Abstract
The cholesterol hydroxylase/lyase (CHL) system, located in the mitochondria of the mammalian adrenal cortex cells, consists of cytochrome P450scc (CYP11A1), adrenodoxin (Adx), and adrenodoxin reductase (AdR) and performs the first stage of the steroidogenesis: AdR and Adx enable the electron transfer between NADPH and cytochrome P450scc, and P450scc catalyzes the conversion of cholesterol into pregnenolone. CHL system was reconstructed in Escherichia coli using the polycistronic plasmid pTrc99A/CHL. In E. coli cells, the recombinant proteins form the catalytically active system. CHL activity towards 22R-hydroxycholesterol was 4.0 ± 1.3 nmol pregnenolone/h per 1 mg homogenate protein. The alteration of the order of heterologous cDNAs in the expression cassette from AdR–Adx–P450scc to P450scc–Adx–AdR results in alteration of stoichiometric ratio P450scc/Adx/AdR from 1:1.45:4.2 to 1:1.67:0.98; the former ratio is more optimal for the functioning of the cytochrome P450scc. The application of modified cDNA of Adx (AdxS112W) does not increase the CHL activity; however, the introduction of the second copy of AdxS112W gene into the expression cassette increases both the expression level of АdxS112W and the CHL activity in comparison with P450scc/АdxS112W/AdR system. In vivo activity of the CHL system in bacteria is limited by the substrate uptake by bacterial cells: it varied in the range of 0.05–0.62 mg pregnenolone/l resting cell suspension per 1-day cultivation, depending on the type and concentration of permeabilizing agents in the medium. The obtained results contribute to the knowledge of CHL system functioning in living bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytochromes P450 (CYP) form a superfamily of heme-containing external monooxygenases. They were discovered in almost all species of living organisms and are involved in a variety of biosynthetic and metabolic processes, providing for the hydroxylation of different hydrophobic compounds. Particularly in mammals, cytochromes P450 provide the synthesis and conversion of steroids and bile acids and play a key role in the detoxification of poisons and biotransformation of drugs. Most of the cytochromes P450 function as a part of the enzymatic systems, which also include proteins that transfer redox equivalents from the NADPH donor to terminal oxidase, cytochrome P450 [1].
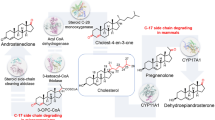
Six cytochromes P450 systems (mitochondrial cytochromes CYP11A1 (P450scc), CYP11B1 and CYP11B2, with their natural partner proteins [2Fe–2S] ferredoxin, called adrenodoxin (Adx), and NADPH-dependent FAD containing reductase, adrenodoxin reductase (AdR); and microsomal cytochromes CYP17, CYP21, and CYP19, functioning in conjunction with NADPH-cytochrome P450 reductase), 3β-hydroxysteroid dehydrogenase/Δ5,4 isomerase, and 17β-hydroxysteroid dehydrogenase are involved in the process of steroidogenesis in mammals [2]. Cytochrome P450scc (CYP11A1) catalyzes the initial reactions in the cascade of cholesterol conversion into steroid hormones, resulting in the cleavage of the side chain of the cholesterol molecule and the formation of pregnenolone, a common precursor of glucocorticoids, mineral corticoids, and sex hormones. Cytochrome P450scc along with physiological redox partners AdR and Adx form a cholesterol hydroxylase/lyase (CHL) system.
Studies of mammalian P450-monooxygenase systems using the cellular models of heterologous proteins expression on the base of microorganisms have become more common. Such transgenic microorganisms are of great interest for medical research (as many cytochromes P450 are involved in the disease progression, such as in the development of cancer) and biotechnology (particularly, for the synthesis of steroid drugs) [3]. Bacteria E. coli and yeast Saccharomyces cerevisiae are widely used as the host microorganisms for the expression of P450 proteins. The advantage of E. coli, in comparison with yeast cells, is the absence of endogenous cytochromes P450, which enables an opportunity to study the recombinant P450 in the absence of a background and reduces the probability of by-product formation [4]. Other advantages include a faster culture growth, a higher expression level, and catalytic activity of P450 proteins [5]. Moreover, mature forms of cytochromes P450 (particularly, mitochondrial and microsomal steroidogenic P450 [6, 7]) can be synthesized in these cells instead of precursor proteins, leading to the reduction of the number of steps between the synthesis of the protein and the formation of its catalytically active form.
The first reports on the expression of functionally active steroidogenic cytochromes P450 in E. coli were published more than 15 years ago [6, 8,9,10,11]. It was discovered that the proteins of E. coli electron transport systems [flavodoxin (Fld), flavodoxin reductase (Fpr), and sulfite reductase (SiR-FP)] can carry electrons to the heme iron of a number of mammalian cytochromes P450 [3, 12], and this feature allows for the functional reconstruction of P450 systems using the carrier proteins present in bacterial cells. However, for several cytochromes P450, it was shown that the interaction of P450 with the proteins of E. coli electron transport systems is less effective than the interaction with natural cytochrome partner proteins [e.g., 13–15]. For instance, the progesterone metabolism rate of the human cytochrome CYP17 expressed in E. coli in vivo is ~ 100 times lower in comparison with the co-expression of CYP17 with its partner protein NADPH-P450 reductase in E. coli cells [14].
The reconstruction of steroidogenic CYP17- and P450scc-systems was performed via the expression of the cDNA encoding the two-component (CYP17-NADPH-reductase) and the three-component (P450scc–AdR–Adx) fusion proteins, respectively [13, 16, 17]. E. coli cells with highly active CYP17-monooxygenase system also were obtained using the bicistronic expression method [14]. We made the first successful attempt to co-express the components of a mitochondrial electron transport chain, bovine cytochrome P450scc, and human AdR and Adx, in E. coli cells using a tricistronic plasmid [18], and then used tricistronic plasmid for reconstruction of the homologous bovine CHL system [19]. In recent years, polycistronic constructions have been used to obtain recombinant strains of E. coli, containing active steroidogenic systems of cytochromes P450C21 [20], CYP11B1 [21], and CYP11B2 [22]. Based on the data gathered [13, 19], the in vivo activity of fused CYP17- and P450scc-systems is significantly lower than the activity of these systems reconstructed in E. coli cells by the co-expression of individual proteins. The efficiency of progesterone metabolism during co-expression of the bovine cytochrome CYP17 and rat NADPH-P450 reductase (50 nmol of hydroxylated steroid/min/nmol of P450) was 3.3 times higher than the efficiency in the case of CYP17-NADPH-reductase fusion protein expression [13]. Co-expression of individual P450scc-system proteins (bovine P450scc and human AdR and Adx) resulted in the cholesterol biotransformation efficiency 30–60 µg of pregnenolone/l of cell culture per 72 h [19]. Concerning P450scc–AdR–Adx fusion protein expression, the efficiency of the reaction was recorded at the limit of detection [19], due to the effective proteolysis of the P450scc–AdR–Adx protein in E. coli cells and steric hindrances for the interaction of catalytic domains [17].
It should be noted that P450 steroidogenic systems, reconstructed in cells of microorganisms, usually demonstrate a low catalytic activity. Possible reasons for this include the low levels of heterologous protein expression, the lack of the necessary cofactors in the cells, low protein ratios of the electron transfer partners to CYP, and the limitations of the transfer of exogenous substrate into the cells. Optimization of these biotechnologically interesting P450 systems functioning towards a higher activity is a difficult task. The optimization approaches include both the modification of the expressed proteins through site-directed mutagenesis and the change of their expression conditions in the whole cell. In previous works [18, 19], we have demonstrated that the efficiency of cholesterol to pregnenolone conversion in bacterial strain containing the bovine CHL system (0.42 ± 0.015 mg pregnenolone/l per 24 h) was almost an order of magnitude higher than the efficiency recorded in the cells expressing P450scc and Adx and AdR from different organisms; nevertheless, it was very low. However, a detailed study of the functioning of heterologous CHL system proteins in living microorganisms has not yet been accomplished.
This work was aimed at searching for the recombinant E. coli cells and cultivation conditions that can provide the most effective biotransformation of cholesterol by bovine CHL system reconstructed in the cells.
Materials and Methods
Materials
Isopropyl-β-d-thiogalactopyranoside (IPTG), δ-aminolevulinic acid (ALA), diaminobenzidine tetrahydrochloride hydrate (DAB), 22R-hydroxycholesterol, progesterone (pregn-4-ene-3,20-dione), cholesterol oxidase, and horseradish peroxidase-conjugated anti-rabbit antibodies were supplied by Sigma-Aldrich (USA). Clarity™ Western ECL blotting substrate was produced by Bio-Rad (USA). Statistically methylated β-cyclodextrin (MCD) was obtained from Wacker Chemie (Germany). Nitrocellulose filters Hybond-C extra were obtained from Amersham Biosciences (UK). Growth media (LB, TB [23]) were prepared using reagents from Difco (USA). DNA-modifying enzymes, Pfu DNA polymerase, RNAse A, and DNA Extraction Kit were purchased from Thermo Fisher Scientific (USA). Oligonucleotides used in the study were synthesized by Evrogen (Russia). Primary antibodies (IgG-fraction) against bovine cytochrome P450scc, AdR, and Adx as well as purified P450scc, AdR, and Adx isolated from bovine adrenal cortex [24, 25] were provided by Prof. V. M. Shkumatov (Research Institute for Physical Chemical Problems, Belarusian State University, Minsk, Belarus).
Bacterial Strain and Plasmids
Escherichia coli strain DH5αc (supE44 Δlac U169 (φ 80 lacZ ΔM15) hsdR17 recA1end A1 gyrA96 thi-1 relA1) was supplied by Gibco-BRL, Germany. Plasmid pTrc99A/mP450scc [6] containing hybrid trp/lac (trc) promoter and cDNA encoding the mature cytochrome P450scc from bovine adrenal cortex was provided by Prof. M. R. Waterman (University of Texas, Dallas, USA). Plasmid pFLuc was provided by New England Biolabs (USA). Plasmid pBar_Twin [26], containing the tac1/tac2/lac promoter and cDNAs of bovine mature AdR and Adx4-108, was provided by Prof. R. Bernhardt (University of Saarlandes, Saabrucken, Germany). Plasmid pBar_Triple [19], which encodes the bovine cytochrome P450scc, AdR и Adx1-108, had been constructed earlier in our laboratory.
Construction of Plasmids
The molecular cloning was performed using E. coli DH5α cells according to standard protocols [23].
pTrc99A/CHL
The pTrc99A/CHL plasmid containing a tricistronic cassette with cDNAs encoding the mature forms of bovine cytochrome P450scc, AdR and Adx1-108 (Fig. 1) was constructed on the base of the pTrc99A/mP450scc [6]. During the first stage, the basic vector was cleaved with restriction endonucleases BglII/KpnI to delete a part of P450scc cDNA (BglII-P450scc′-3′). Then the modified missing 3′-terminal part of the P450 gene which was obtained via PCR amplification, and the RBS and AdR cDNA sequences were inserted into the plasmid to generate the plasmid pTrc99A/P450scc/AdR. PCR amplification was performed using pTrc99A/mP450scc as a template and Primers 1 and 2 (Table 1). The forward primer contained a restriction site BglII, and the reverse primer contained stop-codon and unique restriction sites XhoI and SacII. PCR product was cleaved with BglII/SacII and used in ligation. AdR gene was excised from the vector pBar_Twin with endonucleases NdeI/HindIII and re-cloned into the intermediate vector pFLuc; then, fragment encoding RBS-AdR was excised from this vector with SacII/KpnI digestion and further used in ligation. At the next step, the PCR product including cDNA for RBS-Adx1-108 was amplified from pBar_Triple using Primers 3 and 4 (Table 1) and inserted into the XhoI/SacII-digested vector pTrc99A/P450scc/AdR.
Schematic representation of positions of cDNAs for cytochrome P450scc, Adx, and AdR in the expression cassettes of polycistronic plasmids pBar_Triple (was constructed earlier [19]), pTrc99A/CHL, pTrc99A/CHL(Adx*), and pTrc99A/CHL(2Adx*). Adx, cDNA for Adx1-108, Adx*, cDNA for AdxS112W
pTrc99A/CHL(Adx*) and pTrc99A/CHL(2Adx*)
The plasmid carrying mutated Adx sequence was generated on the base of pTrc99A/P450scc/AdR. PCR fragment containing cDNA for mutated Adx with point substitution of serine to tryptophan in position 112 - AdxS112W (this mutant is ∆113–128, the number of the amino acid residue corresponds to its position in the sequence of the precursor form of the protein), which will hereafter be referred to as Adx*, and preceding RBS sequence, was obtained using pBar_Triple as a template and Primers 5 and 6 (Table 1). Ligation of PCR fragment treated with XhoI/SacII and XhoI/SacII-digested vector pTrc99A/P450scc/AdR resulted in the construction of the plasmid pTrc99A/CHL(Adx*) (Fig. 1). To introduce the second copy of the mutant gene Adx* into pTrc99A/CHL(Adx*), we obtained the sequence XhoI–RBS–Adx*–SacI using Primers 3 and 7 (Table 1). The resulting mutant PCR fragment, cleaved with XhoI/SacI, was inserted into the plasmid pTrc99A/CHL(Adx*), cleaved in the same way. The recombinant plasmid pTrc99A/CHL(2Аdх*) comprised two copies of cDNA encoding AdxS112W (Fig. 1).
All obtained plasmid sequences were validated by restriction analysis and Sanger automatic DNA sequencing. Bacterial cells were transformed with plasmids according to standard protocol [23].
Protein Expression and Preparation of Bacterial Homogenates
Expression of heterologous genes in E. coli cells was induced by adding IPTG (0.5 mM) to the growth medium and carried out as described earlier [17]. The cell culture was incubated at 28 °C with shaking (160 rpm) for 24 h in the presence of ampicillin (100 µg/ml) and ALA (0.5 mM) as an additional source of heme. E. coli cell homogenates were prepared as described previously [27]. Debris was removed by centrifugation (4000g, 10 min). The supernatant (S4 fraction of homogenate, which will hereafter be referred to as “homogenate”) was used for SDS-PAGE analysis and in the experiment aimed at in vitro P450scc activity measurement.
Western blot analysis
Сell homogenates were analyzed using SDS-PAGE technique [28] followed by Western blotting [29]. Samples of homogenates were mixed 1:1 (V/V) with Laemmli buffer (2×) [28] and disrupted by boiling (2 min, 100 °C). 5–150 µg of total protein was loaded into each lane. The protein concentration was measured according to Lowry et al. [30]. During the immunoblotting, the membranes with transferred proteins resolved by SDS-PAGE were incubated with the primary rabbit polyclonal antibodies (IgG-fraction [27]) against P450scc (6.0 mg/ml), AdR (9.5 mg/ml), or Adx (2.5 mg/ml) at 1:7500, 1:7500, and 1:4500 dilution (V/V), respectively. Then the membranes were treated with the conjugate of anti-rabbit secondary antibodies with horseradish peroxidase (1 mg/ml) at 1:15,000 dilution (V/V) and DAB peroxidase (0.25 mg/ml) or ECL substrate. As it has been shown previously, the primary antibodies against bovine P450scc, AdR, and Adx, used in this study, demonstrate affinity to the recombinant P450scc system proteins expressed in E. coli [18]. The membranes treated with DAB were analyzed using ScanImage software (Vidrio Technologies, LLC, USA). To determine the amount of the recombinant proteins using chemiluminescent western blot detection in parallel with the samples under scrutiny, purified P450scc, Adx, and AdR isolated from bovine adrenal cortex with known concentrations as protein standards (precise amounts, 5–50 ng per lane) were loaded. Densitometry analysis of bands was performed using ChemiDoc™ MP imaging system (Bio-Rad, USA) and Image Lab software (Bio-Rad, USA). The calculated amount of CHL proteins in the samples was normalized to 1 mg total protein and reported as mean ± standard deviation from three independent experiments.
Measurement of Cholesterol Hydroxylase/Lyase Activity in the Homogenates of E. coli cells
Cholesterol hydroxylase/lyase activity in the homogenates of E. coli expressing the components of the CHL system for 24 h was determined as described previously [31]. Briefly, the reaction mixture contained the cell homogenate (0.5–1 mg of total protein) and 22(R)-hydroxycholesterol (25 nmol) in 30 mM sodium phosphate buffer (pH 7.2) with 0.05% Tween-20 in a total volume of 0.5 ml. The reaction was induced by adding NADPH-regenerating system and left to proceed for 30 min at 37 °C. Then, cholesterol oxidase (0.5 U) was added to oxidize formed pregnenolone to progesterone. Steroid substances were extracted with ethyl acetate; the precipitate obtained after the evaporation of the extracts (SpeedVac Concentrator, Savant, USA) was dissolved in a sample buffer provided in the reagent kit “IFA PROGESTERONE” (Xema, Russia). The concentration of progesterone in the samples was determined by means of this immunoassay kit based on antiprogesterone antibodies according to the manufacturer’s protocol. The obtained values were normalized to 1 mg of the homogenate protein.
Measurement of Cholesterol Hydroxylase/Lyase Activity of E. coli Cells In Vivo
Expression of heterologous genes was performed as described earlier for 22 h. Later, cells from 50 ml of culture (А600 = 0.34) were centrifuged (3000g, 10 min) and washed with sterile 50 mM sodium phosphate buffer (pH 7.4). The cell suspension was concentrated in the same buffer, and final concentration was 25 g of wet weight/l. Then, IPTG (1 mM), ALA (0.5 mM), ampicillin (50 µg/ml), glycerol (2.0%, V/V), and substrate of P450scc were added and resting cells were cultivated with agitation (160 rpm) at 28 °C for 24 h. Substrate of P450scc cholesterol was added to the final concentration of 0.5 mM in the mixture with different agents: EtOH (1.0%, V/V), DMSO (6.0%, V/V), MCD (0.05%–6%, m/V), 0.5% MCD with saponin (0.2%, m/V), or lysozyme (10 µg/ml). Next, the aliquots of cultures (5 ml) were collected and the organic substances were extracted with ethyl acetate (twice with 2.5 ml). The extracts were evaporated on a vacuum rotor (SpeedVac Concentrator, Savant, USA) and treated with cholesterol oxidase converting the formed pregnenolone to progesterone: the precipitates were washed in 150 µl of 30 mM Na-phosphate buffer (pH 7.4), suspended in 210 µl of 150 mM Na-phosphate buffer (pH 7.4), containing 0.05% Tween 20 (V/V) and 1 U of cholesterol oxidase, and incubated at 37 °C for 1 h. The amount of progesterone in the samples was determined by means of the kit for ELISA, as described above. The obtained values were normalized to 1 l of suspension of resting cells and reported as means ± standard deviation from three or four experiments.
High-Pressure Liquid Chromatography
For the analysis of the samples, we used an Agilent 1260 instrument (Agilent Technologies, USA), equipped with a PDA detector. Reversed-phase HPLC was performed using the Zorbax SB-C8 5 µ column (150 × 4.6 mm, Agilent Technologies, USA) with an acetonitrile gradient. The operating conditions used for HPLC were the following: mobile phase A—H2O, mobile phase B—acetonitrile; gradient elution program − 50% В (10 min), 50–100% В (1 min), 100% В (3 min), flow rate − 1.5 ml/min, t° − 30 °С; injection volume − 20 µl. The amount of the steroid formed in the cells as a result of the substrate biotransformation was evaluated using an external standard method with a control sample of progesterone (1 mg/ml). Absorption was recorded at 242 nm.
Mass Spectrometry
Positive ion mass spectrum was acquired from 200 m/z–3000 m/z using MALDI-TOF mass-spectrometer UltrafleXtreme (BrukerDaltonics, Germany), equipped with UV laser (Nd), in reflectron mode. Accuracy of determined monoisotopic masses was 0.1 Da. Test substance was diluted in 200 µl of 50% (V/V) aqueous acetonitrile solution. 0.5 µl of test sample and 1 µl of 2,5-dihydroxybenzoic acid solution (20 mg/ml in 20% (V/V) aqueous acetonitrile, 0.5% (V/V) trifluoroacetic acid) were mixed on target and left to air dry.
Results and Discussion
Plasmid Construction for the Co-expression of CHL System Proteins
In this work, the co-expression of bovine steroidogenic CHL system proteins in bacterial cells was carried out using polycistronic plasmids containing cDNAs encoding cytochrome P450scc, Adx, and AdR, preceded by ribosome binding sites (RBS). Transcription resulted in the formation of a single hybrid mRNA, but the presence of the RBS, upstream of the start-codons of heterologous genes, ensured independent translation of individual proteins.
It is known that the order of the genes in the expression cassette of the recombinant tandem plasmids may have an effect on gene translation level and, respectively, the ratio of co-expressed proteins in the cell [32, 33]. For instance, when cDNAs for cytochrome P45027B1, Adx, and AdR were co-expressed in E. coli, the change in the order of Adx and AdR cDNA in the expression cassette resulted in a three- to fivefold change of protein levels and, accordingly, in the change in cytochrome P45027B1 activity in the cells by more than 20 times [33]. However, it is obvious that a change in the ratio of co-expressed proteins in case of gene rearrangement cannot be the absolute rule for all co-expression constructs. If polycistronic constructs are used the steady-state levels of the proteins will depend, in particular, on the structure of large artificial mRNA which may not be optimal for translation of the second from the promoter and subsequent proteins due to formation of the hairpins or of the ATG codon availability restriction. Besides, the specific parameters of synthesis of definite proteins and their stability (the half-lives) in the cell are important determinants of the steady-state levels and, respectively, the ratio of co-expressed proteins. For example, when various human microsomal P450s were co-expressed with NADPH-P450 reductase, the expression levels of P450s ranged from 35 to 350 nmol per liter culture, and expression of NADPH-P450 reductase ranging from 50 to 100% of that of P450 resulted in the P450/Red ratio ranging from 3/1 to 1/1 [32].
During this work, we assumed to find out whether the order of genes influences the content of the recombinant proteins and the activity of the CHL system in E. coli cells. To this end, we constructed the vector pTrc99A/CHL on the basis of plasmid pTrc99A/P450scc (which was successfully used for the expression of cytochrome P450scc [6, 15]) where the order of genes has been changed in comparison to pBar_Tiple used earlier [19]: RBS–P450scc–RBS–Adx–RBS–AdR versus RBS–AdR–RBS–Adx–RBS–P450scc, respectively (Fig. 1). The stoichiometry of the components of the CHL system is still not determined with certainty. However, according to the available data, the content of cytochrome P450scc in mitochondria of bovine adrenal cortex exceeds the content of AdR (for example, it was shown that cytochrome P450scc, AdR, and Adx are present in ratio 8:1:3 [34] and 2.9:1:7.7 [35]). We supposed that placing the cytochrome P450scc gene first in the expression cassette of the tandem plasmid could lead to an increase in expression level of this protein and consequently P450scc/AdR ratio. Taking into account the key role of the cytochrome P450scc in the CHL system, we expected to enhance the substrate conversion.
The efficiency of the CHL system depends directly on the content and activity of Adx, which transfers electrons from the AdR molecule to the heme of cytochrome P450scc—the catalyst of the reaction. Adx mutants, including Adx deletion mutants with modified N- or С-terminal regions, have been studied to obtain the Adx versions with an enhanced redox partner interactions and increased reduction rate of CYPs. Particularly, in publication [36] the role of C-terminal regions of Adx molecule was studied by analysis of deletion mutants 4-114 and 4-108 lacking amino acids 1–3 and 115–128 or 109–128, respectively. In this work the kinetics of protein–protein interaction in the mitochondrial CYP11A1(P450scc)- and CYP11B1-systems and heme reduction were studied in detail. It has been demonstrated that C-terminal region of Adx (amino acids 109–128) affects its structural integrity and the interaction with CYP11A1 and CYP11B1 and determines the differences in the rate of electron transfer to cytochromes P450 [36]. As a result, the truncated Adx4-108 provides more efficient hydroxylation of substrates by cytochromes CYP11A1 and CYP11B1 (the efficiency of substrate conversion (kcat/Km) increased up to 3 and 22 times, respectively) when compared to the full-length wild-type Adx1-128 in in vitro experiments in the reconstructed system [36]. Additionally, it was shown that mutated version of the protein AdxS112W, with 16 amino acid truncation on the C-terminus and point substitution of serine to tryptophan in position 112, forms a more stable complex with cytochrome P450scc as compared to the wild-type Adx, resulting in the increase of the electron transfer rate and a 100-fold increase of the in vitro efficiency of the cholesterol conversion [37].
In order to increase the activity of the CHL system, we used a truncated version Adx1-108 during the construction of the vector pTrc99A/CHL. Furthermore, taking into account the data presented above, we obtained two genetic constructs—pTrc99A/CHL(Adx*) and pTrc99A/CHL(2Adx*), containing one or two copies of the mutant version of cDNA encoding Adx with a point mutation S112W (AdxS112W, denoted here as Adx*) (Fig. 1). It is important to determine how this mutation affects the rate of the catalytic reactions during the operation of the complete enzymatic CHL system in living cells. It was assumed that the replacement of Adx1-108 with Adx* in the pTrc99A/CHL expression plasmid would increase the activity of the CHL system, and the introduction of an additional copy of Adx* cDNA, would increase the transcription level of Adx (as well as the relative content of Adx in P450-system, respectively), resulting in a greater increase in the activity.
Analysis of Expression of CHL System Proteins in the Recombinant Cells
Escherichia coli cells, transformed with plasmids pBar_Triple, pTrc99A/CHL, pTrc99A/CHL(Adx*), or pTrc99A/CHL(2Adx*) were grown upon induction of transcription of heterologous cDNAs for 24 h. Western immunoblotting with antibodies against cytochrome P450scc, AdR, or Adx demonstrated that the genes of all the three recombinant proteins have been successfully expressed in cells using all of the indicated plasmids. Figure 2 shows the results of the analysis of the homogenates obtained from the cells transformed with plasmids pBar_Triple, pTrc99A/CHL, and pTrc99A/CHL(Adx*): molecular weights of the proteins corresponded to the expected (AdR-51 kDa, P450scc-53 kDa, and Adx-12 kDa).
Expression of CHL system proteins in E.coli/pBar_Triple, E.coli/pTrc99A/CHL, and E.coli/pTrc99A/CHL(Adx*). SDS-PAGE [10%, 5 µg of total protein/lane (upper and middle panels) or 15%, 10 µg of total protein/lane (lower panel)] and immunoblotting analysis of cell homogenates. Membranes treated with ECL substrate were visualized using ChemiDoc™ MP imaging system. M—standard protein markers
The content of P450scc, AdR, and Adx, determined by densitometric scanning of the Western blotting signals with Image Lab software, is presented in Table 2. The data obtained demonstrate the difference of expression levels of heterologous proteins in E.coli/pBar_Triple and E.coli/pTrc99A/CHL cells. We detected about twofold increase in the content of P450scc (from 0.145 ± 0.025 to 0.290 ± 0.040 nmol/mg total protein) and Adx (from 0.211 ± 0.062 to 0.485 ± 0.157 nmol/mg total protein), and about twofold decrease in the content of AdR (from 0.610 ± 0.019 to 0.283 ± 0.026 nmol/mg total protein) in E. coli/pTrc99A/CHL in comparison with E. coli/pBar_Triple cells. In E. coli, transformed with pBar_Triple and pTrc99A/CHL containing the expression cassettes with different orders of P450scc and AdR cDNAs (Fig. 1), calculated stoichiometric ratios P450scc/Adx/AdR were determined as 1:1.45:4.2 and 1:1.67:0.98, respectively. Therefore, shift of P450scc gene on the first position in the expression cassette of pTrc99A/CHL resulted in change of protein ratio in CHL system.
An analysis of the recombinant proteins content in E. coli/pTrc 99A/CHL strain and E. coli/pTrc 99A/CHL(Adx*) strain, which produces the mutant Adx*, revealed no significant differences in protein expression levels (Fig. 2; Table 2). As follows from the results presented in Fig. 3A, the introduction of a second copy of the Adx* gene into the plasmid resulted in an elevation of the Adx* content: the amount of Adx* in the cells transformed with the plasmid pTrc99A/CHL(2Adx*) was almost twice as high as the amount of Adx* in cells transformed with pTrc99A/CHL(Adx*) (determined using the ScanImage software). The analysis of homogenates obtained from the same cells demonstrated that the introduction of the second Adx* cDNA copy into the plasmid does not affect the expression levels of the cytochrome P450scc and AdR. Figure 3B shows that cells transformed with pTrc99A/CHL(Adx*) and pTrc99A/CHL(2Adx*) contain the same amount of AdR protein, which cDNA is located downstream of the Adx* cDNA in the expression cassette.
Expression of recombinant proteins in E. coli/pTrc99A/CHL(Adx*) and E.coli/pTrc99A/CHL(2Adx*). SDS-PAGE [15% (a) or 10% (b)] and immunoblotting analysis of cell homogenates. The membranes were probed with antibodies against Adx (a) or AdR (b). M—standard protein markers; 1—homogenate of control untransformed cells; 2—homogenate of the E.coli/pTrc99A/CHL(Adx*) cells; 3—homogenate of the E. coli/pTrc99A/CHL(2Adx*) cells
Activity of CHL System Proteins Expressed in E. coli Cells
The cell homogenates of all the strains tested demonstrated CHL activity. The conversion of the 22R-hydroxycholesterol to pregnenolone was examined in an in vitro system using the ELISA method as described in “Materials and Methods” section. Table 3 presents the mean activity values from four independent experiments after subtracting the background value (activity of the homogenate of control E. coli DH5α strain − 0.18 × 10−3 nmol pregnenolone/h per 1 mg homogenate protein). The observed activity of P450scc in the homogenate of cells transformed with pTrc99A/CHL was 4.0 ± 1.3 nmol pregnenolone/h per 1 mg homogenate protein and was considered as 100%. As follows from our data, the samples of the cells obtained using the plasmids pBar_Triple and pTrc99A/CHL show similar CHL activity values of 120% and 100%, respectively. At the same time, specific activity of P450scc in the sample of E. coli/pTrc99A/CHL evaluated as nmol pregnenolone/h per 1 nmol P450scc significantly decreased in comparison with the value detected in the sample of E. coli/pBar_Triple (13.8 versus 33.1 nmol pregnenolone/h per 1 nmol P450scc). Taking into account different stoichiometry of the components of the CHL system in these strains, we can speculate that stoichiometric ratio P450scc/Adx/AdR 1:1.45:4.2 in E. coli/pBar_Triple is more favorable for P450scc system functioning than the ratio 1:1.67:0.98 in E. coli/pTrc99A/CHL. Hence, unfortunately, elevation of P450scc content in E. coli/pTrc99A/CHL cells (Table 2) did not increase the total activity of the CHL system in the cell homogenate. We assume that it can be caused by considerable decrease in AdR content in these cells (Table 2).
As follows from our results, the replacement of Adx1-108 by the mutant version Adx* significantly changes the efficiency of the CHL system. The activity of the system with the mutant Adx* does not increase as it was expected, but decreases dramatically as compared to the activity of the P450scc/AdR/Adx1-108 CHL system: the amount of pregnenolone formed using the E. coli/pTrc99A/CHL(Adx*) homogenate was only about 12% of the total amount of the product produced by the E. coli/pTrc99A/CHL cell homogenate (Table 3). The corresponding value for the sample obtained from cells expressing 2 copies of the Adx* gene was 24%, which is less than the activity of the system expressed using pTrc99A/CHL, but twice as high as the activity in the sample of cells carrying the plasmid with one copy of the Adx* gene. Thus, the comparative analysis did not reveal a positive effect of the application of the mutated version Adx* on the efficiency of the recombinant CHL system. At the same time, the introduction of an additional copy of Adx* results in an increase in the recorded activity; this is apparently due to a change in the stoichiometric ratio of proteins in the system towards an increase in the Adx* content.
Cholesterol Bioconversion by Recombinant E. coli/pTrc99A/CHL Cells
We have checked the ability of recombinant E. coli cells, transformed with pTrc99A/CHL, as a resting cell suspension in Na–phosphate buffer, to perform cholesterol hydroxylation. Heterologous proteins were expressed in E. coli cells cultured in a TB medium. Then, the cell culture was concentrated in 50 mM Na–phosphate buffer (pH 7.4), and a suspension of resting cells (25 g/l) was incubated for 24 h in the presence of cholesterol (0.5 mM), which was added into the medium as the ethanol solution. Cells were incubated in Na–phosphate buffer because of the possible accumulation of toxic endogenous E. coli metabolite, indole, during the prolonged cultivation of cells in the TB medium, which can inhibit the reactions of biotransformation catalyzed by cytochromes P450 [38, 39]. For instance, it was shown that the product yield of a 24-h biotransformation of abietic acid by Streptomyces griseolus cytochrome CYP105A1 in resting cells, incubated in 50 mM K–phosphate buffer (pH 7.4), was twice greater than the product yield in cells cultivated in the TB medium [39].
HPLC and mass spectrometry were used to identify the CHL reaction product formed in the in vivo system. Organic compounds were extracted from the cell culture and treated with cholesterol oxidase, catalyzing the conversion of pregnenolone, the product of the CHL reaction, to progesterone (see “Materials and Methods” section) prior to analysis. Figure 4 shows the fragments of chromatograms (patterns comprising the retention time for progesterone), for a sample of the progesterone standard (Fig. 4a) as well as a sample obtained from a culture of recombinant cells cultivated in the presence of cholesterol (Fig. 4b). The comparison of HPLC profiles showed the presence of a peak, with the retention time of 8.9 min, corresponding to progesterone in the organic extract under investigation. The presence of progesterone in the sample was confirmed using mass spectrometry. The peak 315.3 corresponding to progesterone with the molecular weight of 314.4 was detected in the obtained organic extract (Fig. 5). Thus, direct instrumental analysis showed that the recombinant E. coli/pTrc99A/CHL strain converts cholesterol to pregnenolone (Figs. 4, 5).
Effect of Various Chemicals on the Cholesterol Biotransformation Efficiency
Essential factors affecting the in vivo activity of steroidogenic systems reconstructed in bacterial cells are as follows: the poor solubility of hydrophobic steroid substrates in the aquatic environment and the consequent inefficient transport of substrates into the cells (for example, see [13, 40]). The productivity of transgenic microorganisms can be significantly increased by using agents that increase the solubility of steroids and/or permeabilize the cell membrane (various organic solvents, detergents, surface-active substances, liquid polymers, cyclodextrins, antibiotics, etc.) [40]. β-Cyclodextrins, in particular, are used to increase the solubility of the cytochrome P450scc substrates—cholesterol and vitamin D3—due to formation of the water-soluble inclusion complexes [41]. Moreover, cyclodextrins can influence the properties of microorganism cell membranes, facilitating transport of various compounds into the cells [40].
We have discovered earlier that the addition of the cholesterol into the cell growth medium in an MCD aqueous solution (the final concentration of MCD was 0.35%, m/V) resulted in ~ 30 times an elevation of the cholesterol biotransformation yield by E. coli/pBar_Triple cells as compared to the addition of cholesterol in the form of suspension, stabilized by Tween 80 (final concentration of Tween 80 − 1 g/l) [19]. In the present study, we have compared the effect of several compounds, used in standard concentrations, on the CHL activity of the E. coli/pTrc99A/CHL incubated as resting cells in 50 mM Na-phosphate buffer (pH 7.4). Figure 6A shows the results of measuring the CHL activity of induced cells in the presence of EtOH (1%, V/V), DMSO (6%, V/V), or MCD (1.35%, m/V). The activity determined in the presence of 1.35% MCD was 108 µg (0.34 µM) of pregnenolone/l of cell suspension in 24 h; this was considered as 100%. It was observed that the addition of MCD resulted in a higher yield of the biotransformation product than the addition of EtOH or DMSO. Thus, MCD most likely provides a more efficient transportation of cholesterol for P450scc localized inside the cell than Tween 80 [19], EtOH, and DMSO.
Effect of permeabilizing agents on CHL activity of E.coli/pTrc99A/CHL resting cells. a Сells were incubated in the presence of EtOH, DMSO, and MCD; b cells were incubated in the presence of different concentrations of MCD. The content of the cholesterol biotransformation product in organic extracts, obtained from cell cultures, was measured by ELISA. Mean values and standard deviations for 3–5 independent experiments are presented. The activity measured in the samples of cells grown in the presence of 1.35% MCD was considered as 100%
In the next step, we incubated E. coli/pTrc99A/CHL resting cells in the presence of cholesterol and various concentrations of MCD (in the range of 0.05–6% (m/V)). With a decrease in the MCD concentration in the culture medium in the range of 6–1.35%, efficiency of the pregnenolone production by the cells increased approximately fivefold (Fig. 6b). During further decrease in the concentration of MCD, the activity continued to increase and reached 146 µg of pregnenolone/l of the resting cells suspension in 24 h at MCD concentration of 0.2%. Then, the pregnenolone yield in the samples decreases by 1.4 and 6 times, respectively, compared with the maximum value at the MCD concentration of 0.15% and 0.05%, probably due to a non-optimal ratio of MCD to cholesterol. The study [42] showed that the addition of β-cyclodextrin to the culture medium substantially increased the cholesterol bioconversion efficiency by Mycobacterium sp.; at the same time, the optimal molar ratio of MCD to cholesterol was two moles of β-cyclodextrin per one mole of substrate. From the results presented in Fig. 6, it can be inferred that the addition of the MCD also significantly affects the efficiency of cholesterol conversion by E. coli/pTrc99A/CHL, where the maximum yield of pregnenolone was detected at the ratio of 1.4 mM (0.2%) MCD to 0.5 mM cholesterol.
If two chemical agents—0.5% (m/V) MCD and 6% DMSO (m/V)—were added to the cell culture medium, the recorded activity varied greatly from 100 to 623 µg of pregnenolone/l, which is probably due the problems with the formation of a stable suspension. In this case, the maximum activity value was greater than four times of the activity recorded in the presence of 0.5% (m/V) MCD. At the same time, the addition of the lysozyme hydrolase (10 µg/ml) or a surfactant saponin (0.2%, m/V), which decreases the barrier function of the cell membrane, to the culture medium containing 0.5% (m/V) MCD did not affect the efficiency of the cholesterol biotransformation.
Escherichia coli bacterial cells can synthesize the functional active mammalian mitochondrial proteins, owing to particular features such as the similarity of bacterial cells and mitochondria with respect to the main factors involved in the protein topogenesis control (chaperones and proteinases) [43] and the ability to perform post-translational modifications of heterologous proteins (for example, binding of prosthetic groups) [44]. In this study, we obtained E. coli/pTrc99A/CHL cells that include mammalian mitochondrial CHL complex—AdR/Adx/P450scc. Heterologous proteins form a functionally active enzymatic system in bacteria. Moreover, the CHL activity measured in the homogenate of recombinant E. coli/pTrc99A/CHL cells is close to the expressed in E. coli cytochrome P450scc activity measured in the cell homogenate containing Adx and AdR isolated from the adrenal cortex [17]. It was found that the alteration of the order of heterologous P450scc and AdR cDNAs in the plasmid expression cassette resulted in alteration of the content of the heterologous proteins, but did not change notably the catalytic activity of the CHL system detected in the cell homogenate (evaluated as nmol pregnenolone/h per 1 mg homogenate protein). Based on the comparison of the specific activity evaluated as nmol pregnenolone/h per 1 nmol P450scc in cell homogenates (see section “Activity of CHL system proteins expressed in E. coli cells”), we can suppose that stoichiometric ratio P450scc/Adx/AdR 1:1.45:4.2 is more optimal for P450scc system functioning than the ratio 1:1.67:0.98.
During this work, we performed enzyme engineering of Adx—alteration of S112 to W112—and evaluated the effect of this mutation on Adx functioning as a part of CHL system. In vitro activity of CHL system comprising mutant Adx* (AdxS112W) decreased dramatically as compared to that of P450scc/Adx1-108/AdR. This decrease was not associated with a decrease in the expression level of proteins, since the expression levels of mutated Adx* and unmutated Adx in homogenates of E. coli/pTrc99A/CHL(Adx*) and E. coli/pTrc99A/CHL cells were comparable. At the same time, introduction of a second copy of the Adx* gene into the plasmid increased the Adx* expression level and, consequently, the CHL activity, by almost twice in comparison with the P450scc/AdR/Adx* system (Fig. 3A; Table 3). Thus, our data indicate that Ser112 is the crucial amino acid residue in the Adx molecule and the mutation S112W significantly affects the enzymatic activity of the whole recombinant CHL system. Perhaps the discrepancy between these results and the data, obtained for AdxS112W in vitro in the reconstructed system using isolated proteins (see above [37]), is caused by the influence of some intracellular factors on the interaction of recombinant Adx* with AdR or P450scc in the cell, which in turn affects the electron transfer efficiency and cytochrome P450scc functioning.
We report here the successful use of resting recombinant E. coli/pTrc99A/CHL cells for cholesterol biotransformation and conducting experiments to determine the effect of various chemicals on CHL activity in vivo. HPLC and mass spectrometry results (Figs. 4, 5) demonstrated the presence of biotransformation product in culture medium. A comparative analysis of CHL activity in vivo revealed that the amount of pregnenolone formed as a result of the 24-h cultivation of resting cells in the presence of cholesterol varies in the range of approximately 0.05–0.62 mg/l, depending on the type and concentration of permeabilizing agents present in the culture medium. Screening for an appropriate co-solvent identified MCD as the most promising candidate for activation of cholesterol transforming activity in these reaction conditions. It can be noted that CHL activity in vivo using E. coli/pTrc99A/CHL resting cells is comparable to the value of CHL activity using growing culture of E. coli/pBar/Triple cells, determined earlier in our laboratory [19]. However, it is obvious that the use of a concentrated suspension of resting cells in phosphate buffer to carry out the experiments to optimize the functioning of heterologous enzymatic systems in living bacterial cells is more convenient and cheaper in terms of cultivation, the use of a smaller sample volume for analysis, and a smaller amounts of necessary reagents.
Overall, the data obtained in this study demonstrate that the activity of the CHL system localized in E. coli cells is mainly limited by the solubility and inefficient uptake of exogenous substrates into the cell, while the formation of active proteins, synthesized in E. coli cells, proceeds without any particular complications. These results contribute to the knowledge of CHL system functioning and to understanding its activity towards sterols in living recombinant E. coli cells and also represent the basis for further improvement of the whole-cell biotransformation using E. coli cells.
References
Bernhard, R. (2006). Cytochromes P450 as versatile biocatalysts. Journal of Biotechnology, 124, 128–145. https://doi.org/10.1016/j.jbiotec.2006.01.026.
Miller, W. L. (2008). Steroidogenic enzymes. Endocrine Development, 13, 1–18. https://doi.org/10.1159/000134751.
Urlacher, V. B., & Girhard, M. (2012). Cytochrome P450 monooxygenases: An update on perspectives for synthetic application. Trends in Biotechnology, 30, 26–36. https://doi.org/10.1016/j.tibtech.2011.06.012.
Nelson, D. R., Kamataki, T., Waxman, D. J., Guengerich, F. P., Estabrook, R. W., Feyereisen, R., Gonzalez, F. J., Coon, M. J., Gunsalus, I. C., Gotoh, O., et al. (1993). The P450 superfamily: Update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cellular Biology, 12, 1–51. https://doi.org/10.1089/dna.1993.12.1.
Hanlon, S. P., Friedberg, T., Wolf, C. R., Chisalba, O., & Kittelmann, M. (2012). Recombinant yeast and bacteria that express human P450s: Bioreactors, for drug discovery, development, and biotechnology. In R. D. Schmid & V. B. Urlacher (Eds.), Modern Biooxidation: Enzymes, Reactions and Applications (pp. 233–252). New York: Wiley.
Wada, A., Mathew, P. A., Barnes, H. J., Sanders, D., Estabrook, R. W., & Waterman, M. R. (1991). Expression of functional bovine cholesterol side chain cleavage cytochrome P450 (P450scc) in Escherichia coli. Archives of Biochemistry and Biophysics, 290, 376–380.
Sagara, Y., Barnes, H., & Waterman, M. R. (1993). Expression in Escherichia coli of functional cytochrome P450c17 lacking its hydrophobic amino-terminal signal anchor. Archives of Biochemistry and Biophysics, 304, 272–278. https://doi.org/10.1006/abbi.1993.1349.
Barnes, H. J., Arlotto, M. P., & Waterman, M. R. (1991) Expression and enzymatic activity of recombinant cytochrome P450 17 alpha-hydroxylase in Escherichia coli. Proceedings of National Academy Science United states of America, 88, 5597–5601.
Hu, M. C., & Chung, B. C. (1990). Expression of human 21-hydroxylase (P450c21) in bacterial and mammalian cells: A system to characterize normal and mutant enzymes. Molecular Endocrinology, 4, 893–898. https://doi.org/10.1210/mend-4-6-893.
Osawa, Y., Higashiyama, T., Toma, Y., & Yarborough, C. (1997). Diverse function of aromatase and the N-terminal sequence deleted form. Journal of Steroid Biochemistry and Molecular Biology, 61, 117–126.
Nonaka, Y., Fujii, T., Kagava, N., Waterman, M. R., Takemori, H., & Okamoto, M. (1998). Structure/function relationship of CYP11B1 associated with Dahl’s salt-resistant rats—expression of rat CYP11B1 and CYP11B2 in Escherichia coli. European Journal of Biochemistry, 258, 869–878.
Novikova, L. A., Faletrov, Y. V., Kovaleva, I. E., Mauersberger, S., Luzikov, V. N., & Shkumatov, V. M. (2009). From structure and functions of steroidogenic enzymes to new technologies of gene engineering. Biochemistry, 74, 1482–1504.
Shet, M. S., Fisher, C. W., & Estabrook, R. W. (1997). The function of recombinant cytochrome P450s in intact Escherichia coli cells: The 17 alpha-hydroxylation of progesterone and pregnenolone by P450c17. Archives of Biochemistry and Biophysics, 339, 218–225. https://doi.org/10.1006/abbi.1996.9868.
Ehmer, P. B., Jose, J., & Hartmann, R. W. (2000). Development of a simple and rapid assay for the evaluation of inhibitors of human 17alpha-hydroxylase-C(17,20)-lyase (P450cl7) by coexpression of P450cl7 with NADPH-cytochrome-P450-reductase in Escherichia coli. The Journal of Steroid Biochemistry and Molecular Biology, 75, 57–63.
Shkumatov, V. M., Radyuk, V. G., Falertov, Y. V., Vinogradova, A. A., Luzikov, V. N., & Novikova, L. A. (2006). Expression of cytochrome P450scc in Escherichia coli cells: Intracellular location and interaction with bacterial redox proteins. Biochemistry, 71, 884–892.
Shet, M. S., Fisher, C. W., Arlotto, M. P., Shackleton, C. H., Holmans, P. L., Martin-Wixtrom, C. A., Saeki, Y., & Estabrook, R. W. (1994). Purification and enzymatic properties of a recombinant fusion protein expressed in Escherichia coli containing the domains of bovine P450 17A and rat NADPH-P450 reductase. Archives of Biochemistry and Biophysics, 311, 402–417.
Nazarov, P. A., Drutsa, V. L., Miller, W. L., Shkumatov, V. M., Luzikov, V. N., & Novikova, L. A. (2003). Formation and functioning of fused cholesterol side-chain cleavage enzymes. DNA Cellular Biology, 22, 243–252. https://doi.org/10.1089/104454903321908638.
Shashkova, T. V., Luzikov, V. N., & Novikova, L. A. (2006). Coexpression of all constituents of the cholesterol hydroxylase/lyase system in Escherichia coli cells. Biochemistry, 71, 810–814.
Makeeva, D. S., Dovbnya, D. V., Donova, M. V., & Novikova, L. A. (2013). Functional reconstruction of bovine P450scc steroidogenic system in Escherichia coli. American Journal of Molecular Biology, 3, 173–182. https://doi.org/10.4236/ajmb.2013.34023.
Brixius-Anderko, S., Schiffer, L., Hannemann, F., Janocha, B., & Bernhardt, R. (2015). A CYP21A2 based whole-cell system in Escherichia coli for the biotechnological production of premedrol. Microbiology Cell Fact, 14, 135. https://doi.org/10.1186/s12934-015-0333-2.
Schiffer, L., Anderko, S., Hobler, A., Hannemann, F., Kagawa, N., & Bernhardt, R. (2015). A recombinant CYP11B1 dependent Escherichia coli biocatalyst for selective cortisol production and optimization towards a preparative scale. Microbiology Cell Fact, 14, 25. https://doi.org/10.1186/s12934-015-0209-5.
Schiffer, L., Müller, A.-R., Hobler, A., Brixius-Anderko, S., Zapp, J., Hannemann, F., & Bernhardt, R. (2016). Biotransformation of the mineralocorticoid receptor antagonists spironolactone and canrenone by human CYP11B1 and CYP11B2: Characterization of the products and their influence on mineralocorticoid receptor transactivation. Journal of Steroid Biochemistry and Molecular Biology, 163, 68–76. https://doi.org/10.1016/j.jsbmb.2016.04.004.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual (2nd edn.). Cold Spring Harbor: Cold Spring Harbor Laboratory Press.
Akhrem, A. A., Lapko, V. N., Lapko, A. G., Shkumatov, V. M., & Chashchin, V. L. (1979). Isolation, structural organization and mechanism of action of mitochondrial steroid hydroxylating systems. Acta Biology Medicine of Germany, 38, 257–273.
Chashchin, V. L., Vasilevsky, V. I., Shkumatov, V. M., Lapko, V. N., Adamovich, T. B., Berikbaeva, T. M., & Akhrem, A. A. (1984). The domain structure of the cholesterol side-chain cleavage cytochrome P-450 from bovine adrenocortical mitochondria. Localization of haem group and domains in the polypeptide chain. Biochim Biophysics Acta, 791, 375–383.
Hannemann, F., Virus, C., & Bernhardt, R. (2006). Design of an Escherichia coli system for whole cell mediated steroid synthesis and molecular evolution of steroid hydroxylases. Journal of Biotechnology, 124, 172–181. https://doi.org/10.1016/j.jbiotec.2006.01.009.
Efimova, V. S., Isaeva, L. V., Makeeva, D. S., Rubtsov, M. A., & Novikova, L. A. (2017). Expression of cholesterol hydroxylase/lyase system proteins in yeast s. cerevisiae cells as a self-processing polyprotein. Molecular Biotechnology, 59, 394–406. https://doi.org/10.1007/s12033-017-0028-5.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Towbin, H., Staehelin, T., & Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceeding of National Academy Science United States of America. 76, 4350–4354.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–270.
Vinogradova, A. A., Luzikov, V. N., & Novikova, L. A. (2007). Comparative study of topogenesis of cytochrome P450scc (CYP11A1) and its hybrids with adrenodoxin expressed in Escherichia coli cells. Biochemistry, 72, 208–214.
Parikh, A., Gillam, E. M., & Guengerich, F. P. (1997). Drug metabolism by Escherichia coli expressing human cytochromes P450. Nature Biotechnology, 15, 784–788. https://doi.org/10.1038/nbt0897-784.
Sawada, N., Sakaki, T., Kitanaka, S., Takeyama, K., Kato, S., & Inouye, K. (1999). Enzymatic properties of human 25-hydroxyvitamin D3 1α-hydroxylase. European Journal of Biochemistry, 265, 959–956.
Hanukoglu, I., & Hanukoglu, Z. (1986). Stoichiometry of mitochondrial cytochromes P-450, adrenodoxin and adrenodoxin reductase in adrenal cortex and corpus luteum. Implications for membrane organization and gene regulation. European Journal of Biochemistry, 157, 27–31.
Usanov, S. A., Chernogolov, A. A., Petrashin, A. I., Akhrem, A. A., & Chashchin, V. L. (1987). Immunochemical study of the cholesterol hydroxylating system topology and stoichiometry of the electron transport chain components in mitochondrial membrane. Biological Membranes, 4, 1102–1116.
Uhlmann, H., Kraft, R., & Bernhardt, R. (1994). C-terminal region of adrenodoxin affects its structural integrity and determines differences in its electron transfer function to cytochrome P-450. Journal of Biological Chemistry, 269, 22557–22564.
Schiffler, B., Kiefer, M., Wilken, A., Hannemann, F., Adolph, H. W., & Bernhardt, R. (2001). The interaction of bovine adrenodoxin with CYP11A1 (cytochrome P450scc) and CYP11B1 (cytochrome P45011beta). Acceleration of reduction and substrate conversion by site-directed mutagenesis of adrenodoxin. Journal of Biological Chemistry, 276, 36225–36232. https://doi.org/10.1074/jbc.M102320200.
Ringle, M., Khatri, Y., Zapp, J., Hannemann, F., & Bernhardt, R. (2013). Application of a new versatile electron transfer system for cytochrome P450-based Escherichia coli whole-cell bioconversions. Applied Microbiology and Biotechnology, 97, 7741–7754. https://doi.org/10.1007/s00253-012-4612-0.
Janocha, S., & Bernhardt, R. (2013). Design and characterization of an efficient CYP105A1-based whole-cell biocatalyst for the conversion of resin acid diterpenoids in permeabilized Escherichia coli. Applied Microbiology and Biotechnology, 97, 7639–7649. https://doi.org/10.1007/s00253-013-5008-5.
Donova, M. V., & Egorova, O. V. (2012). Microbial steroid transformations: Current state and prospects. Applied Microbiology and Biotechnology, 94, 1423–1447. https://doi.org/10.1007/s00253-012-4078-0.
Wallimann, P., Marti, T., Furer, A., & Diederich, F. (1997). Steroids in molecular recognition. Chemical Reviews, 97, 1567–1608.
Hesselink, P. G. M., van Vliet, S., de Vries, H., & Witholt, B. (1989). Optimization of steroid side-chain cleavage by Mycobacterium sp. in the presence of cyclodextrins. Enzyme Microb. Technol, 11, 398–404. https://doi.org/10.1016/0141-0229(89)90133-6.
Suzuki, C. K., Rep, M., van Dijl, J. M., Suda, K., Grivell, L. A., & Schatz, G. (1997). ATP-dependent proteases that also chaperone protein biogenesis. Trends in Biochemical Sciences, 22, 118–123.
Guengerich, F. P., Gillam, E. M. J., Ohmori, S., Sandhu, P., Brian, W. R., Sari, M.–A., & Iwasaki, M. (1993). Expression of human cytochrome P450 enzymes in yeast and bacteria and relevance to studies on catalytic specificity. Toxicology, 1993, 82, 21–37.
Acknowledgements
The authors would like to thank M. S. Serebryakova for conducting the mass spectrometry experiments, and V. N. Tashlitsky for conducting the experiments using the HPLC.
Funding
This research was supported by Russian Foundation for Basic Research (Grant No. 16-54-00139).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors state that they have no Conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Efimova, V.S., Isaeva, L.V., Rubtsov, M.A. et al. Analysis of In Vivo Activity of the Bovine Cholesterol Hydroxylase/Lyase System Proteins Expressed in Escherichia coli. Mol Biotechnol 61, 261–273 (2019). https://doi.org/10.1007/s12033-019-00158-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-019-00158-6