Abstract
Natural colchicinoids and their semisynthetic derivatives are important active ingredients for pharmaceutical applications. Thiocolchicoside (3-demethoxy-3-glucosyloxythiocolchicine) is used in several countries as standard therapy for the treatment of diseases of the muscle–skeletal system, due to its potent antiinflammatory and myorelaxant properties. Manufacturing of thiocolchicoside requires a key step, the regioselective demethylation and glucosylation of chemically derivative thiocolchicine. High selectivity and efficiency of this transformation cannot be achieved in a satisfactory way with a chemical approach. In particular, the chemical demethylation, a part from requiring toxic and aggressive reagents, generates a complex mixture of products with no industrial usefulness. We report herein an efficient, direct and green biotransformation of thiocolchicine into thiocolchicoside, performed by a specific strain of Bacillus megaterium. The same process, with minor modifications, can be used to convert the by-product 3-O-demethyl-thiocolchicine into thiocolchicoside. In addition, we describe the B. megaterium strain selection process and the best conditions for this effective double biotransformation. The final product has a pharmaceutical quality, and the process has been industrialised.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colchicinoids are alkaloids of particular importance as active ingredients with anti-inflammatory and, in some cases, anticancer activities, occurring in plants of the Family Colchicaceae (Colchicum sp., Sandersonia sp., Gloriosa sp., Iphigenia sp., Merendera sp., etc.).
Commercially important examples are Colchicine [I], Colchicoside (3-demethoxy-3-glucosyloxycolchicine) [II] and their semisynthetic thioderivatives, Thiocolchicine [III] and Thiocolchicoside (3-demethoxy-3-glucosyloxythiocolchicine) [IV] (Fig. 1).
Colchicine is a well-known traditional active principle, whose antimitotic activity is mainly exploited in the treatment of gout. Its thio- and glucosyl-derivatives show superior pharmacological properties and decreased toxicity. They are also useful intermediates for the preparation of new medicaments [1, 2].
In particular, thiocolchicoside (3-demethoxy-3-glucosyloxythiocolchicine) is an interesting active pharmaceutical ingredient, used in the therapy of diseases of the muscle–skeletal system and under evaluation for the preparation of novel antitumour and anti-inflammatory medicaments [3].
Thiocolchicoside can be obtained by thiomethylation of colchicoside, which is in turn extracted from the seeds of Colchicaceae. On the other hand, colchicine, which is present in good amount in the same seeds and other parts of the plant, can also be a precursor of thiocolchicoside.
Colchicine is firstly converted into the more stable thiocolchicine [III] by chemical thiomethylation. Subsequent multi-step conversion of the latter into thiocolchicoside can be carried out by chemical methods. Such an approach needs a preliminary demethylation at C-3 and a subsequent glucosylation at the same site (Fig. 2).
However, because of the lack of regioselectivity of the chemical demethylation, conversion yields are low and a mixture of different 1-,2-,3-demethyl derivatives and di-demethyl derivatives are obtained [4].
Furthermore, most reagents used for both reactions, demethylation and glucosylation, are expensive and toxic, and the resulting process requires complex and expensive downstream-processing steps.
On the other hand, demethylation followed by glucosylation is a typical biological pathway for drug detoxification in many living cells of animals, plants and bacteria [5]. We thus decided to explore the possibility to identify and select a specific microorganism able to perform these processes on thiocolchicine on an industrial scale.
In a previous paper, we described the regioselective demethylation of colchicine and thiocolchicine, based on a biotransformation process with strains of Bacillus megaterium isolated from soil samples [6]. This preliminary work, leading to the formation of 3-O-demethyl colchicine and 3-O-demethyl thiocolchicine, respectively, was unsatisfactory for industrial applications, due to low conversion yields and poor productivity. Recently, Dubey et al. [7] and Dubey and Behera [7, 8] reported a similar improved process for the regioselective demethylation of colchicine and thiocolchicine into their corresponding 3-demethyl derivatives. However, from an industrial point of view, final conversion to thiocolchicoside is obtained with an inefficient chemical glucosylation step.
Solet et al. [9] described the conversion of colchicine and thiocolchicine into the corresponding C-3 glucosides by selected cell cultures of Centella asiatica. On the other hand, the biotransformation was characterized by very low conversion yield (less than 10 %), low regioselectivity and extended process time (12 days), resulting in a process not suitable for a commercial/economical production of glycosylated colchicinoids like thiocolchicoside.
After a new thorough strain selection, we succeeded in identifying a B. megaterium strain able to perform the double biotransformation of thiocolchicine into thiocolchicoside, thus performing the regioselective demethylation of the methoxy-group at C-3 and the following glucosylation at the same site. The fine-tuning of the biotransformation conditions allowed the definition of an efficient, industrially green and cost effective route to thiocolchicoside [10, 11].
Materials and Methods
Chemicals
Reagents for microbial culture and product isolation and identification were from Merck, Darmstadt, Germany. Tryptone, Agar and yeast extract were purchased from Difco, UK. XAD 1180 resin was from Sigma. Thiocolchicine was from Indena SpA (Milan, Italy). Thiocolchicoside and thiocolchicine reference standards were prepared in house and chemically characterized by MS, 1H NMR, 13C NMR, IR and UV/Vis spectra. UHPLC potency (P %) was assigned taking into account chromatographic purity (A %), water content (KF), total residual organic solvents (RS) and total ash (TA) according to the following formula: P % = A % × 100/(100 − KF − RS − TA).
Microbial Strain Selection and Cultivation
Selected strains of B. megaterium, obtained from soil samples as previously described [6], were conserved in liquid nitrogen as frozen suspensions in LB (Luria–Bertani) medium added with 20–50 % glycerol.
Enrichment cultures were prepared for the selection of high-producing strains. Aliquots of frozen suspension were inoculated into 50 mL of SF2 medium composed of 4.0 % (w/v) glucose, 2.0 % (w/v) casein peptone, 0.5 % (w/v) yeast extract, 0.3 % (w/v) sodium chloride, 0.3 % (w/v) ammonium sulphate, 0.8 % (w/v) potassium hydrogen phosphate, 0.3 % (w/v) potassium di-hydrogen phosphate, 0.05 % (w/v) magnesium sulfate; pH 6.9–7.1.
Suspension cultures were incubated overnight at 30 °C, at 250 rpm on a rotary shaker (seed culture). After growth, 10 mL of seed cultures were transferred into 40 mL of fresh SF2 medium in a 300-mL Erlenmeyer flask, added with thiocolchicine to a final concentration of 2.5 g/L. The resulting cultures were incubated for 72 h at 30 °C, 250 rpm. Aliquots of broth from each subculture were plated on LB Agar (DSMZ medium N. 381), containing thiocolchicine at the same concentration used in the suspension, and on LB Agar without colchicinoid as control.
The single colonies, grown on agar plates at the highest thiocolchicine concentration, were transferred on LB Agar medium without colchicinoids, in glass tubes, incubated overnight at 30° C and subsequently conserved at 4° C for biotransformation experiments.
Biotransformation Experiments
Preliminary experiments for the selection of the best producing strains were performed in 300-mL Erlenmeyer flasks, containing 50 mL of SF2 medium, at 30 °C on a rotary shaker at 250 rpm for 4 days. Colchicine and thiocolchicine were added at growth start, at different final concentrations, up to 2.5–3 g/L. The best performing cultures were frozen and conserved in liquid nitrogen for further experiments.
Aliquots of frozen culture of B. megaterium, selected as described above, were utilized for the inoculum of seed cultures (i.e. preculture) in 1,000-mL Erlenmeyer flasks, containing 250 mL of medium SF2. Precultures were incubated overnight at 30 °C on a rotary shaker at 250 rpm. After incubation, 500 mL of preculture were transferred in sterile into a 14 L fermenter, containing 9.0 L of fresh medium SF2, added with thiocolchicine (1.5 g/L) or 3-O-demethyl-thiocolchicine (1.0 g/L). Fermentations were carried out at 30 °C, keeping suitable levels of stirring-aeration (stirring up to 700 rpm; aeration up to 1.8 vvm (volume of air per volume of fermentation broth per minute; in SI accepted units, L/L/min) depending on the culture growth level). Culture samples were withdrawn for the following analysis:
-
Growth level, measured as optical density (OD) at 550 nm;
-
Axenicity and purity analysis of the strain on LB Agar;
-
Microscopic morphology (Gram stain);
-
Analysis of thiocolchicoside content, by TLC and UHPLC.
Biotransformation experiments were stopped when no significant increase of glucosylated product was detected by UHPLC analysis.
Analytical Methods
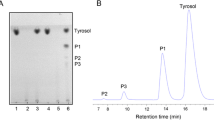
Bioconversion level was monitored by TLC and UHPLC analysis.
TLC analysis was performed on silica gel, with an acetone:ethylacetate:water 5:4:1 eluent system. The content in thiocolchicoside of the supernatant was determined by UHPLC as described hereupon. Apparatus: Waters Acquity UPLC H-Class. Column: Waters Acquity BEH Phenyl (100 mm × 2.1 mm, particle size 1.7 μm). Column temperature: 25 °C. Detection: 370 nm. Injection volume: 1.0 μL. Flow: 0.400 mL/min. Linear gradient program, solvent A: 99 % at 0 min, 99 % at 1.0 min, 92 % at 5.0 min, 70 % at 6.5 min, 50 % at 7.8 min, 2 % at 8.2 min, 2 % at 9.0 min, 99 % at 9.1 min and 99 % at 10.0 min. Solvent A: water (with ammonium formate 10 mM, adjusted to pH 3.0 with formic acid) and tetrahydrofuran in ratio 93/7 (V/V). Solvent B: acetonitrile (with formic acid 50 ppm) and tetrahydrofuran in ratio 90/10 (V/V).
Product Isolation
The final culture broth from biotransformation (10.8 L) was subjected to cross-flow microfiltration on a 0.22-μm ceramic cartridge, to separate cells from soluble fraction. After an initial volume reduction to 3.0 L of retentate, the culture medium was replaced by dilution with saline solution (NaCl 0.9 %; 3 × 3.0 L) and further concentrated to a final volume of 3.0 L. The permeate (microfiltered broth and saline solution) was adsorbed on a column filled with Amberlite XAD 1180 adsorption resin (650 mL of bed volume, BV). After washing with water (2.0 L) at a flow rate of 1 BV/h, the product was eluted with methanol (2.0 L) at a flow rate of 1 BV/h. The methanol was concentrated to dryness under vacuum, yielding 47.0 g of solid. The latter was dissolved in 50 % v/v methanol (230 mL) and extracted with dichloromethane (10 × 60 mL). The hydroalcoholic phase was concentrated to dryness, providing 24.2 g of solid. The latter was dissolved in a 1:1 ethanol-dichloromethane mixture (470 mL). The solution was clarified on a pad of silica gel (5.0 g), which was then washed with the same solvent mixture (100 mL). Dichloromethane was removed by evaporation, the resulting solution concentrated to 140 mL and left to crystallize overnight at 25 °C. The solid was filtered and dissolved in a 1:1 ethanol-chloroform mixture (470 mL). The solution was clarified on a pad of silica gel (5.0 g), which was then washed with the same solvent mixture (100 mL). Chloroform was removed by evaporation, the resulting solution was concentrated to 140 mL and left to crystallize overnight at 25 °C. Thiocolchicoside was obtained as a yellow solid (18.4 g).
Molecular Identification
All NMR experiments were performed on a VARIAN INOVA300 FT MHz spectrometer (Varian, Palo Alto, California USA) operating at 7.05 T. 1H and 13C NMR experiments were recorded at 300 MHz for 1H and 75 MHz for 13C. Chemical shifts are reported in part per million (ppm) downfield from TMS, and all the spectra were recorded on DMSO-d6 solution. Raw files were processed with VNMRJ™ Software.
Results
Screening for High-Activity Strains
The selection of microbial cultures of B. megaterium, in the presence of increased concentrations of thiocolchicine in selective growth medium, led to the isolation of colchicinoids resistant strains, able to grow at high level of toxic thiocolchicine (2.5 g/L) in 48–72 h. Strains with increased resistance to thiocolchicine occurred in the original population of B. megaterium with a frequency of about 1:500: viable count on LB Agar supplemented with 2.5 g/l of thiocolchicine was 1 × 106 versus 6.2 × 108 cfu/mL on LB Agar without additives.
Some selected strains, tested in preliminary bioconversion assay on thiocolchicine, showed a good growth on the substrate and a sensibly higher bioconversion activity, if compared with the original strain. In particular a strain, described as B375/11, was selected for its high-biotransforming activity, leading to an almost total conversion of thiocolchicine into thiocolchicoside.
In comparison with the new strain the original one revealed a different behaviour, with markedly lower growth rate and final productivity (conversion rate of about 60 %, with about 35 % residual, unconverted thiocolchicine).
Biotransformation Experiments in Fermenter
Frozen cultures of the selected strain were utilized for biotransformation experiments in medium SF2, in a 14 L fermenter. In such condition the strain confirmed its ability to perform a quantitative conversion of thiocolchicine: 20 g of thiocolchicoside were obtained from 15 g of thiocolchicine, with a molar yield of about 98 %, in a process lasting about 30 h (Fig. 3). The bioconversion in lab-scale experiments clearly shows a partial and transitory accumulation of 3-O-demethyl-thiocolchicine (Fig. 4a) followed by the formation of the glucosyl-derivative, thiocolchicoside (Fig. 4b).
Given the nature of this process, based on two independent steps, it was consequent to apply the same approach for the biotransformation of 3-O-demethyl-thiocolchicine obtained from 3-O-demethyl-colchicine, another metabolite commonly present in the seeds of Colchicaceae. An amount of 10 g of 3-O-demethyl-thiocolchicine was converted into about 13 g of thiocolchicoside, in a 30-h fermentation process, with a molar yield of about 93 %.
Product Isolation and Identification
The product contained in the final culture broth from fermentation (total volume: about 10.8 L, containing about 20 g of thiocolchicoside, as determined by UHPLC analysis), was purified as described in “Materials and Methods”.
A total amount of 18.4 g of product was obtained after purification, with a purification yield of 92 % and a purity >99.9 %.
Thiocolchicoside was identified on the basis of the 1H and 13C-NMR spectra. 1H-NMR spectrum showed an exchangeable broad doublet at δ 8.60, which was assigned to the NH group. The AB system at δ 7.25 and δ 7.13 was attributed to H-12 and H-11. The singlets at δ 7.00 and δ 6.84 were assigned to H-8 and H-4, respectively. Finally, the two singlets at δ 3.82 and δ 3.52 were attributed at the OMe groups.
13C-NMR: (DMSO-d6) 181.0 s, 168.5 s, 157.3 s, 150.9 s, 150.3 s, 141.0 s, 137.3 s, 133.9 d, 127.7 d, 126.3 d, 126.3 s, 110.9 d, 100.2 d, 77.1 d, 76.8 d, 73.3 d, 69.8 d, 60.9 t, 60.9 q, 51.3 d, 35.5 t, 29.2 t, 22.3 q and 14.3 q.
The NMR spectral assignments of thiocolchicoside were confirmed by the literature data [12].
Discussion
Thiocolchicine can be biotechnologically converted into thiocolchicoside with high conversion yields and purity, quality consistency and reasonable costs. Furthermore, the mild operational conditions, typical of a biotransformation system, offer an interesting environmental-friendly production process.
By targeted selection of thiocolchicine-resistant strains of B. megaterium, thiocolchicine conversion yield was improved tenfold compared to the wild-type bacterial strain and, moreover, it was possible to obtain straightly the pharmacologically active principle (thiocolchicoside) instead of its precursor (3-O-demethyl-thiocolchicine). Further optimization works are in progress, envisaging even higher productivity (data not shown).
Thanks to its high regioselectivity, the key role is expressed by a monooxygenase, the cytochrome P450BM-3, whose gene CYP102A1 has been recently cloned and functionally expressed in E. coli [13]; it is similar to the enzyme present in human liver (CYP3A4), which catalyses the demethylation of colchicine and thiocolchicine [14–16], and could represent a detoxification system for the bacterial cell in this particular species. The glucosylating activity, on the other hand, allows the prompt conversion of 3-O-demethyl thiocolchicine into the final product, avoiding the accumulation of the intermediate and any inhibitory effect on the demethylation step.
For its regioselectivity and flexibility such biocatalytic system, supported by a preliminary, well-targeted screening of colchicinoid resistant strains of B. megaterium, has been applied for the biosynthesis of glycosylderivatives of other colchicinoids as well [10], and therefore offer an interesting and useful tool for further biotechnological applications, in the industrial production of plant-derived active ingredients.
Recently, a new bioconversion method—based on a strain of the Gram-negative bacterium Providencia vermicola, first isolated from the gut of a nematode—has been described; also in this case, the authors claim to convert thiocolchicine directly into thiocolchicoside in a single fermentation process [17]. Providencia is a genus of the family Enterobacteriaceae, strictly related to the genera Proteus and Morganella, including human opportunistic pathogens isolated from human diarrheal stools, urinary tract infections, wounds, burns and bacteremias, and from penguins; furthermore, there are indications of Providencia strains capable of developing resistance against an extended antibiotic spectrum [18, 19].
We have selected a safe, non-recombinant microbial source, B. megaterium [20, 21], non-pathogenic for humans (Biosafety Level 1), non-invasive, as demonstrated by our consolidated experience at industrial fermentation scale; last but not least, thanks to its particular cellular size and morphology, it can be easily differentiated from possible contaminants by simple and fast microscopic analysis, thus allowing a practical and efficient in process control of fermentation broth axenicity.
The chemical characterization of the purified fermentation product confirms its pharmaceutical quality, so that the herein described biotransformation process has been scaled at 20 m3 volume and is currently applied for the industrial production of thiocolchicoside as API in compliance with current Good Manufacturing Practices, under approval of the Italian Health Authority.
References
Boye, O., & Brossi, A. (1992). Tropolonic colchicine alkaloids and allocongeners. In A. Brossi & G. Cordell (Eds.), The alkaloids, chemistry and pharmacology. New York: Academic Press.
Brossi, A., et al. (1988). Colchicine and its analogues: Recent findings. Medicinal Research Reviews, 8, 77–94.
Reuter, S., Prasad, S., Phromnoi, K., et al. (2010). Thiocolchicoside exhibits anticancer effects through downregulation of NF-kB pathway and its regulated gene products linked to inflammation and cancer. Cancer Prev Res, 3, 1462–1472.
Roussel UCLAF (1956). Colchicine derivatives. Patent GB762706 (A).
Gibson, G. G., & Skett, P. (2001). Introduction to drug metabolism. Cheltenham: Nelson Thornes.
Poulev, A., Bombardelli, E., Ponzone, C., & Zenk, M. H. (1995). Regioselective bioconversion of colchicine and thiocolchicine into their corresponding 3-demethyl derivatives. Journal of Fermentation and Bioengineering, 79, 33–38.
Dubey, K. K., Ray, A. R., & Behera, B. K. (2008). Production of demethylated colchicine through microbial transformation and scale-up process development. Process Biochemistry, 43(3), 251–257.
Dubey, K. K., & Behera, B. K. (2011). Statistical optimization of process variables for the production of an anticancer drug (colchicine derivatives) through fermentation: at scale-up level. New Biotechnology, 28(1), 79–85.
Solet, J. M., Bistermiel, F., Galons, H., Spagnoli, R., Guignard, J. L., & Cosson, L. (1993). Glucosylation of thiocolchicine by a cell-suspension culture of Centella asiatica. Phytochemistry, 33(4), 817–820.
Bombardelli E., & Ponzone C. (1996). A process for the biotransformation of colchicinoid compounds into the corresponding 3-glycosylderivatives. European Patent No EP 0 931 161 B1.
Ponzone C. (2004). Biotransformation of colchicinoid compounds. European Patent No EP 1 745 140 B1.
Clerici, F., Mottadelli, S., & Rossi, L. M. (1995). 1H- and 13C-NMR spectra of thiocolchicine and derivatives: A complete analysis. Journal of Natural Products, 58(2), 259–263.
Dubey, K. K., Haque, S., Jawed, A., Singh, B. P., & Behera, B. K. (2010). Construction of recombinant Escherichia coli for enhanced bioconversion of colchicine into 3-demethylated colchicine at 70 l bioreactor level. Process Biochemistry, 45, 1036–1042.
Urlacher, V. B., & Schmid, R. D. (2004). Protein engineering of the cytochrome P450 monooxygenase from Bacillus megaterium. Methods in Enzymology, 388, 208–220.
Urlacher, V. B., Lutz-Wahl, S., & Schmid, R. D. (2004). Microbial P450 enzymes in biotechnology. Applied Microbiology and Biotechnology, 64, 317–325.
Tateishi, T., Soucek, P., Caraco, Y., Guengerich, F. P., & Wood, A. J. J. (1997). Colchicine biotransformation by human liver microsomes: identification of CYP3A4 as the major isoform responsible for colchicine demethylation. Biochemical Pharmacolology, 53, 111–116.
Prabakaran, K., et al. (2012). A microbial method for the biotransformation of colchicinoid compounds. Patent No WO 2012/038982 A2.
Bergey, D. H., & Holt, J. G. (1994). Bergey’s manual of determinative bacteriology (9th ed., Vol. 185). Baltimore: Lippincott Williams & Wilkins.
Mohr O’Hara, C., Brenner, F. W., & Miller, J. M. (2000). Classification, identification and clinical significance of Proteus, Providencia, and Morganella. Clinical Microbiology Reviews, 13(4), 534–546.
Vary, P. S. (1992). Development of genetic engineering in Bacillus megaterium: an example of the versatility and potential of industrially important bacilli. In Doi & McGloughlin (Eds.), Biology of bacilli: Applications to industry (pp. 251–310). Boston: Butterworths-Heinemann.
Vary, P. S. (1994). Prime time for Bacillus megaterium. Microbiology, 140, 1001–1013.
Acknowledgments
The authors are thankful to Dr. Eric De Combarieu (Indena SpA) for giving experimental details regarding NMR analysis, Dr. Francesco Villa (Indena SpA) for reference standard characterization and Dr. Stephen Beszant (Indena SpA) for his linguistic help in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ponzone, C., Berlanda, D., Donzelli, F. et al. Biotransformation of Colchicinoids into Their Corresponding 3-O-Glucosyl Derivatives by Selected Strains of Bacillus megaterium . Mol Biotechnol 56, 653–659 (2014). https://doi.org/10.1007/s12033-014-9741-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-014-9741-5