001}\right \rangle }\) direction. The optical absorption of the grown NWs shows a sharp absorption edge with a blueshift, which indicates an expansion of the optical band gap. All prepared samples show two emission peaks in their photoluminescence spectra. The emission peak location depends on the reaction temperature and duration. The CdS NWs prepared at 160 °C show a sharp band–band emission compared with those prepared at 200 °C. Raman analysis indicates that the optical properties of the grown NWs are enhanced with increased temperature and reaction duration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Considerable interest is focused on the synthesis of nanocrystalline materials because of their unique physical and chemical properties that distinguish them from bulk-phase materials. The particle size and shape of materials significantly affect their properties. Thus, control of the size and morphology of nanocrystalline materials can lead to the discovery of new physical and chemical properties (Wang et al 2011). One-dimensional (1D) semiconductors such as nanowires (NWs), nanorods and nanoribbons have several unique advantages, including high crystallinity, self-assembly, high surface-to-volume ratio, quantum confinement effects and slow electron–hole recombination (Choi et al 2010; Mahdi et al 2012a; Rai et al 2012). The ability to grow 1D, single-crystal and nanostructured semiconductors has led to the fabrication of functional nanoscale devices (Li et al 2005, 2010; Yingkai et al 2006; Xi et al 2010; Mahdi et al 2012a). Among group II–VI semiconductor materials, cadmium sulfide (CdS; direct band gap = 2⋅42 eV), is considered to be an excellent material for various optoelectronic applications within the visible range of the electromagnetic spectrum (Yan et al 2009; Mahdi et al 2012b). 1D CdS has been prepared usingvarious methods, such as D.C. electrochemical techniques (Mondal et al 2007a), thermal evaporation (Kar and Chaudhuri 2006), chemical vapour deposition (Ma et al 2007) and hydro/solvothermal methods (Qingqing et al 2005; Xu et al 2005; Nirmala Jothi et al 2011). Solvothermal synthesis is widely used to prepare semiconductor NWs, particularly CdS. This synthesis can enable the control wire morphology and dimensions via reaction variables such as solvent type, temperature and duration (Datta et al 2009). The solvo/hydrothermal method is also simple, easy to scale up, low-energy consuming and cost-effective (Yan et al 2009; Chen and Wu 2011). In the solvothermal method, preparation parameters such as reaction temperature, duration and solvent type have crucial functions in determining the nature of the product as well as its physical properties. The solvothermal method can produce high-purity nanocrystalline materials with a high degree of crystallinity compared with other solution-based methods (Dalvand et al 2011). In the present work, CdS NWs were grown by the solvothermal method. The effects of the reaction temperature and duration on NW morphology, crystalline structure and optical properties were investigated.
2 Materials and methods
Cadmium nitrate [Cd(NO3)2⋅4H2O] and sulfur (S) powder were used as Cd2+ and S2−ion sources, respectively. Approximately 0⋅22 g of [Cd(NO3)2⋅4H2O] and 0⋅066 g of Spowder were dissolved in 50 ml of ethylenediamine en and stirred for 10 min. The solution was poured into a 100 ml teflon-lined stainless steel autoclave filled with en to 60% of its total volume capacity. The autoclave was sealed and subsequently loaded into an oven. Two sets of samples were prepared at different reaction temperatures and durations. The first set was prepared at 160 °C for 3⋅5, 7 and 24 h, whereas the other set was grown at 200 °C for the same durations. After removing the autoclave from the oven and cooling naturally, the resulting yellow precipitates were filtered, washed several times with absolute ethanol as well as distilled water and then air dried at 60 °C for 2 h. A field-emission scanning electron microscopy (FESEM) system (FEI Nova NanoSEM 450) was used to determine the morphology of the products. The microstructures were investigated by a transmission electron microscopy (TEM) system (Libra 120-Carl Zeiss). The crystalline structure of the prepared CdS nanostructures was investigated by X-ray diffraction (XRD) using a PANalytical X’Pert PRO MRD PW3040 instrument with CuKα radiation. Optical properties such as absorption, photoluminescence (PL) and Raman spectra were measured using a Shimadzu UV–Vis spectrophotometer (UV-1800) and Horiba Jobin Yvon H 800 UV equipment.
3 Results and discussion
3.1 Surface morphology
Figure 1 shows the FESEM images of the CdS NWs grown at 160 and 200 °C for various reaction durations. Figure 1(A–C) shows the CdS NWs prepared at 160 °C for 3⋅5, 7 and 24 h, respectively; figure 1(D–F) displays the CdS NWs prepared at 200 °C for the same durations. The results show that the dimensions of the grown CdS NWs depend on the reaction temperature and duration. The grown NWs exhibit uniform shapes and similar diameters, as clearly shown by the TEM images (figure 2). The TEM image of the sample prepared at 160 °C for 3⋅5 h contains a number of nanoparticles in addition to the NW structure. In the solvothermal method, two mechanisms are involved in the formation of the 1D structure. Cd2+ ions interact with the lone pair of electrons of the en nitrogen atoms to form a Cd-en [Cd(en)2]2+ complex. S2− ions are then slowly released into the solution, consequently, increasing the temperature and resulting in the combination of the Cd2+ and S2− ions to form a two-dimensional (2D), CdS–0⋅5en complex structure, as shown in the following reaction:
This complex has an organic–inorganic lamellar structure with inorganic CdS layers separated by organic en spacers (Kar et al 2008). The 2D complex structure becomes unstable with increased temperature until around 120 °C; thus, the complex fragments into a 1D needle-like structure to achieve stability. The needle-like structure then grows to form NWs due to the high surface energy. Mahdi et al (2012c) grown CdS nanostructures by solvothermal and they noted that the CdS prepared at 100 and 120 °C grew into nanosheets, and those prepared at 160 and 200 °C developed a nanowire structure. On the other hand, a number of uncoupled Cd2+ ions directly react with S2− ions to form CdS nanoparticles. These nanoparticles also serve as nuclei for the growth of CdS NWs in solution. A three-armed nanorod structure is also shown in the TEM images (figure 3). This three-armed structure frequently appears in all the samples prepared at 160 and 200 °C. The 1D CdS structure grows into nanorods, as clearly shown by the hexagonal shape in figure 3. The three-armed structure is due to the presence of CdS nanoparticles in the solution, which acts as nuclei for the growth of CdS nanorods. Chen et al (2002) grew CdS nanorods by solvothermal synthesis using CdS nanoparticles as seeds to nucleate CdS nanorods, and they obtained tetrapod structures. The NW diameter distribution analysis shows that the diameters of CdS NWs grown at 160 °C for 3⋅5 and 24 h range from 10 to 30 nm, whereas those of CdS nanorods prepared for 7 h range from 15 to 40 nm (figure 4). This difference in diameter may be due to the higher concentration of the three-armed structure in the sample prepared for 7 h than the other samples. Interestingly, the NWs shorten with increased reaction duration to 24 h and some CdS nanosheets appear. The longer duration may have caused the wire to break into shorter pieces. The appearance of a number of CdS nanosheets may have been due to the growth of sheets that do not break into the needle structure. However, the reason for the absence of nanosheet structures in the sample prepared for 7 h is unclear. Figure 2(D–F) show that the CdS NWs grown at 200 °C for 3⋅5, 7 and 24 h, respectively, are longer than those prepared at 160 °C. The wire diameters of these NWs range from 15 to 35 nm, as indicated by the wire diameter distribution results in figure 4. Jang et al (2007) prepared 1D CdS by the solvothermal method at different reaction temperatures and durations. They noted that with increased duration, CdS NWs grown at 160 °C increase in length but not in diameter. Moreover, Datta et al (2009) studied the reaction temperature (150–250 °C) and duration (5–48 h) effect on the morphology, crystalinity and the optical properties of CdS NWs prepared via sovothermal on Cd foil substrate. They noted that the wires were grown vertically on the substrate and the diameter average was 200–400 nm. The elemental ratios (Cd/S) of all grown CdS NWs range from 1⋅10 to 1⋅12, as determined by energy dispersive X-ray measurements.
3.2 Crystalline structure
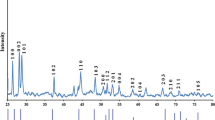
The crystalline structure of the solvothermally prepared CdS NWs was determined by XRD analysis, and the results are shown in figure 5(A and B). Compared with a standard XRD database (PDF-4, 00-001-0780), the grown CdS NWs exhibit hexagonal wurtzite structures belonging to the \(C_{6v}^4 \) CdS space group. The preferred orientation of the grown NWs can be predicted from the following formula (Phuruangrat et al 2010):
where I (hkl) and \(I^{\prime }_{(\text {hkl})}\) are the integrated intensities of the experimental and random (simulated) XRD diffractions, respectively. For a random orientation, P equals one. For a particular (hkl) plane with a preferred orientation, P is greater than one. The calculated P hkl values of the grown CdS NWs prepared at 160 and 200 °C are listed in table 1. The preferred orientation of the NWs prepared at 160 °C for 3⋅5 h is along the \(\left \langle {001} \right \rangle \) direction. This preferred orientation changes to the \(\left \langle {100} \right \rangle \) direction with increased reaction duration to 7 and 24 h. The XRD patterns of the CdS NWs prepared at 200 °C indicate that the samples are more crystalline than those prepared at 160 °C (figure 5(B)). The preferred orientation of the CdS NWs grown at 200 °C also depends on the reaction duration. The preferred orientation of the CdS NWs prepared for 3⋅5 h is along the \(\left \langle {110} \right \rangle \) direction. Increasing the reaction duration to 7 and 24 h changes this orientation to the \(\left \langle {100} \right \rangle \) direction. Dalvand and Mohammadi (2011) studied the effect of preparation parameters on the crystalline structure, morphology and optical properties of solvothermally grown CdS NWs using en as a solvent as well as Cd(Ac)2⋅2H2O and S powder as ion sources. They concluded that the preferred orientation of the NWs prepared at 160 °C for 12 h was along \(\left \langle {110} \right \rangle \). This orientation changes to the \(\left \langle {100} \right \rangle \) direction with increased reaction temperature to 200 °C. They also noted that the preferred orientation changes from \(\left \langle {110} \right \rangle \) to \(\left \langle {100} \right \rangle \) with increased reaction duration from 12 to 24 h. Datta et al (2009) noted that the CdS nanowires grown along (001) direction and the wires became more crystalline when the temperature of the preparation increased from 150 to 250 °C. Surface atoms have higher energies than interior atoms and thus significantly affect the growth direction. For the hexagonal structure, the atomic density is higher on the (001) surface than on the (100) and (110) surfaces. Therefore, atoms on the (002) face have significantly lower excess energy than atoms on other faces (Phuruangrat et al 2009). The preferred orientation in all prepared CdS NWs is along the \(\left \langle {100} \right \rangle \) or \(\left \langle {110} \right \rangle \) direction, which makes a 90° angle with \(\left \langle {001} \right \rangle \). Hence, the growth direction of the grown CdS NWs is along \(\left \langle {001} \right \rangle \). The (002) peak intensity is higher than those of the (100) and (101) planes of the CdS NWs grown at 160 °C for 3⋅5 h, which may be due to the lower aspect ratio (Zhou et al 2011). Growth along the c-axis of the NW wurtzite structure benefits optoelectronic and electro-optic applications due to the polarized light emission along the c-axis of the wire (Thupakula et al 2012). Growth along the \(\left \langle {001} \right \rangle \) direction indicates that the grown NWs are bipolar because of the non-centrosymmetric charge distribution in the wurtzite crystal along the c-axis (Thupakula et al 2012). The lattice constants a and c for the hexagonal structure are calculated using the following equation (Nien et al 2008):
where d hkl is the interplanar spacing of the atomic planes; and h, k and l are the Miller indices.
The calculated lattice parameter values of the prepared samples are almost identical to standard data (table 1). The particle size, P S can be calculated from the XRD peakbroadening using the Debye–Scherer equation (Thupakula et al 2012):
where K is a shape factor, λ the X-ray radiation wavelength (∼0⋅15 nm), β the full-width at half-maximum (FWHM) of the XRD diffraction peak and 𝜃 the half of the diffraction angle. The CdS NW growth direction is along \(\left \langle {001} \right \rangle \). Thus, the FWHM of the (002) plane is used to calculate P S. The diameters (R w) of the grown CdS NWs are estimated using P S = 2R w. The estimated R w values are listed in table 1. The calculated R w found close to the dominated diameter of the grown CdS NWs that estimated by TEM images. Thus, XRD can be used as a method to estimate the diameter of the grown wires.
3.3 Optical properties
3.3.1 Optical absorption
Optical studies were performed by measuring the absorption of the prepared CdS NWs within the 300–900 nm wavelength range. Figure 6 shows the UV–Vis absorption of the CdS NWs prepared at 160 and 200 °C for different durations. The absorption edge of CdS NWs grown at 160 °C for 3⋅5 and 7 h is observed at around 480 nm; this peak shifted to ∼485 nm for the NWs prepared for 24 h (figure 6A). Thus, the optical band gap for the NWs prepared for 3⋅5 and 7⋅0 h is 2⋅58 eV, and that for the NWs prepared for 24 h is 2⋅55 eV. The absorption band peaks for the NWs grown at 200 °C for all durations peak at ∼485 nm (figure 6B). The obtained optical band gap values are higher than the bulk value (2⋅42 eV and ∼515 nm) (Mahdi et al 2012b). The sharp absorption peak of the CdS NWs can be attributed to their size uniformity and high crystallinity (Jang et al 2007; Yan et al 2009). Using the solvothermal method, Yan et al (2009) prepared CdS NWs with diameters ranging from 23 to 37 nm and an optical energy gap of 2⋅56 eV.
3.3.2 Photoluminescence spectra analysis
The room-temperature PL emission spectra of the CdS NWs prepared at 160 and 200 °C are shown in figure 7. The CdS NWs prepared at 160 °C for 3⋅5 and 7 h show a sharp PL emission band at around 496 nm (2⋅5 eV), which correspond to a typical band–band transition. The observed PL band at 496 nm is blue shifted from bulk CdS (500–700 nm). However, the emission bands comes either from band edge or recombination of surface states energy level (Sadhu et al 2008). The PL emission of the NWs prepared for 24 h redshifts to 519 nm (2⋅39 eV), as shown in figure 7(A). A broad yellow emission is observed at 668 nm (1⋅85 eV) for the NWs prepared for 3⋅5 and 7 h; this emission peaks at ∼690 nm (1⋅8 eV) for the NWs grown for 24 h. Excess Cd in CdS can lead to the formation of an acceptor level within the CdS optical band gap. Thus, the defect band is attributed to the transition of Cd-interstitial donors to the valence band (Tsai et al 1996). Ahmad-Bitar (2000) attributed the appearance of the defect emission band to recombination via surface localized states, the transition from I Cd to the valance band, and the transition from the interstitial Cd–Cd vacancy complex that acts as a donor to an accepter level. The shift in the defect band position may also be related to the effect of the concentration of the defect levels. Interestingly, the CdS NWs prepared at 200 °C for all durations show a sharp PL emission band at 512 nm and another broad emission band at 690 nm. The PL intensities of the CdS NWs prepared for 3⋅5 h at 160 and 200 °C are the highest among all the samples. Table 2 summarizes the optical properties of the CdS NWs prepared at 160 and 200 °C. However, Datta and his workers (2009) noted that the solvothermally grown CdS NWs showed a distinct blue shift of the PL peak position which centered at ∼530 nm from low temperature (180 °C) to the higher temperature (250 °C) prepared samples.
3.3.3 Raman spectra analysis
Figure 8(A and B) shows the room-temperature Raman spectra of all prepared CdS NWs excited by 515 nm Ar+ laser light. The two observed peaks in the Raman spectra correspond to the first-order (1LO) and second-order (2LO) longitudinal optical phonon modes of CdS, which are polarized in the x–z face and strongly coupled to the exciton along the c-axis (Arguello et al 1969; Wang et al 2002; Pan et al 2005; Mondal et al 2007b). For all samples prepared at 160 °C, the 1LO peak is located at 298 cm−1 The 2LO peak of the samples prepared for 3⋅5 and 7 h appear at 600 cm−1 and redshifts to 590 cm−1 for the sample prepared at 24 h (figure 8(A)). The Raman spectra show that the 1LO and 2LO peaks of all the samples prepared at 200 °C are located at 299 and 600 cm−1, respectively. For bulk CdS, the fundamental 1LO and overtone 2LO modes are located at 305 and 605 cm−1, respectively (Mahdi et al 2012b). Thus, the redshift in the positions of the 1LO and 2LO modes in all prepared CdS NWs samples occurs because of the phonon confinement effect (Owens and Poole 2008). The exciton–phonon coupling strength in semiconductors can be determined from the intensity ratio of the phonon overtone to the fundamental(I 2LO/I 1LO)(Mahdi et al 2012d). The CdS NWs prepared at 160 °C for 24 h show higher (I 2LO/I 1LO) intensity ratios than the samples prepared for 3⋅5 and 7 h. On the other hand, the NWs prepared at 200 °C for 7 h have the highest (I 2LO/I 1LO) intensity ratio (table 2). NWs with high (I 2LO/I 1LO) intensity ratios exhibit strong exciton–LO phonons because of phonon confinement in the transverse directions and the transfer of elementary excitation (carriers, excitons and phonons) to the longitudinal direction (Cao et al 2007). These single-crystal CdS NWs possess high optical properties and are promising building blocks for photonic devices (Yan et al 2009).
4 Conclusions
CdS NWs/nanorods were prepared by the solvothermal method at 160 and 200 °C for 3⋅5, 7 and 24 h and using en as a solvent. The dimensions of the grown CdS NWs are found to depend on the reaction temperature and duration. Analysis of the diameter distribution of the CdS NWs grown at 160 and 200 °C shows that the reaction duration does not significantly affect the wire diameter. A three-armed CdS nanorod structure frequently appears in all the samples prepared at 160 and 200 °C. Appearing three-armed CdS nanorod structure can be attributed to the CdS nanoparticles in the solution, which acts as nuclei for the growth of this structure. The CdS NWs grown at 200 °C for 3⋅5, 7 and 24 h are longer than those prepared at 160 °C. The crystalline structure of all grown CdS NWs is wurtzite, and the NW/nanorod growth direction is along the \(\left \langle {001} \right \rangle \) plane. The NW diameters estimated from the XRD results are almost identical to those calculated from the TEM images, thus the XRD method can be used efficiently to calculate the diameter of the NWs. Sharp absorption peaks of the CdS NWs with blueshifts in the absorption edge are observed in all the prepared samples. The optical band gap of the NWs prepared at 160 °C decreases from 2⋅58 to 2⋅55 eV with increased reaction duration from 3⋅5 to 24 h. However, the band gaps of the samples grown at 200 °C are unaffected. The PL emission spectra of the CdS NWs prepared at 160 and 200 °C for 3⋅5 h show a sharp green emission. The ratios of the band–band emission to the defect intensity for the samples prepared at 200 °C are lower than those for the samples prepared at 160 °C. Raman results show that the optical properties of the nanostructures are enhanced with increased reaction duration for all prepared CdS NWs.
References
Ahmad-Bitar R N 2000 Renew. Energ. 19 579
Arguello C A, Rousseau D L and Porto S P S 1969 Phys. Rev. 181 1351
Cao B L, Jiang Y, Wang C, Wang W H, Wang L Z, Niu M, Zhang W J, Li Y Q and Lee S T 2007 Adv. Funct. Mater. 17 1501
Chen S W and Wu J M 2011 Acta Mater. 59 841
Chen M, Xie Y, Lu J, Xiong Y, Zhang S, Qian Y and Liu X 2002 J. Mater. Chem. 12 748
Choi Y J, Park K S and Park J G 2010 Nanotechnol. 21 509901
Dalvand P and Mohammadi M R 2011 J. Nanopart. Res. 13 3011
Dalvand P, Mohammadi M R and Fray D J 2011 Matter. Lett. 65 1291
Datta A, Chavan P G, Sheini F J, More M A, Joag D S and Patra A 2009 Cryst. Growth Des. 9 4157
Jang J S, Joshi U A and Lee J S 2007 J. Phys. Chem. C 111 13280
Kar S and Chaudhuri S 2006 J. Phys. Chem. B 110 4542
Kar S, Santra S and Heinrich H 2008 J. Phys. Chem. C 112 4036
Li Q H, Gao T and Wang T H 2005 Appl. Phys. Lett. 86 193109
Li L, Wu P, Fang X, Zhai T, Dai L, Liao M, Koide Y, Wang H Q, Bando Y and Golberg D 2010 Adv. Mater. 22 3161
Ma R M, Wei X L, Dai L, Huo H D and Qin G G 2007 Nanotechnol. 18 205605
Mahdi M A, Hassan J J, Ng S S and Hassan Z 2012a Phys. E 44 1716
Mahdi M A, Hassan Z, Ng S S, Hassan J J and Mohd Bakhori S K 2012b Thin Solid Films 520 3477
Mahdi M A, Hassan J J, Ng S S and Hassan Z 2012c J. Cryst. Growth 359 43
Mahdi M A, Asmiet Ramzy, Hassan Z, Ng S S, Hassan J J and Kasim S K 2012d Chalcogen. Letts. 9 19
Mondal S P, Dhar A and Ray S K 2007a Mat Sci. Semicon. Proc. 10 185
Mondal S P, Das K, Dhar A and Ray S K 2007b Nanotechnol. 18 095606
Nien Y T, Chen P W and Chen I J 2008 J. Alloys Compd. 462 398
Nirmala Jothi N S, Chisty P D, Baby Suganthi A R, Ramalingam G and Sagayaraj P 2011 J. Crys. Growth 316 126
Owens F J and Poole C P 2008 The physics and chemistry of nanosolids (Hoboken, New Jersey, USA: John Wiley & Sons Inc.)
Pan A L, Liu R B, Yang Q, Zhu Y C, Yang G Z, Zou B S and Chen K Q 2005 J. Phys. Chem. B 109 24268
Phuruangrat A, Thongtoem T and Thongtoem S 2009 Mater. Lett. 63 1538
Phuruangrat A, Thongtem T and Thongten S 2010 Chalcogen. Letts. 7 605
Qingqing W, Gang X and Gaorong H 2005 J. Solid State Chem. 178 2680
Rai P, Song H M, Kim Y S, Song M K, Oh P R, Yoon J M and Yu Y T 2012 Mater. Lett. 68 90
Sadhu S, Chowdhury P S and Patra A 2008 J. Lumin. 128 1235
Thupakula U, Jena A, Khan A H, Dalui A and Acharya S 2012 J. Nanopart. Res. 14 701
Tsai C T, Chuu D S, Chen G L and Yang S L 1996 J. Appl. Phys. 79 9105
Wang Y W, Meng G W, Zhang L D, Liang C H and Zhang J 2002 Chem. Mater. 14 1773
Wang X, Liu W, Yang H, Li X, Li N, Shi R, Zhao H and Yu J 2011 Acta Mater. 59 1291
Xi Y, Hu C, Zheng C, Zhang H, Yang R and Tian Y 2010 Mater. Res. Bull. 45 1476
Xu D, Liu Z, Liang J and Qian Y 2005 J. Phys. Chem. B 109 14344
Yan S, Sun L, Qu P, Huang N, Song Y and Xiao Z 2009 J. Solid State Chem. 182 2941
Yingkai L, Xiangping Z, Dedong H and Hui W 2006 J. Mater. Sci. 41 6492
Zhou J, Zhao G, Yang J and Hano G 2011 J. Alloys Compd. 509 6731
Acknowledgements
The authors gratefully acknowledge the support of the Research University (RU) grant and the University Sains Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MAHDI, M.A., HASSAN, J.J., KASIM, S.J. et al. Solvothermal growth of single-crystal CdS nanowires. Bull Mater Sci 37, 337–345 (2014). https://doi.org/10.1007/s12034-014-0655-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-014-0655-4