Abstract
Quartz and kaolin were partially substituted by sand stone dust (a siliceous byproduct of Indian stone cutting and polishing industries) in a traditional triaxial porcelain composition consisting of kaolin, quartz and feldsper. The effect of substitution upon heating at different temperatures (1050–1150\(\boldsymbol{^\circ}\)C) were studied by measuring the linear shrinkage, bulk density, porosity and flexural strength. Qualititative phase and microstructural analysis on selected samples were carried out using XRD and SEM/EDX technique. The results show that the samples of all the batches achieved higher density (2\(\boldsymbol\cdot\)50 g/cc) and almost full vitrification (\(\boldsymbol <\)0\(\boldsymbol{\cdot}\)1% apparent porosity) at around 1115\(\boldsymbol{^\circ}\)C compared to around 1300\(\boldsymbol{^\circ}\)C for traditional triaxial porcelain composition. As high as 70 MPa flexural strength was obtained in most of the vitrified samples. No significant variation in physico-mechanical properties was observed in between the composition. XRD studies on selected samples show presence of mainly quartz phase both at low and high temperatures. SEM photomicrographs of the 1115\(\boldsymbol{^\circ}\)C heated specimen show presence of quartz grain and glassy matrix. Few quartz grains (20–40\(\boldsymbol{\mu}\)m) are associated with circumferential cracks around them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A traditional porcelain composition consists of around 50 wt% kaolin, 25 wt% quartz and 25 wt% feldspar. The role of each of these raw materials on the physico-mechanical behaviour of such triaxial porcelain has been studied in detail by several researchers (Sane and Cook 1951; Mattyasovsky 1957; Kingery 1976; Hamano et al 1992; Maity and Sarkar 1996; Carty and Senapati 1998). Due to gradual depletion of naturally occurring minerals, there is a strong need to evolve alternate source of raw materials which are abundantly available as overburden wastes. In such attempts, many workers have utilized solid industrial wastes as alternative source of alumino silicate and fluxing mineral by replacing a part of quartz, clay and feldspar in triaxial porcelain composition (Marghussian and Yekta 1994; Das et al 2000; Kumar et al 2001; Shah and Maiti 2001; Ghosh et al 2002; Basin et al 2003; Dana and Das 2003, 2004; Dana et al 2004; Sarkar et al 2010). Dana et al (2004) substituted a part of quartz by fly ash in triaxial porcelain composition and found higher strength due to better mullitization. Kumar et al (2001) and Shah and Maiti (2001) observed that replacement of 25–30 wt% of kaolinitic clay by fly ash is beneficial as it has close resemblance with clay in their chemistry and due to inherent presence of micro-crystalline components such as quartz and mullite. In another study, the same authors produced anorthite porcelain by replacing part of quartz by fly ash and part of feldspar by blast furnace slag which has several advantageous properties. Tai et al (2002) also reported the presence of anorthite phases using non-plastic raw material. Few workers (Sersale et al 1976; Colella et al 1981; Marghussian and Yekta 1994; Ghosh et al 2002) observed that alkaline earth oxide present in slag favours early maturing of porcelain bodies due to their strong fluxing action. It was reported by Das et al (2000) that iron ore tailing (solid waste generated by iron ore mining industries) with high silica content favours formulation of ceramic tile body composition. In the recent work of Sarkar et al (2010), high strength vitrified ceramic tile has been developed utilizing electric arc furnace slag of iron and steel industry and other highly siliceous clayey minerals.
India is endowed with high quality natural stones like sandstone, marble stone, granite etc. The country produces more than 27% of the total stones produced all over the world and has more than 11% export share in World’s total stone export. This massive industry operates mainly through stone crushing and polishing operation which generates crystalline silica dust and has been linked to chronic lung disease and increased risk of tuberculosis. Moreover, exposure to respirable crystalline silica causes silicosis, which ultimately leads to lung cancer and other chronic renal diseases including rheumatoid arthritis (Gottesfeld et al 2008). Hence, this deadly solid waste has been the subject of great concern to the public and government agencies.
In the present study, an attempt has been made to utilize sand stone dust as one of the inexpensive sources of raw materials as a full replacement of quartz and part replacement of clay in triaxial porcelain body. The stone dust has been characterized with respect to chemical composition, mineral phases, particle size distribution etc. Several batch compositions were formulated using sand stone dust, different types of locally available clays and feldspar. The products developed have been characterized with respect to physico-mechanical properties using standard techniques. XRD and SEM techniques have been used to identify the phases and to study their microstructural features.
2 Experimental
The raw materials used in the present study were sand stone dust generated by one of the Indian stone cutting and polishing industries. Different varieties of clays and feldspar available were collected from Indian sources. Gravimetric method was utilized to determine SiO2 and Al2O3 whereas Fe2O3, CaO and MgO were estimated volumetrically (Hillebrand and Lundell 1953). Na2O and K2O were determined by flame photometry and loss on ignition by usual technique. Phase analysis of stone dust, feldspar and one representative clay were done by X-ray diffraction method (Philips make X-ray diffractometer, model PW 1730) using Cu-K\(_{\upalpha}\) radiation at a scanning speed of 2°/min. Particle size of stone dust and one representative clay have been measured using particle size analyser (Malvern make). Based on the chemical analysis and XRD results, six batch compositions were formulated. The batch compositions are shown in table 1.
1 kg batch of each composition as per table 1 were prepared by the common ceramic processing. The batches were separately pot milled with water for 4 h in a porcelain pot using alumina balls to obtain homogeneous mix and required fineness. The slurry obtained was screened and oven dried at 110 ±10 °C for 24 h. Loosely agglomerated powder was passed through 60 mesh BS sieve and mixed uniformly with 6–8% water and then uniaxially pressed at 350 kg/cm2 specific pressure. Adequate number of bars of dimension 100 ×15 ×5 mm were fabricated and oven dried at 110 ±10°C for 24 h. Few samples were initially fired at 1000°C, however, no strength development was observed. Then the samples were heated at different temperatures in the range of 1050–1150°C with soaking for 2 h. The rate of heating was kept at 3°C/min in all the cases and a total heating schedule of 6 h was maintained. Few tiles of commercial size (200 ×100 mm) and pavement blocks of hexagonal and dumb bell shaped were also fabricated utilizing optimum composition. The heated samples were characterized in terms of physico-mechanical properties along with microstructural analysis. Result reported here is the average of 5 samples with ± 0·5% variation in between the samples. Linear shrinkage of the samples was measured after firing at different temperatures by calculating the difference in length (after and before firing) in terms of percentage. Bulk density and apparent porosity were determined by conventional liquid displacement method using Archimedes’s Principle in water medium. Flexural strength was measured as three-point bending strength using an Universal Testing Machine (Make Instron, Model 5500 R) for the samples having a dimension of 100 ×15×5 mm. The phases formed in vitrified samples were identified using X-ray diffraction method. Microstructural analysis was done on the polished section of fractured surface of the samples in a scanning electron microscope (make Leica model S430i).
3 Results and discussion
3.1 Raw materials and composition
Chemical analysis of raw materials excluding loss on ignition are given in table 2. Stone dust powder sample collected from one source of stone cutting and polishing industry contains 72·7 wt% SiO2, 10·19 wt% Al2O3, 4·1 wt% Fe2O3, 4·5 wt% (K2O + Na2O) and 4·43 wt% alkaline earth oxides (CaO+ MgO). Clays of different varieties are highly siliceous and ferruginous type with higher amount of Fe2O3. Feldspar is normal potash type generally used in porcelain body composition.
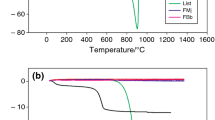
X-ray diffraction pattern of stone dust, one clay sample and feldspar is shown in figure 1. It may be observed that stone dust contains mainly quartz phase. The identification of this phase by XRD is very important as one of the major phase quartz in a triaxial porcelain system is substituted by this stone dust material. Quartz is found as a major phase in clay sample. Hence, stone dust is suitable for partial replacement of quartz and clay also. Feldspar was found as orthoclase (K2O·Al2O3·6SiO2) in the XRD pattern and it is commonly accepted as a fluxing mineral in the triaxial porcelain system. The particle size spectra of stone dust and one typical clay have been given in figures 2 and 3, respectively. Figure 2 depicts d 50 (mean diameter) of sand stone to be of 40 micron and the mean diameter (d 50) of clay sample is 18 microns (figure 3). The overall particle size distribution calculated from the above figures is given in table 3. The results revealed that stone dust generated during cutting and polishing is sufficiently fine and clay is finer than stone dust.
The oxide compositions of each batch excluding loss on ignition are shown in table 4. It may be observed that all the compositions are highly siliceous in nature with considerable amount of alkali, alkaline earth and Fe2O3 content. Such high SiO2 containing body in presence of higher amount of alkali, alkaline earth oxides and Fe2O3 may favour formulation of ceramic tile body as reported in literature (Das et al 2000; Sarkar et al 2010).
3.2 Densification and flexural strength
A general trend of increasing bulk density (BD) with increase in heating temperature up to 1115°C was observed for all the batches beyond which the values fall down slightly which may be due to formation of more glassy phases (figure 4). The increasing trend of densification is due to decrease in apparent porosity on heating. Combined presence of Fe2O3, alkali and alkaline earth minerals promoted the densification at early temperature and full vitrification is obtained at 1115°C. Johnson and Park (1982) and Choudhuri (1974) also observed similar effect of Fe2O3, alkali and alkaline earth oxides towards earlier vitrification of porcelain bodies. Highest density (2·5 g/cc) is achieved with almost nil porosity at 1115°C for all the batches. Similarly, apparent porosity at 1080°C varies (figure 5) between 7 and 25% but at 1115°C it is almost nil for all the batches. Table 5 compared true density and relative density of all the batches heated at different temperatures. Almost 100% relative density is obtained in all the samples heated at 1115°C.
In general, linear shrinkage in such type of composite body is mainly due to sintering within and between the components present and generated in the system. In the present study, the linear shrinkage of all the batches increases up to 1115°C (figure 6) beyond which the value became almost constant. The shrinkage value at the vitrified temperature (1115°C) varies between 10 and 13% which seems to be at higher side than normal porcelain body. This is mainly due to the formation of more glassy phases in the system.
The flexural strength of all the batches increases with increase in heating temperature as expected and very high strength in the range of 70–75 MPa is obtained at a vitrified temperature of 1115°C (figure 7). Beyond this temperature, the strength drastically reduced due to formation of glassy phases at higher temperature which support the observation on decrease in B.D. beyond 1115°C. C3 batch obtained highest strength (approx. 75 MPa) due to their lowest R2O:R′O ratio (R = K, Na & R′ = CaO, MgO) and moderately higher SiO2:Al2O3 ratio. Other batches with slightly higher R2O:R′O ratio possesses slightly lower strength (closer to 70 MPa) than C3 batch. Dana et al (2005) also observed similar effect of fly ash and slag in specific combination on strength development at higher temperature. The superior strength value of such vitrified tiles will be highly useful for application on floor subjected to heavy load.
3.3 Phase and microstructural studies
Samples of C3 batch heated at 1080°C and 1115°C were selected for phase analysis as a typical case. XRD patterns are given in figures 8 and 9, respectively. No major differences have been observed in both XRD patterns. The major phase was found to be quartz and this was due to the presence of silica which was as high as 70–73%. Compared to figure 8 (1080°C heated C3 specimen), the number of quartz peaks and their intensity was found to be lesser in figure 9 (1115°C heated C3 specimen). At the vitrified temperature of 1115°C, a small portion of SiO2 must have dissolved in the glassy matrix.
The microstructural analysis of C3 samples heated at 1080°C and 1115°C are given in figures 10 and 11, respectively. Microstructure of the samples heated at 1080°C (figure 10) reveals very little vitrification and the matrix shows mainly quartz grains loosely bounded but uniformly distributed. Lots of pores are also seen. Figure 11 represents compact microstructure of the samples vitrified at 1115°C. In this case, quartz grains are distributed uniformly in the glassy matrix. Circumferential cracks are also seen in some of the quartz grains due to thermal expansion mismatch between quartz and glassy phases. Such type of cracks are not seen in quartz grain of 1080°C heated sample since glassy matrix is not predominant in this structure. EDX analysis of various phases of the specimens heated at 1115°C are indicated in figure 11. The composition of the glassy phases is not uniform throughout the matrix.
3.4 Prototype development
In order to study the thermal stability and dimensional tolerance of C3 composition, few larger sized prototype samples (tiles of 200 ×100 mm) size and pavement block of hexagonal and dumb bell shape were produced using higher capacity hydraulic press. The common die available in the laboratory was used for this development. The process parameters were kept similar to those used during laboratory experimentation. Few samples were also polished to see the surface appearance. The general product properties obtained after heating at 1115°C are given below. The dimensional tolerance was found to be within specifications and strength value is much superior in comparison to commercially available similar kind of products. The vitrified tiles on polishing show no surface pores and other visible defects.
Major properties | Values in developed samples |
|---|---|
Dimensional tolerance: | ± 0·5% |
Thickness tolerance: | ± 0·5% |
Rectangularity: | ± 0·6% |
Apparent porosity: | < 0·5% |
Flexural strength: | > 600 kg/cm2 |
Moh’s scale hardness: | 7 (minimum) |
Photographs of vitrified pavement blocks and floor tiles produced in the laboratory are given in figure 12a and b, respectively.
4 Conclusions
Partial substitution of quartz and kaolin by sand stone dust in a triaxial porcelain composition was found to be beneficial towards improvement in flexural strength and early vitrification at 1115°C. Presence of considerable amount of alkali and alkaline earth minerals in the sandstone dust and clayey minerals were responsible for early vitrification. Further, use of such overburden industrial byproduct reduces the cost of raw materials and thermal energy without altering the required physico-mechanical properties of ceramic tiles for application in building industries.
References
Basin S, Annitphale S S and Chandra S 2003 Br. Ceram. Trans. 102 83
Carty W M and Senapati U 1998 J. Am. Ceram. Soc. 81 3
Choudhuri S P 1974 Am. Ceram. Soc. Bull. 53 169, 251
Colella C, Mascolo G, Nastra A and Aiello R 1981 La Ceramica 2 12
Dana K and Das S K 2003 J. Mater. Sci. Lett. 12 387
Dana K and Das S K 2004 J. Eur. Ceram. Soc. 24 3833
Dana K, Das S and Das S K 2004 J. Eur. Ceram. Soc. 24 3169
Dana K, Dey J and Das S K 2005 Ceram. Int. 31 147
Das S K, Kumar S and Ramchandrarao P 2000 Waste Management 20 725
Ghosh S, Das M, Chakraborti S and Ghatak S 2002 Ceram. Int. 28 393
Gottesfeld P, Nicas M, Kephart W, Balakrishnan K and Rinehart R 2008 Int. J. Occup. Environ. Health. 14 94
Hamano K, Nakagawa Z and Hasegawa M 1992 J. Ceram. Soc. Jap. 100 1066
Hillebrand W F and Lundell G C E 1953 Applied inorganic analysis (New York: John Wiley & Sons) 2nd ed.
Johnson S M and Park J A 1982 Am. Ceram. Soc. Bull. 61 838
Kingery W D 1976 Introduction of ceramics (New York: Wiley)
Kumar S, Singh K K and Rao P R 2001 J. Mater. Sci. 36 5917
Maity S and Sarkar B K 1996 J. Eur. Ceram. Soc. 16 1083
Marghussian V K and Yekta B E 1994 Br. Ceram. Trans. 93 141
Mattyasovsky L Z 1957 J. Am. Ceram. Soc. 40 299
Sane S C and Cook R L 1951 J. Am. Ceram. Soc. 34 145
Sarkar R, Singh N and Das S K 2010 Bull. Mater. Sci. 33 293
Sersale R, Aiello R, Colella C and Frigione G 1976 Silic. Ind. 12 513
Shah H M and Maiti V N 2001 Trans. Ind. Ceram. Soc. 60 145
Tai W, Kimura K and Jinnai K 2002 J. Eur. Ceram. Soc. 22 463
Acknowledgements
The authors wish to thank XRD and SEM section of this Institute for phase identification and microstructural studies. The authors thankfully acknowledge the financial support received from M/s Diversified Vyapar Private Limited, Jharkhand, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haldar, M.K., Das, S.K. Effect of substitution of sand stone dust for quartz and clay in triaxial porcelain composition. Bull Mater Sci 35, 897–904 (2012). https://doi.org/10.1007/s12034-012-0377-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-012-0377-4