Abstract
Pyroptosis is an inflammation-triggered cell death caused by certain inflammasomes, and long non-coding RNAs (lncRNAs) are related to cell pyroptosis. This study evaluated the mechanism of lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1) on lipopolysaccharide (LPS)-induced trophoblastic cells pyroptosis. HTR-8/Svneo trophoblastic cells were treated with LPS. The expression of lncRNA NEAT1 was decreased using siRNAs, followed by the evaluation of cell proliferation, Caspase-1 activity, levels of Cleaved Caspase-1 and gasdermin D-N, and the concentrations of Interleukin (IL)-1β and IL-18. We found that LPS promoted the pyroptosis of HTR-8/Svneo cells, and lncRNA NEAT1 was highly expressed in LPS-treated HTR-8/Svneo cells while silencing lncRNA NEAT1 inhibited LPS-induced trophoblastic cells pyroptosis. The subcellular localization of lncRNA NEAT1 was detected. Dual-luciferase gene experiment and RNA pull-down assay detected that lncRNA NEAT1 bound to miR-302b-3p and could inhibit miR-302b-3p, and toll-like receptor 4 (TLR4) was the target gene of miR-302b-3p. Then, a joint experiment was designed for detection, which found that miR-302b-3p downregulation partially reversed the inhibition of silencing lncRNA NEAT1 on LPS-induced trophoblastic cells pyroptosis and overexpression of TLR4 annulled the inhibition of silencing lncRNA NEAT1 on LPS-induced trophoblastic cells pyroptosis. Therefore, lncRNA NEAT1 promoted the transcription of TLR4 by competitively binding to miR-302b-3p, thus promoting LPS-induced trophoblastic cells pyroptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The trophoblastic cells, as the epithelial cell of the placenta, plays a primary role in fetal development during pregnancy by coordinating placental functions such as digestion, respiration, and immunity [1]. However, the inflammatory or death of trophoblastic cells can lead to pregnancy complications, such as recurrent miscarriage and preeclampsia (PE) [2, 3]. PE is a severe complication with increasing morbidity and fatality rate [4]. Studies have shown that pyroptosis is an important inflammatory pathway in the placenta and human trophoblastic cells of PE [5]. Pyroptosis, as a distinct death pathway of inflammatory cells, happens in the placenta mainly of the early stage of PE [6], and the pyroptosis of trophoblastic cells has a great impact on the development of PE [5, 7]. The relative study of trophoblastic pyroptosis can offer a new direction for the treatment of PE.
Long non-coding RNAs (lncRNAs) are > 200 nucleotides long [8] and exert their functions through interactions with proteins, RNA, and DNA [9]. A recent study suggested that the upregulation of lncRNA nuclear-enriched abundant transcript 1 (NEAT1) was associated with an increase in pyroptosis [10]. And lncRNA NEAT1 may participate in the development of PE by regulating the proliferation and apoptosis of trophoblastic cells [11, 12]. However, further evaluation is needed.
Competing endogenous RNAs (ceRNAs) are transcripts that can regulate each other at the post-transcription level by competing for shared microRNAs (miRNAs) and ceRNAs networks link the function of protein-coding mRNAs with that of non-coding RNAs such as miRNAs and lncRNAs [13]. In addition, lncRNAs can act as miRNAs “sponges”, and miRNAs can regulate lncRNAs [14]. miRNAs are small non-coding RNAs that regulate gene expression via recognition of cognate sequences and interference of transcriptional, translational, or epigenetic processes [15]. miR-302b-3p has been identified to have a significant role in regulating the activity, apoptosis, and oxidative stress in a variety of cells [16], and miR-302b-3p shows low expression in PE [17]. Nonetheless, the role of miR-302b-3p on the pyroptosis of trophoblastic cells is unclear.
During the activation of congenital and adaptive immune responses, toll-like receptor 4 (TLR4) plays a primary role [18], and the activation of the TLR4 receptor would induce intracellular inflammation, thus leading to pyroptosis [19]. More importantly, TLR4 has profound influences on pregnancy-related complications, such as PE and the death of the fetus [20].
As lipopolysaccharide (LPS) could act on the trophoblast and further affect PE, we decided to introduce LPS to our study [21, 22]. In this study, we focused on the mechanism of lncRNA NEAT1 in regulating the pyroptosis of LPS-induced trophoblastic cells.
Materials and Methods
Cell Culture and Transfection
The trophoblastic cell lines HTR-8/Svneo were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured in RMPI-1640 (Thermo Fisher Scientific, Waltham, MA, USA) medium supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific) and 1% penicillin/streptomycin (Thermo Fisher Scientific) at 37℃ with 5% CO2. Two siRNAs (si-NEAT1-1 and si-NEAT1-2) and their negative control (si-NC), as well as plasmid pcDNA3.1-TLR4 and its negative control pcDNA3.1-NC were all designed and synthesized by GenePharma (Shanghai, China). miR-302b-3p inhibitor and inhibitor NC were provided by Thermo Fisher Scientific. HTR-8/Svneo cells were seeded in 6-well plates (1 × 105 cells/mL). siRNAs with a final concentration of 60 nM, pcDNA 3.1 with a final concentration of 0.8 μg/mL, or miR-302b-3p inhibitor or inhibitor NC with a final concentration of 50 nM were, respectively, transfected into HTR-8/Svneo cells via Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). After 24 h of transfection, the medium was cultured with 200 ng/mL LPS (Sigma-Aldrich, St. Louis, MO, USA) for 48 h [21]. After that, the cells were harvested for the following experiments.
Cell Counting Kit-8 (CCK-8) Assay
The activity of the cells was detected by the CCK-8 assay kit (Dojindo, Kumamoto, Kyushu, Japan), and cells were seeded in 96-well plates at 4 × 103 cells/well and incubated with 5% CO2 at 37℃. CCK-8 solution was added at 0, 24, 48, and 72 h, respectively, and incubated for 1.5 h. Then the absorbance at 450 nm was measured by a microplate reader (Bio-Rad 680, Hercules, CA, USA).
Caspase-1 Activity Detection
The activity of Caspase-1 in HTR-8/Svneo cells was examined using the FAM-FLICCA Caspase-1 assay kit (Imminochemistry Technologies, Bloomington, MN, USA). After several washes with PBS, HTR-8/Svneo cells were treated with 10 μL FLICA reagent and 300 μL DMEM containing 1% FBS for 1 h and then washed several times with PBS. The fluorescence intensity was measured at Ex492/Em520 nm using SpectraMax Gemini EM Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). Final results were compared to the controls and were presented as relative percentages.
Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted from the cells by using Trizol (Takara, Dalian, China) and reversely transcribed into cDNA by using PrimeScript RT Master Mix. RT-qPCR was adopted using SYBR Premix Ex Taq (Takara, Japan) on ABI 7500 fast system (Applied Biosystems, CA, USA). With U6 and GAPDH as internal references, the relative expressions were calculated based on the 2−ΔΔCt method [23]. The primer sequences are shown in Table 1.
Enzyme-Linked Immunosorbent Assay (ELISA)
The concentrations of IL-1β and IL-18 in cell culture supernatants were determined according to the manufacturer's instructions using Human IL-1β ELISA kit (ab214025, Abcam) and Human IL-18 ELISA kit (ab215539, Abcam, Cambridge, UK). The cells were centrifuged at 14,000 g for 15 min to obtain the supernatant. According to the manufacturer's instructions, the absorbance at 450 nm was determined using a microplate reader (Bio-Rad, CA, USA).
Western Blot
Total protein was extracted using cell lysis buffer RIPA (Thermo Fisher). The concentration of protein was measured using bicinchoninic acid kits (Beyotime) which loaded the protein onto a 12% sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), separated by electrophoresis, and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% skim milk for 1 h at ordinary temperature and incubated overnight with primary antibodies at 4℃. The primary antibodies are as follows: Anti-Cleaved Caspase-1 antibody (1:1000, #4199, CST, Cell Signaling Technology, Danvers, MA, USA), Anti-GSDMD antibody (1:1000, ab215203, Abcam), and Anti-β-actin (1:1000, ab8227, Abcam). The membrane was incubated with the secondary antibody (1:2000, ab205718, Abcam) at ordinary temperature for 1 h on the next day, and the blot was tested using Pierce ECL Plus Substrate (Thermo Fisher Scientific). Grayscale values were verified using Image J (National Institutes of Health, Bethesda, Maryland, USA), and β-actin was defined as the internal reference.
Bioinformatics
The binding sites of lncRNA NEAT1 and miR-302b-3p were predicted online by the Starbase website (http://starbase.sysu.edu.cn/) [24]. The downstream target gene of miR-302b-3p is predicted online by Starbase and TargetScan (http://www.targetscan.org/vert_71/) [25].
Nuclear/Cytosol Fractionation Assay
The nuclear and cytoplasmic portions of HTR-8/Svneo cells were separated by using nuclear fractionation buffer (140 mmol/L NaCl, 1.5 mmol/L MgCl2, 10 mmol/L Tris HCl, pH 8.5, 0.5% NP40). The cell particles were re-suspended in the nuclear fractionation buffer and incubated on ice for 5 min, centrifuged at 5000 g for 5 min, and the supernatant was collected, e.g., nuclear precipitate. Nuclear and cytoplasmic RNA were extracted using TRIzol reagent (Invitrogen). The expression of lncRNA NEAT1 in the nucleus and cytoplasm was detected by RT-qPCR, with GAPDH and U6 as controls.
Dual-Luciferase Report Gene Experiment
According to the prediction, the binding sites of lncRNA NEAT1 and miR-302b-3p and miR-302b-3p and TLR4 were attained. A wild-type sequence or a mutant sequence of lncRNA NEAT1 or TLR4 containing the binding site with miR-302b-3p was, respectively, inserted into a pMIR-REPORT plasmid (Thermo Fisher Scientific), and wild-type plasmids lncRNA NEAT1 WT and TLR4 WT and mutant plasmids lncRNA NEAT1 MUT and TLR4 MUT were, respectively, constructed. The above plasmids were co-transfected into HTR-8/Svneo cells with miR-302b-3p mimic or mimic NC. At 48 h after transfection, the cells were collected and lysed and luciferase activity was detected using the dual-luciferase reporter gene analysis system (Promega, Madison, WI, USA) according to the manufacturer's instructions.
RNA Pull-Down
The biotin-labeled miR-302b-3p and the negative control (Sangon Biotech, Shanghai, China) were designated as bio-miR-302b-3p and bio-NC, respectively, and incubated with HTR-8/Svneo cell lysates. Streptavidin-coated beads were then added. The pull-down assay was performed using a biotin-coupled RNA complex (Pierce Biotechnology, USA). The expressions of lncRNA NEAT1 and TLR4 detected by RT-qPCR.
Statistical Analysis
Statistical analysis and mapping of the data were performed using SPSS21.0 statistical software (IBM, Armonk, NY, USA) and GraphPad Prism 8.0 software (GraphPad Software Inc., San Diego, CA, USA). The measurement data were expressed in the form of mean ± standard deviation. First, the normality and homogeneity of variance tests were performed to verify that the data conformed to the normal distribution and the variances were homogeneous. The data between two groups were compared using t test. One-way ANOVA or two-way ANOVA was used for comparisons among multiple groups, and Tukey’s multiple comparisons test was used for the post-hoc test. The p value was obtained from two-sided test and p < 0.05 indicated the difference was statistically significant, and p < 0.01 indicated the difference was highly statistically significant.
Results
LPS-Induced Trophoblastic Cell Pyroptosis and High Expression of lncRNA NEAT1
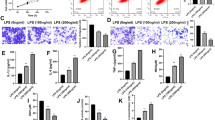
HTR-8/Svneo cells were treated with LPS and it was found that the proliferation of LPS-treated HTR-8/Svneo cells was decreased (p < 0.01, Fig. 1A). Next, the level of cell pyroptosis was detected. After LPS treatment, the activity of Caspase-1 in cells was increased (p < 0.01, Fig. 1B), the expressions of Cleaved Caspase-1 and GSDMD-N were increased (p < 0.01, Fig. 1C), and the concentrations of IL-1β and IL-18 were increased (p < 0.01, Fig. 1D). These results suggested that LPS treatment promoted the pyroptosis of HTR-8/Svneo cells. The upregulation of lncRNA NEAT1 is associated with an increase in pyroptosis in a diabetic nephropathy model, and lncRNA NEAT1 might be involved in the progression of PE by regulating the proliferation and apoptosis of trophoblastic cells [10,11,12]. As determined by RT-qPCR, lncRNA NEAT1 expression in LPS-treated HTR-8/Svneo cells was markedly increased (p < 0.01, Fig. 1E). The above results indicated LPS-induced trophoblastic cell pyroptosis and high expression of lncRNA NEAT1.
LPS-induced trophoblastic cell pyroptosis and high expression of lncRNA NEAT1. HTR-8/Svneo cells induced by LPS were prepared with normal cultured HTR-8/Svneo cells were used as the control. A The proliferation of HTR-8/Svneo cells was detected by the CCK-8 method. B The activity of Caspase-1 in cells was tested by FAM-FLICA Caspase-1 detection. C The protein expressions of Cleaved Caspase-1 and GSDMD-N in the cells were tested by Western blot. D The concentrations of IL-1β and IL-18 in the cells were detected by ELISA. E RT-qPCR was used to detect the expression of lncRNA NEAT1 in the cells. The experiment was repeated 3 times independently. Data were expressed as mean ± standard deviation. Data in panels B and E were examined by t test. Data in panels A and C–D were analyzed by two-way ANOVA and Tukey’s multiple comparisons test was used for the post-hoc test. **p < 0.01
Silencing lncRNA NEAT1 Inhibited LPS-Induced Trophoblastic Cell Pyroptosis
To investigate the regulation of lncRNA NEAT1 on trophoblastic cell pyroptosis, two siRNAs (si-NEAT1-1 and si-NEAT1-2) were designed and transfected into LPS-treated HTR-8/Svneo cells, and the expression of lncRNA NEAT1 in the cells was successfully reduced (p < 0.05, Fig. 2A). si-NEAT1-1, with the best intervention efficiency, was selected for subsequent testing. The results showed that after the expression of lncRNA NEAT1 was inhibited, the proliferation capacity of LPS-treated HTR-8/Svneo cells was increased (p < 0.01, Fig. 2B). After silencing of lncRNA NEAT1, the activity of intracellular Caspase-1 was decreased (p < 0.05, Fig. 2C), the expressions of Cleaved Caspase-1 and GSDMD-N were decreased (p < 0.05, Fig. 2D), and the concentrations of IL-1β and IL-18 were also significantly reduced (p < 0.05, Fig. 2E). The above results indicated that silencing lncRNA NEAT1 could inhibit LPS-induced trophoblastic cell pyroptosis.
Silencing lncRNA NEAT1 inhibited LPS-induced trophoblastic cell pyroptosis. Two NEAT1 siRNAs (si-NEAT1-1 and si-NEAT1-2) were transfected into LPS-treated HTR-8/Svneo cells using transfected NC siRNA (si-NC) as the control. A The expression of lncRNA NEAT1 in HTR-8/Svneo cells was detected by RT-qPCR. si-NEAT1-1 with the best intervention efficiency was selected for subsequent testing. B The cell proliferation capacity was measured by the CCK-8 method. C The activity of Caspase-1 was examined by FAM-FLICA Caspase-1 detection kit. D The protein expressions of Cleaved Caspase-1 and GSDMD-N were tested by Western blot. E The concentrations of IL-1β and IL-18 were detected by ELISA. The experiment was repeated 3 times independently. Data were expressed as mean ± standard deviation. Data in panels A and C were analyzed by one-way ANOVA. Data in panels B and D–E were analyzed by two-way ANOVA and Tukey’s multiple comparisons test was used for the post hoc test. *p < 0.05, **p < 0.01
LncRNA NEAT1 Bound to miR-302b-3p
To further study the mechanism of lncRNA NEAT1 in regulating the pyroptosis of trophoblastic cells, through the results of nuclear fractionation assay, it was found that lncRNA NEAT1 was mainly expressed in the cytoplasm of trophoblastic cells (Fig. 3A). It indicated that lncRNA NEAT1 might regulate the pyroptosis of trophoblastic cells through the ceRNA mechanism. miRNAs downstream of lncRNA NEAT1 were predicted, in which miR-302b-3p was lowly expressed in PE [17]. A binding relationship between lncRNA NEAT1 and miR-302b-3p was found based on the binding site of lncRNA NEAT1 and miR-302b-3p and the dual-luciferase experiment (p < 0.01, Fig. 3B). The RNA pull-down experiment further verified this binding relationship (p < 0.01, Fig. 3C). Next, the expression of miR-302b-3p in each group of cells was detected by RT-qPCR, and the results showed that miR-302b-3p showed low expression in LPS-treated HTR-8/Svneo cells, while after silencing lncRNA NEAT1, the expression of miR-302b-3p was increased (p < 0.01, Fig. 3D). The above results indicated that lncRNA NEAT1 could bind to miR-302b-3p and inhibit the expression of miR-302b-3p.
LncRNA NEAT1 bound to miR-302b-3p. A The subcellular localization of lncRNA NEAT1 was verified by the nuclear/cytosol fractionation assay. B, C The binding relationship between lncRNA NEAT1 and miR-302b-3p was tested by dual-luciferase gene assay and RNA pull-down assay. D The expression of miR-302b-3p in HTR-8/Svneo of each group was examined by RT-qPCR. The experiment was repeated 3 times independently. Data were expressed as mean ± standard deviation. Data in panel C were examined by t test. Data in panel D were analyzed by one-way ANOVA. Data in panel B were analyzed by two-way ANOVA and Tukey’s multiple comparisons test was used for the post hoc test. **p < 0.01
miR-302b-3p Downregulation Alleviated the Inhibitory Effect of Silencing lncRNA NEAT1 on LPS-Induced Trophoblastic Cell Pyroptosis
To verify the role of miR-302b-3p in LPS-induced trophoblastic cell pyroptosis which was regulated by lncRNA NEAT1, miR-302b-3p inhibitor (inhibitor) was transfected into HTR-8/Svneo cells and it successfully reduced the expression of miR-302b-3p in cells (p < 0.01, Fig. 4A), and then a joint experiment with si-NEAT1 was conducted. The results showed that compared with lncRNA NEAT1 silencing alone, the proliferation ability of HTR-8/Svneo cells in the combination group was decreased (p < 0.01, Fig. 4B). In addition, the degree of cell pyroptosis was detected, and the results showed that after the combination treatment with silencing lncRNA NEAT1 and miR-302b-3p downregulation, the intracellular Caspase-1 activity was distinctly increased (p < 0.05, Fig. 4C). The expressions of Cleaved Caspase-1 and GSDMD-N were notably increased (p < 0.05, Fig. 4D), and the concentrations of IL-1β and IL-18 were increased (p < 0.05, Fig. 4E). These results indicated that miR-302b-3p downregulation weakened the inhibitory effect of silencing lncRNA NEAT1 on LPS-induced trophoblastic cell pyroptosis.
miR-302b-3p downregulation alleviated the inhibitory effect of silencing lncRNA NEAT1 on LPS-induced trophoblastic cell pyroptosis. miR-302b-3p inhibitor (inhibitor) was transfected into HTR-8/Svneo cells, with inhibitor NC as the control. A The expression of miR-302b-3p was detected by RT-qPCR, and the LPS-induced HTR-8/Svneo cells were treated with miR-302b-3p inhibitor and si-NEAT1. B The cell proliferation capacity was measured by the CCK-8 method. C The activity of Caspase-1 was tested by FAM-FLICA Caspase-1 detection kit. D The protein expressions of Cleaved Caspase-1 and GSDMD-N were examined by Western blot. E The concentrations of IL-1β and IL-18 in the cells were detected by ELISA. The cell experiment was repeated 3 times independently. Data were expressed as mean ± standard deviation. Data in panels A and C were analyzed by one-way ANOVA. Data in panels B and D–E were analyzed by two-way ANOVA and Tukey's multiple comparisons test was used for the post hoc test. *p < 0.05, **p < 0.01
miR-302b-3p-Targeted TLR4
To explore the downstream mechanism of miR-302b-3p, the downstream target genes of miR-302b-3p were predicted via the Starbase and TargetScan databases, and the intersection was taken (Fig. 5A). TLR4 is associated with cell pyroptosis and is highly expressed in PE [18, 19, 21, 22]. Through the dual-luciferase experiment and RNA pull-down assay, it was found that TLR4 was the target gene of miR-302b-3p (p < 0.01, Fig. 5B-C). In addition, RT-qPCR was adopted to verify the mRNA level of TLR4 in each group of cells, and it was found that TLR4 was highly expressed in LPS-treated HTR-8/Svneo cells. Silencing lncRNA NEAT1 inhibited the transcription of TLR4, while miR-302b-3p downregulation could reduce the inhibition of silencing lncRNA NEAT1 on the transcription of TLR4 (p < 0.05, Fig. 5D). The above results indicated that TLR4 was a target gene of miR-302b-3p.
miR-302b-3p-targeted TLR4. A The downstream target gene of miR-302b-3p was predicted Starbase and TargetScan databases (http://www.targetscan.org/vert_71/), and the intersection was taken. B, C The binding relationship between miR-302b-3p and TLR4 was verified by the dual-luciferase experiment and an RNA pull-down experiment. D mRNA level of TLR4 in HTR-8/Svneo of each group was detected by RT-qPCR. The experiment was repeated 3 times independently. Data were expressed as mean ± standard deviation. Data in panel C were examined by t test. Data in panel D were analyzed by one-way ANOVA. Data in panel B were analyzed by two-way ANOVA and Tukey’s multiple comparisons test was used for the post hoc test. *p < 0.05, **p < 0.01
Overexpression of TLR4 Alleviated the Inhibitory Effect of Silencing lncRNA NEAT1 on LPS-Induced Trophoblastic Pyroptosis
To verify the role of TLR4 in LPS-induced trophoblastic cell pyroptosis which was regulated by lncRNA NEAT1, a joint experiment was designed. First, pcDNA3.1-TLR4 was transfected into HTR-8/Svneo cells and the mRNA level of TLR4 in the cells was successfully upregulated (p < 0.01, Fig. 6A), and then a joint experiment with si-NEAT1 was conducted. The results showed that after the overexpression of TRL4, the LPS-induced cell proliferation was significantly downregulated (p < 0.05, Fig. 6B). In addition, compared with the lncRNA NEAT1 silencing alone, the activity of Caspase-1 in cells of the combination group was increased (p < 0.05, Fig. 6C), the protein expressions of Cleaved Caspase-1 and GSDMD-N were increased (p < 0.05, Fig. 6D), and the concentrations of IL-1β and IL-18 were markedly increased (p < 0.01, Fig. 6E). The results indicated that lncRNA NEAT1 upregulated TLR4 transcription by competitively binding to miR-302b-3p and further promoted LPS-induced trophoblastic cell pyroptosis.
Overexpression of TLR4 alleviated the inhibitory effect of silencing lncRNA NEAT1 on LPS-induced trophoblastic pyroptosis. pcDNA3.1-TLR4 (pc-TLR4) was transfected into HTR-8/Svneo cells using pcDNA3.1-NC (pc-NC) as a control. A mRNA level of TLR4 was detected by RT-qPCR, and then combined with si-NEAT1 to conduct a joint experiment. B The cell proliferation ability was verified by the CCK-8 method. C The activity of Caspase-1 in HTR-8/Svneo cells was detected by FAM-FLICA Caspase-1 detection kit. D The protein expression of Cleave Caspase-1 and GSDMD-N in cells was detected by western blot. E The concentration of IL-1β and IL-18 in cells was examined by ELISA. The experiment was repeated 3 times independently. Data were expressed as mean ± standard deviation. Data in panels A and C were analyzed by one-way ANOVA. Data in panels B and D–E were analyzed by Two-way ANOVA. Data in panel B were analyzed by two-way ANOVA and Tukey’s multiple comparisons test was used for the post-hoc test. *p < 0.05, **p < 0.01
Discussion
Trophoblastic cells, as the functional epithelial cells of the placenta, have distinct functions for placentation [26, 27]. The abnormity of trophoblastic cells, such as pyroptosis would cause the PE [2]. Pyroptosis is defined as a cell death that is featured by programmed processes and is dependent on the caspases and protein family of gasdermin [28], and it is also a pivotal pathway of inflammation mainly in the placenta of an early stage of PE and human trophoblasts that subject to hypoxia as well as endoplasmic reticulum stressors [5]. LncRNA NEAT1 has been proved to be related to pyroptosis [29]. In this study, the effect of lncRNA NEAT1 on LPS-induced pyroptosis of trophoblastic cells was investigated to provide new theoretical knowledge for the treatment of PE.
A previous study has shown that Caspase-11/4/5 would trigger pyroptosis upon recognizing intracellular LPS [30]. Hence, HTR-8/Svneo cells were treated with LPS to verify the function of LPS on trophoblastic cell pyroptosis. We found that the cell proliferation was decreased, the activity of Caspase-1 was increased, the expressions of Cleaved Caspase-1 and GSDMD-N were increased, and the concentrations of IL-1β and IL-18 were increased, indicating the pyroptosis in HTR-8/Svneo cells. A study showed that LPS could lead to the activity of Caspase-1 and pyroptosis with an increase of the levels of IL-1β and IL-18, and an increase of the activity of Caspase-1 [31], which were consistent with our test results. The above demonstrated that LPS could induce the pyroptosis of trophoblastic cells. Previous studies showed that lncRNAs were involved in the process of pyroptosis, such as lncRNA H19, lncRNA KLF3 AS1, and lncRNA MEG3 [32,33,34]. Subsequently, RT-qPCR was adopted to examine the expression of lncRNA NEAT1, which showed that lncRNA NEAT1 was highly expressed in LPS-treated HTR-8/Svneo cells. LncRNA NEAT1 involves in the progression of PE by regulating the proliferation and apoptosis of trophoblastic cells [10,11,12]. In short, LPS induced trophoblastic cell pyroptosis and lncRNA NEAT1 was highly expressed in LPS-treated HTR-8/Svneo cells.
To investigate the roles of lncRNA NEAT1 in trophoblastic cell pyroptosis, siRNAs were transfected into LPS-induced HTR-8/Svneo cells to decrease the expression of lncRNA NEAT1. The maturation and secretion of Caspase-1, IL-1β, and IL-18 would initiate pyroptosis, and the decrease of Cleaved-Caspase-1, GSDMD, and GSDMD-N could alleviate pyroptosis [35, 36]. As the results of our experiments showed, silencing lncRNA NEAT1 could inhibit LPS-induced trophoblastic cell pyroptosis with the Caspase-1 activity, the expression of Cleaved Caspase-1, and GSDMD-N decreased, and the concentrations of IL-1β and IL-18 declined. Literature has shown that the upregulation of lncRNA NEAT1 was involved in the increase of pyroptosis in a diabetic nephropathy model and a model of ischemia/reperfusion [10, 37]. But the regulation of lncRNA NEAT1 in trophoblastic cell pyroptosis is not studied yet. Overall, we first concluded that silencing lncRNA NEAT1 promoted the proliferation of LPS-induced HTR-8/Svneo cells and limited LPS-induced trophoblastic cell pyroptosis.
LncRNAs could function by acting as molecular sponges of miRNAs to regulate gene expression and cell growth [38, 39]. Through the nuclear/cytosol fractionation assay, we found that lncRNA NEAT1 was mainly expressed in the cytoplasm of trophoblastic cells. Then we predicted the miRNA downstream of lncRNA NEAT1, among which we focused on miR-302b-3p. miR-302b-3p was lowly expressed in PE [17]. Through dual-luciferase assay and RNA pull-down assay, we identified that lncRNA NEAT1 bound to miR-302b-3p. RT-qPCR revealed that miR-302b-3p was lowly expressed in LPS-induced HTR-8/Svneo cells, while after silencing lncRNA NEAT1, miR-302b-3p was increased. Briefly, lncRNA NEAT1 targeted miR-302b-3p.
miRNAs have been identified to be involved in the cell pyroptosis mechanism [40,41,42]. Hence, to further verify the effect of miR-302b-3p on LPS-induced trophoblastic cell pyroptosis, we transfected HTR-8/Svneo cells with miR-302b-3p inhibitor and conducted a joint experiment with si-NEAT1. The results showed that after inhibiting miR-302b-3p, the proliferation of HTR-8/Svneo cells was inhibited, the activity of Caspase-1, and the expressions of Cleaved Caspase-1 and GSDMD-N as well as the concentrations of IL-1β and IL-18 were increased. There is no report on the regulation of miR-302b-3p on HTR-8/Svneo cells or pyroptosis. Our results firstly suggested that miR-302b-3p downregulation reduced the inhibitory effect of silencing lncRNA NEAT1 on LPS-induced trophoblastic cell pyroptosis.
Then through website prediction, dual-luciferase reporter gene assay, RNA pull-down, and RT-qPCR assay, we found TLR4 was highly expressed in the LPS-treated HTR-8/Svneo cells, and silencing lncRNA NEAT1 inhibited the transcription of TLR4 while miR-302b-3p downregulation elevated the transcription of TLR4. Hence, we confirmed that TLR4 was a target gene of miR-302b-3p. Furthermore, we designed a joint experiment to test the effect of TLR4 on LPS-induced trophoblastic cell pyroptosis. After overexpressing TRL4, the cell proliferation of LPS-induced was greatly downregulated and Caspase-1 activity was increased, Cleaved Caspase-1 and GSDMD-N were also increased, while IL-1β and IL-18 were increased. TLR4 can induce GSDMD-mediated pyroptosis [18, 19, 43] and was related to LPS-induced and PE-like symptoms by damaging trophoblast invasion [21], and was highly expressed in PE (22). In conclusion, miR-302b-3p targeted TLR4, and overexpression of TLR4 alleviated the inhibitory effect of silencing lncRNA NEAT1 on LPS-induced trophoblastic cell pyroptosis.
This study highlighted that lncRNA NEAT1 promoted the transcription of TLR4 by competitively binding to miR-302b-3p, thus promoting LPS-induced trophoblastic cell pyroptosis, which may provide a promising target for PE and other obstetrical and gynecologic related diseases. However, this study still has its limitations. There is a variety of downstream miRNAs and target genes of lncRNA NEAT1, but we only studied the lncRNA NEAT1-miR-302b-3p-TRL4 axis. Besides, this study only involved cell pyroptosis, and the effects of lncRNA NEAT1 on trophoblastic cell apoptosis and iron death need further exploration. Only the transcriptional level of TLR4 was studied in our study, and further research is warranted to explore the effects of TLR4 protein level on trophoblastic cell pyroptosis. And, we did not separately observe the effect of the miR-302b-3p inhibitor. In future studies, we will investigate the effects of TLR4 protein level and other regulatory mechanisms of lncRNA NRAT1 on trophoblast cell pyroptosis. Meanwhile, we will conduct an individual intervention with miR-302b-3p inhibitor to study the role of miR-302b-3p in LPS-induced trophoblast pyroptosis more specific.
Data Availability
The data that support this study are available from the corresponding author upon reasonable request.
References
Staud, F., & Karahoda, R. (2018). Trophoblast: The central unit of fetal growth, protection and programming. International Journal of Biochemistry and Cell Biology, 105, 35–40.
Rampersad, R., & Nelson, D. M. (2007). Trophoblast biology, responses to hypoxia and placental dysfunction in preeclampsia. Frontiers in Bioscience, 12, 2447–2456.
Horii, M., Touma, O., Bui, T., & Parast, M. M. (2020). Modeling human trophoblast, the placental epithelium at the maternal fetal interface. Reproduction, 160, R1–R11.
Tao, J., Xia, L. Z., Liang, L., Chen, Y., Wei, D., Meng, J., Wu, S., & Wang, Z. (2020). MiR-124-3p promotes trophoblast cell HTR-8/SVneo pyroptosis by targeting placental growth factor. Placenta, 101, 176–184.
Cheng, S. B., Nakashima, A., Huber, W. J., Davis, S., Banerjee, S., Huang, Z., Saito, S., Sadovsky, Y., & Sharma, S. (2019). Pyroptosis is a critical inflammatory pathway in the placenta from early onset preeclampsia and in human trophoblasts exposed to hypoxia and endoplasmic reticulum stressors. Cell Death and Disease, 10, 927.
Yu, S. Y., & Li, X. L. (2021). Pyroptosis and inflammasomes in obstetrical and gynecological diseases. Gynecological Endocrinology, 37, 385–391.
Quan, X. Z., Ye, J. H., Yang, X. Z., & Xie, Y. (2021). HOXA9-induced chemerin signals through CMKLR1/AMPK/TXNIP/NLRP3 pathway to induce pyroptosis of trophoblasts and aggravate preeclampsia. Experimental Cell Research, 408, 112802.
Zhang, S., Duan, J., Du, Y., Xie, J., Zhang, H., Li, C., & Zhang, W. (2021). Long non-coding RNA signatures associated with liver aging in senescence-accelerated mouse prone 8 model. Frontiers in Cell and Developmental Biology, 9, 698442.
Robinson, E. K., Covarrubias, S., & Carpenter, S. (2020). The how and why of lncRNA function: An innate immune perspective. Biochimica et Biophysica Acta (BBA)-Gene Regulatory Mechanisms, 1863, 194419.
Zhan, J. F., Huang, H. W., Huang, C., Hu, L. L., & Xu, W. W. (2020). Long non-coding RNA NEAT1 regulates pyroptosis in diabetic nephropathy via mediating the miR-34c/NLRP3 axis. Kidney & Blood Pressure Research, 45, 589–602.
Xufei, F., Xiujuan, Z., Jianyi, L., Liyan, Y., Ting, Y., & Min, H. (2020). Up-regulation of LncRNA NEAT1 induces apoptosis of human placental trophoblasts. Free Radical Research, 54, 678–686.
Teng, L., Liu, P., Song, X., Wang, H., Sun, J., & Yin, Z. (2020). Long non-coding RNA nuclear-enriched abundant transcript 1 (NEAT1) represses proliferation of trophoblast cells in rats with preeclampsia via the microRNA-373/FLT1 axis. Medical Science Monitor, 26, e927305.
Qi, X., Zhang, D. H., Wu, N., Xiao, J. H., Wang, X., & Ma, W. (2015). ceRNA in cancer: Possible functions and clinical implications. Journal of Medical Genetics, 52, 710–718.
Chen, L., Zhou, Y., & Li, H. (2018). LncRNA, miRNA and lncRNA-miRNA interaction in viral infection. Virus Research, 257, 25–32.
Chen, L., Heikkinen, L., Wang, C., Yang, Y., Sun, H., & Wong, G. (2019). Trends in the development of miRNA bioinformatics tools. Briefings in Bioinformatics, 20, 1836–1852.
Zhang, Z., Wang, N., Zhang, Y., Zhao, J., & Lv, J. (2019). Downregulation of microRNA-302b-3p relieves oxygen-glucose deprivation/re-oxygenation induced injury in murine hippocampal neurons through up-regulating Nrf2 signaling by targeting fibroblast growth factor 15/19. Chemico-Biological Interactions, 309, 108705.
Akgor, U., Ayaz, L., & Cayan, F. (2021). Expression levels of maternal plasma microRNAs in preeclamptic pregnancies. Journal of Obstetrics and Gynaecology, 41, 910–914.
Wang, Y., Zhu, X., Yuan, S., Wen, S., Liu, X., Wang, C., Qu, Z., Li, J., Liu, H., Sun, L., & Liu, F. (2019). TLR4/NF-kappaB signaling induces GSDMD-related pyroptosis in tubular cells in diabetic kidney disease. Front Endocrinol (Lausanne), 10, 603.
Tavakoli Dargani, Z., & Singla, D. K. (2019). Embryonic stem cell-derived exosomes inhibit doxorubicin-induced TLR4-NLRP3-mediated cell death-pyroptosis. American Journal of Physiology-Heart and Circulatory Physiology, 317, H460–H471.
Firmal, P., Shah, V. K., & Chattopadhyay, S. (2020). Insight into TLR4-mediated immunomodulation in normal pregnancy and related disorders. Frontiers in Immunology, 11, 807.
Fan, M., Li, X., Gao, X., Dong, L., Xin, G., Chen, L., Qiu, J., & Xu, Y. (2019). LPS induces preeclampsia-like phenotype in rats and HTR8/SVneo cells dysfunction through TLR4/p38 MAPK pathway. Frontiers in Physiology, 10, 1030.
Xue, P., Fan, W., Diao, Z., Li, Y., Kong, C., Dai, X., Peng, Y., Chen, L., Wang, H., Hu, Y., & Hu, Z. (2020). Up-regulation of PTEN via LPS/AP-1/NF-kappaB pathway inhibits trophoblast invasion contributing to preeclampsia. Molecular Immunology, 118, 182–190.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods, 25, 402–408.
Li, J. H., Liu, S., Zhou, H., Qu, L. H., & Yang, J. H. (2014). starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Research, 42, D92-97.
Agarwal, V., Bell, G. W., Nam, J. W., & Bartel, D. P. (2015). Predicting effective microRNA target sites in mammalian mRNAs. Elife. https://doi.org/10.7554/eLife.05005
Baines, K. J., & Renaud, S. J. (2017). Transcription factors that regulate trophoblast development and function. Progress in Molecular Biology and Translational Science, 145, 39–88.
Gamage, T., Schierding, W., Hurley, D., Tsai, P., Ludgate, J. L., Bhoothpur, C., Chamley, L. W., Weeks, R. J., Macaulay, E. C., & James, J. L. (2018). The role of DNA methylation in human trophoblast differentiation. Epigenetics, 13, 1154–1173.
Wu, D., Wei, C., Li, Y., Yang, X., & Zhou, S. (2021). Pyroptosis, a new breakthrough in cancer treatment. Frontiers in Oncology, 11, 698811.
Zhang, P., Cao, L., Zhou, R., Yang, X., & Wu, M. (2019). The lncRNA Neat1 promotes activation of inflammasomes in macrophages. Nature Communications, 10, 1495.
Shi, J., Gao, W., & Shao, F. (2017). Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends in Biochemical Sciences, 42, 245–254.
Qiu, Z., He, Y., Ming, H., Lei, S., Leng, Y., & Xia, Z. Y. (2019). Lipopolysaccharide (LPS) aggravates high glucose- and hypoxia/reoxygenation-induced Injury through activating ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2 cardiomyocytes. Journal of Diabetes Research, 2019, 8151836.
Liang, J., Wang, Q., Li, J. Q., Guo, T., & Yu, D. (2020). Long non-coding RNA MEG3 promotes cerebral ischemia-reperfusion injury through increasing pyroptosis by targeting miR-485/AIM2 axis. Experimental Neurology, 325, 113139.
Mao, Q., Liang, X. L., Zhang, C. L., Pang, Y. H., & Lu, Y. X. (2019). LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138-5p/Sirt1 axis. Stem Cell Research and Therapy, 10, 393.
Wan, P., Su, W., Zhang, Y., Li, Z., Deng, C., Li, J., Jiang, N., Huang, S., Long, E., & Zhuo, Y. (2020). LncRNA H19 initiates microglial pyroptosis and neuronal death in retinal ischemia/reperfusion injury. Cell Death and Differentiation, 27, 176–191.
Xu, X. E., Liu, L., Wang, Y. C., Wang, C. T., Zheng, Q., Liu, Q. X., Li, Z. F., Bai, X. J., & Liu, X. H. (2019). Caspase-1 inhibitor exerts brain-protective effects against sepsis-associated encephalopathy and cognitive impairments in a mouse model of sepsis. Brain, Behavior, and Immunity, 80, 859–870.
Li, N., Zhou, H., Wu, H., Wu, Q., Duan, M., Deng, W., & Tang, Q. (2019). STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biology, 24, 101215.
Zhang, H. S., Ouyang, B., Ji, X. Y., & Liu, M. F. (2021). Gastrodin alleviates cerebral ischaemia/reperfusion injury by inhibiting pyroptosis by regulating the lncRNA NEAT1/miR-22-3p axis. Neurochemical Research, 46, 1747–1758.
Zhao, Z., Sun, W., Guo, Z., Zhang, J., Yu, H., & Liu, B. (2020). Mechanisms of lncRNA/microRNA interactions in angiogenesis. Life Sciences, 254, 116900.
Paraskevopoulou, M. D., & Hatzigeorgiou, A. G. (2016). Analyzing MiRNA-LncRNA interactions. Methods in Molecular Biology, 1402, 271–286.
Yang, F., Qin, Y., Wang, Y., Li, A., Lv, J., Sun, X., Che, H., Han, T., Meng, S., Bai, Y., & Wang, L. (2018). LncRNA KCNQ1OT1 mediates pyroptosis in diabetic cardiomyopathy. Cellular Physiology and Biochemistry, 50, 1230–1244.
Li, X., Zeng, L., Cao, C., Lu, C., Lian, W., Han, J., Zhang, X., Zhang, J., Tang, T., & Li, M. (2017). Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Experimental Cell Research, 350, 327–335.
Song, Y., Yang, L., Guo, R., Lu, N., Shi, Y., & Wang, X. (2019). Long noncoding RNA MALAT1 promotes high glucose-induced human endothelial cells pyroptosis by affecting NLRP3 expression through competitively binding miR-22. Biochemical and Biophysical Research Communications, 509, 359–366.
Zhang, Y. H., Aldo, P., You, Y., Ding, J., Kaislasuo, J., Petersen, J. F., Lokkegaard, E., Peng, G., Paidas, M. J., Simpson, S., Pal, L., Guller, S., Liu, H., Liao, A. H., & Mor, G. (2020). Trophoblast-secreted soluble-PD-L1 modulates macrophage polarization and function. Journal of Leukocyte Biology, 108, 983–998.
Acknowledgements
We thank all of members in our team for the excellent work.
Funding
This work was supported by Research Project of Maternal and Child Health in Jiangsu Province under Grant Number F201944.
Author information
Authors and Affiliations
Contributions
Conceptualization: DF, YJ, and CZ; data curation: DF and Yu Pan; methodology: DF, YJ, and SZ; validation: CZ and Yu Pan; writing—original draft: DF and YJ; writing—review and editing: CZ and SZ.
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm they have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, D., Ju, Y., Zhu, C. et al. LncRNA NEAT1 Promotes TLR4 Expression to Regulate Lipopolysaccharide-Induced Trophoblastic Cell Pyroptosis as a Molecular Sponge of miR-302b-3p. Mol Biotechnol 64, 670–680 (2022). https://doi.org/10.1007/s12033-021-00436-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00436-2