Abstract
A small number of stress-responsive genes, such as those of the mitochondrial F1F0-ATP synthase complex, are encoded by both the nucleus and mitochondria. The regulatory mechanism of these joint products is mysterious. The expression of 6-kDa subunit (MtATP6), a relatively uncharacterized nucleus-encoded subunit of F0 part, was measured during salinity stress in salt-tolerant and salt-sensitive cultivated wheat genotypes, as well as in the wild wheat genotypes, Triticum and Aegilops using qRT-PCR. The MtATP6 expression was suddenly induced 3 h after NaCl treatment in all genotypes, indicating an early inducible stress-responsive behavior. Promoter analysis showed that the MtATP6 promoter includes cis-acting elements such as ABRE, MYC, MYB, GTLs, and W-boxes, suggesting a role for this gene in abscisic acid-mediated signaling, energy metabolism, and stress response. It seems that 6-kDa subunit, as an early response gene and nuclear regulatory factor, translocates to mitochondria and completes the F1F0-ATP synthase complex to enhance ATP production and maintain ion homeostasis under stress conditions. These communications between nucleus and mitochondria are required for inducing mitochondrial responses to stress pathways. Dual targeting of 6-kDa subunit may comprise as a mean of inter-organelle communication and save energy for the cell. Interestingly, MtATP6 showed higher and longer expression in the salt-tolerant wheat and the wild genotypes compared to the salt-sensitive genotype. Apparently, salt-sensitive genotypes have lower ATP production efficiency and weaker energy management than wild genotypes; a stress tolerance mechanism that has not been transferred to cultivated genotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abiotic stresses can decrease the yield potential to less than 50 % of that possible under typical growing conditions [4]. One of the most yield-threatening important stresses is salt stress [10]. About 20 % of the all cultivated land and nearly half of the irrigated land are affected by soil salinity [13]. Soil salinity is increasing, especially in arid and semiarid climates as example Iran, where 12.5 % of the agricultural lands are affected by soil salinity [2].
Wheat is the major crop among all grains in the world and is well adapted to the Mediterranean environments, where it often suffers salt stress due to increasing soil salinity [28]. Iran is one of the main regions where wild relatives of wheat evolved [35]. These species are valuable recourses for wheat improvement, distributed in various regions of central Asia [49], especially in arid and semiarid regions of Iran [2].
Extensive genetic studies of wild germplasm have indicated considerable variation in biotic and abiotic stress tolerance, but this valuable variation has been difficult to exploit due to the relatively poor understanding of the underlying molecular basis of stress tolerance in wild wheat species [26]. Another limitation is that their genomes have not been completely sequenced. Many stress tolerance mechanisms of the wild genotypes have not been transferred to the current cultivated genotypes. For example, Gorham et al. [20] tested several accessions of Ae. crassa and showed they have much lower Na+ concentrations and higher K+/Na+ ratios in leaves than durum wheat, similar to bread wheat. They suggested that genome D imparts the Na+ ‘exclusion’ and enhances the K+/Na+ discrimination in bread wheat.
Another stress tolerance mechanism in plants is the synthesis of compatible solutes such as sucrose, proline, and glycine betaine [22]. These compounds play vital roles in higher stress tolerance of the wild genotypes than cultivated genotypes. Compatible solutes synthesis mechanisms are associated with energy cost and therefore involve a potential growth penalty. For example, the ATP requirement for the synthesis or accumulation of solutes has been estimated as 3.5 for Na+, 34 for mannitol, 41 for proline, 50 for glycine betaine, and 52 for sucrose [41].
Mitochondria are the unique and important organelles in terms of ATP (energy) production for the eukaryotic cell [6]. The mitochondrion is often one of the first recognition sites of stress within the cell, as its activities and responses are crucial in maintaining cell viability during these conditions [32]. The central role of the mitochondria in stress tolerance has been well-documented [37]. ATP, the universal biological energy currency, is synthesized by the mitochondrial F1F0-ATP synthase complex that is final complex of the electron transport chain [6]. This complex generally provides the required ATP for osmoticum synthesis under stress. Communications among the nucleus, mitochondria, and chloroplasts are essential in stress tolerance. In eukaryotic organisms, all of the subunits of the F1 (α3β3γδε) and a subset of the F0 part are encoded by the nucleus, and only two or three of the hydrophobic components of the F0 part are encoded by mitochondrial DNA [1].
It seems that MtATP6 subunit (mitochondrial F1F0-ATP synthase 6-kDa subunit) as a anterograde signal protein, encoding by nucleus is a subunit of the F0 part of the plant mitochondrial F1F0-ATP synthase complex but also may be a subunit of the chloroplastic F1F0-ATP synthase complex, probably playing the same function in activating the complex in both organelles. In other words, 6-kDa subunit is a communicating protein between the nucleus and these two organelles in the regulation of energy metabolism of cell under stress.
Some evidences come from expression and transformation experiments. Hamilton et al. [21] reported that exposure of wheat (T. aestivum) to aluminum increases the mitochondrial F1F0-ATP synthase complex activity only in aluminum-tolerant genotype. Zhang and Liu [59] identified a salt-responsive gene (RMtATP6, NCBI accession no. AB05576) from rice that is a subunit of F0F1-ATP synthase. MtATP6 from Arabidopsis (AtMtATP6, AK117680 and NM_148152), rice (RMtATP6, HM173320), wheat (WMtATP6, GQ503255), and Ae. crassa (AcMtATP6, GU183146) and T. monococcum ssp. aegilopoides (T. boeoticum) (TmMtATP6, GU183145) have been isolated [31, 33]. Overexpression of AtMtATP6 and RMtATP6 significantly enhanced the tolerance of transgenic Arabidopsis, transgenic tobacco and yeast under salt, cold, oxidative, and drought stresses [60, 61]. For example, functional evaluation of the mitochondrial F1F0-ATP synthase 6-kDa subunit (MtATP6) in salt-hypersensitive mutant yeast showed that it confers NaCl tolerance up to 1,000 mM NaCl [12]. The expression of the ApNa +-atp operon (a putative F1F0-type Na+-ATP synthase) of Aphanothece halophytica in a heterologous cyanobacterium Synechococcus sp. PCC7942 increases salt stress tolerance [46]. These findings demonstrate that 6-kDa subunit plays a vital role in energy homeostasis in response to wide range of environmental stresses.
In this study, a particular attention was paid to 6-kDa subunit, a nucleus-encoded subunit of the F0 part of the plant mitochondrial F1F0-ATP synthase complex, whose role is not clearly understood in abiotic stress tolerance mechanisms [1]. The MtATP6 expression in Mahuti (salt-tolerant) and Alamut (salt-sensitive) cultivated wheat genotypes, (2n = 6x = AABBDD), in the wild wheat plants T. boeoticum (2n = 2x = AA), and Ae. crassa (2n = 4x = MMDD) was analyzed by real-time PCR under salt stress to investigate its possible biological role in salt tolerance in wheat. Another reason for using Ae. crassa and T. boeoticum was to examine and compare the activity of 6-kDa subunit in the A and D wheat genomes.
In addition, regarding the prominent role of promoter analysis in identifying the unknown function and underlying regulatory mechanism in silico promoter analysis carried out on MtATP6 orthologous genes. The promoter of a drought-, high salinity-, and cold-inducible gene contains the major cis-acting elements, Abscisic acid response element (ABRE); Myelocytomatosis oncogene cellular homolog/Myeloblastosis viral oncogene homolog (MYB/MYC), and dehydration-responsive element (DRE), which are involved in stress-inducible gene response [55]. The existence of these elements can explain how MtATP6 respond to several types of stresses, especially abiotic stresses, via an intricate mechanism. In addition, protein structure analysis is another strategy that can help to identify functions of 6-kDa subunit. In fact, another our hypothesis is that 6-kDa subunit may be a nuclear regulatory factor transferring to mitochondria.
With regards to the recent findings indicating a prominent role for 6-kDa subunit, the data presented here will provide further evidences of 6-kDa subunit contribution to environmental stress tolerance based on nuclear and mitochondrial communications.
Materials and Methods
Plant Materials and Growth Conditions
In this experiment, Mahuti (salt-tolerant) and Alamut (salt-sensitive) genotypes were used as cultivated wheat (2n = 6x = 42, AABBDD), and T. boeoticum (2n = 2x = 14, AA) and Ae. crassa (2n = 4x = 28, MMDD) were used as wild wheat relatives. Imbibed seeds were kept in the dark for 24 h at 4 °C and germinated for 3 days (d) at 25 °C. Then, germinated seeds were uniformly selected and transferred to the hydroponic culture medium. Seedlings were irrigated with a modified Hoagland solution with pH 6.8 [22]. A 16 h light/8 h photoperiod and 25 °C temperature were used in a stabilized greenhouse.
Stress Conditions
After plants reached to the “Zadoks code” 13 (3 leaves stage) (http://www.lethamshank.co.uk/gs.htm), salinity treatments, including control 0, 50, 100, and 200 mM NaCl, were used in combination with Hoagland solution. All solutions contained CaCl2 to maintain the Na+:Ca2+ ratio below 10:1. Sampling was carried out after 0, 3, 6, 10, 24, and 72 h from leaves of all treatments.
RNA Extraction
Total RNA was extracted from leaf samples using RNX-Plus buffer (Cinnagen, Tehran, Iran). Briefly, about 100 mg of leaf was grounded in liquid nitrogen. The ground powder was transferred to 1 ml of RNX-Plus buffer in an RNase-free microtube, mixed thoroughly and left at room temperature for 5 min. Chloroform (0.2 ml) was added to the slurry, and mixed gently. The mixture was centrifuged at 10,000×g at 4 °C for 15 min, and the supernatant was transferred to a new tube and precipitated with an equal volume of isopropanol for 15 min on ice. The RNA pellet was washed using 75 % ethanol, briefly dried, and resuspended in 50 μl of RNase-free water. The integrity and quantity of RNA was checked by visual observation of 28S and 18S rRNA bands on a 1.2 % agarose gel. Then, the quantity and concentration of RNA was measured using a NanoDrop ND 1000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). Prior to use, RNA samples were stored at −80 °C.
DNase Treatment and cDNA Synthesis
DNase treatment was carried out using a Fermentas DNase Kit (Fermentas, Leon-Rot, Germany) according to the manufacturer’s instructions. Again, the integrity of RNA was checked by visual observation of 28S and 18S rRNA bands on a 1.2 % agarose gel. Then, 1 μg of DNase-treated RNA was used for first-strand cDNA synthesis using 100 pmol oligo-dT (18-mer), 15 pmol dNTPs, 20 U RNase inhibitor and 200 U M-Mulv reverse transcriptase (all from Fermentas) in a 20 μl final volume. The cDNA samples were stored at −20 °C prior to use.
Primer Design
Primers were designed using Allele ID 7 and Vector NTI 10 software for the reference genes and MtATP6 (Table 1). MtATP6 primers were designed based on the aligned nucleotide file, including AB055076 [60], HM173320, GQ503255, GU183145, and GU183146 [33]. The wheat elongation factor-1α (ef1α) (M90077) and beta tubulin (β-tubulin) (U76745) genes were used as the reference genes for data normalization because salt stress does not influence their expression [24]. In this project in order to higher precise, we quantified the final expressions based on the means of two reference genes of ef1α and β-tubulin.
qRT-PCR Analysis
First, primer specificity was confirmed by PCR and sequence analysis. To minimize pipetting error, the cDNA samples were diluted 1:10 by using nuclease-free water, and 5 μl cDNA was used for qRT-PCR.
Relative qRT-PCR was performed in a 20 μl volume containing 5 μl cDNA (diluted), 10 μl 2× Sybr Green buffer, 0.5 μl of 10 μM primers (forward and reverse), and 0.12 U of Taq DNA polymerase (5 U/μl) (all from Bioer, China). The amplification reactions were carried out in a Line-gene K thermal cycler (Bioer, China) under the following conditions: 2 min at 94 °C, 40 cycles of 94 °C for 10 s, 57 °C for 15 s, and 72 °C for 30 s. After 40 cycles, the specificity of the amplifications was tested by melting curve analysis by heating from 50 to 95 °C. All amplification reactions were repeated two times under identical conditions and included a negative control and 5 standard samples. To ensure that the PCR products were generated from cDNA and not genomic DNA, proper control reactions were carried out without reverse transcriptase treatment.
Statistical Data Analysis
For qRT-PCR data, the relative expression of MtATP6 was calculated based on the threshold cycle (CT) method. The CT for each sample was calculated using the Line-gene K software and the Larionov et al. [27] method. When replicate PCRs are run on the same sample, it is more appropriate to average C T data before performing the 2 −ΔΔCT calculation [24].
The determined mean C T values for both the target (MtATP6) and internal control (elongation factor-1α and β-tubulin) genes were used in equation 2 −ΔΔCT = (C T, MtATP6 − C T, housekeeping genes)Time x − (C T, MtATP6 − C T, housekeeping genes)Time 0. Time x represents the expression of the MtATP6 any time point after salt stress and Time 0 represents the expression of the MtATP6 before salt stress. The fold change ratios of MtATP6 were normalized to internal control genes and were calculated relative to the expression at time zero.
Finally, statistical analysis was carried out from the replicate samples at each time point using MINITAB 14 (Minitab, Inc., Pennsylvania, USA) and SAS6.12 (SAS institute Inc., Cary, NC, USA). Analysis of variance was employed to investigate significant differences in the MtATP6 expression profile under different NaCl concentrations (0, 50, 100, and 200 mM) and different times (0, 3, 6, 10, 24, 72 h) after NaCl treatment. Mean comparisons were carried out by Duncan’s multiple range test in SAS. Pearson’s correlation coefficient was used to quantify the relationship between gene expression levels under different NaCl concentrations and different times after NaCl treatment. A paired t test was used to determine the significance of differences in the gene expression profiles between wheat genotypes.
Promoter Identification and Promoter Analysis
To identify the promoter region of the 6-kDa subunit gene in rice (RMtATP6, AB05576) and Arabidopsis (AtMtATP6, AK1176809) and its paralogous genes (AtMtATP6, AK117680, and NM_148152), CDS (coding sequence) of MtATP6 were blasted in the Phytozome database (http://www.phytozome.net/). Then, the 2000 bp upstream of the transcriptional start point of rice (RMtATP6) and Arabidopsis (AtMtATP6 homologous genes) 6-kDa subunit were considered as promoters [33] (Supplementary data 2).
The obtained 2000 bp upstream region of the MtATP6 CDS was analyzed using the PlantCARE database [29]. This database focuses on cis-acting elements, aiming to identify the regulatory mechanism that determines the expression profiles [29].
Results
The Quantitative Expression Patterns of MtATP6 in Wheat Genotypes Under Different Salt Concentrations
The differences in the MtATP6 expression profile at different times after NaCl treatment in each wheat genotype were investigated (Fig. 1). A sudden significant increase (P ≤ 0.05) in MtATP6 transcript 3 h after NaCl treatment happened in all genotypes except salt-sensitive genotype Alamut (Fig. 1). Seventy-two hours (72 h) after NaCl treatment, MtATP6 expression in Ae. crassa was significantly higher compared to Mahuti and Alamut (Table 2 part A), but T. boeoticum was not different from Mahuti or Alamut (Table 2 part A). There was a sudden decrease in MtATP6 72 h after NaCl treatment in all of the genotypes (Table 2 part A; Fig. 1). Expression analysis showed significant differences between all four genotypes at each of the 50, 100, and 200 mM NaCl concentrations (Table 2 part B; Fig. 1). There was a significant difference in MtATP6 level between 72 h and every other time point at 200 mM (Table 2 part B). Finally, there was a significant difference between the MtATP6 expression pattern at 10 and 24 h compared to 72 h at 50 mM (Table 2 part B; Fig. 1).
MtATP6 expression pattern under different NaCl concentrations (control, 50, 100, and 200 mM NaCl) and different times after NaCl treatment (0, 3, 6, 10, 24, 72 h) in Mahuti (salt-tolerant) and Alamut (salt-sensitive) genotypes as cultivated wheat (2n = 6x = 42, AABBDD), and T. boeoticum (2n = 2x = 14, AA) and Ae. crassa (2n = 4x = 28, MMDD) as wild wheat relatives. The x-axis shows time after NaCl treatment and y-axis shows the MtATP6 relative expression
Investigation of Differences in MtATP6 Expression Profile Under NaCl Concentrations in Each Wheat Genotype
Analysis of variance showed significant differences between MtATP6 expression in Ae. crassa and Alamut under different NaCl concentrations. Ae. crassa had significantly higher expression of MtATP6 in 100 and 200 mM NaCl compared to 50 mM, while T. boeoticum and Mahuti did not have significantly higher MtATP6 expression in 100 mM NaCl compared to 50 or 200 mM (Table 3). In contrast, Alamut had significantly higher expression of MtATP6 in 50 mM NaCl compared to 100 and 200 mM. In all NaCl concentrations, in general, wild genotypes had greater MtATP6 expression than cultivated genotypes. In all the NaCl concentrations, Ae. crassa had the highest MtATP6 expression and Alamut the lowest (Table 3; Fig. 1).
Analysis of Variance for the Differences in MtATP6 Expression in Wheat Genotypes Under NaCl Concentrations
We observed significant differences (P ≤ 0.05) in MtATP6 expression level between all four genotypes at 50, 100, and 200 mM NaCl. The MtATP6 expression level of Ae. crassa was significantly different from every other genotype at 100 and 200 mM. There was no significant difference between Mahuti and Alamut at any NaCl concentration. Nor was there a significant difference between Ae. crassa and T. boeoticum at 50 mM (Table 4; Fig. 1). Thus, it seems that Ae. crassa, due to the presence of genome D, shows a different expression pattern of MtATP6 than other genotypes.
Comparison of the MtATP6 Expression Profile in Different Wheat Genotypes
The comparison of the MtATP6 expression profiles in different wheat genotypes using the paired t test showed significant (P ≤ 0.05) differences between T. boeoticum and Mahuti, Ae. crassa and Mahuti, T. boeoticum and Alamut, Ae. crassa and Alamut, and Ae. crassa and T. boeoticum (Table 5). The two cultivated genotypes (Alamut and Mahuti) were not significantly different. MtATP6 showed the greatest expression fold change (3.67, P ≤ 0.01) between Ae. crassa and Alamut (salt-sensitive cultivar). MtATP6 also showed a greater expression fold change in T. boeoticum than the cultivated genotypes (Table 5; Fig. 1). All together, it seems that longer-term MtATP6 expression is one of the reasons for the superiority of wild relatives. It is possible that the mechanism of energy management of wild genotypes have not been fully transferred to cultivated genotypes.
Relationships Between MtATP6 Expression in Different NaCl Concentrations
The MtATP6 expression levels in different NaCl concentrations were compared using Pearson’s correlation coefficient. There was a significant (P ≤ 0.05) positive correlation in gene expression profile between 50 and 100 mM in T. boeoticum (P = 0.01) and between 100 and 200 mM NaCl in Alamut (P ≤ 0.01). In contrast, there was no significant relationship between gene the expression profiles of Mahuti and Ae. crassa at any NaCl concentration.
Correlations Between the Expression Levels of MtATP6 at Different Times After NaCl Treatment
In Alamut, significant correlations were observed between MtATP6 expression at 3 and 72 h (P ≤ 0.01), 6 and 10 h (P ≤ 0.01), and 6 and 24 h after NaCl treatment (P ≤ 0.05). Similar results were obtained between 3 and 72 h (P ≤ 0.05) and 6 and 24 h (P ≤ 0.05) in T. boeoticum. However, there were no temporal correlations in Mahuti or Ae. crassa (Fig. 1). It seems that MtATP6 expression profiles of Mahuti and Ae. crassa (salt-tolerant genotypes) are similar over time (Fig. 1).
Comparison of the MtATP6 Expression Fold Changes Before and After Salt Stress in Each Genotype
The MtATP6 expression fold changes before and after salt stress in each genotype was compared by using the paired t test. There were significant (P ≤ 0.05) differences between 0 and 100 mM and between 0 and 200 mM in Ae. crassa only; MtATP6 expression did not change significantly in Mahuti, Alamut or T. boeoticum after salt stress compared to before. Finally, the wild genotypes had greater expression of MtATP6 than the cultivated genotypes after stress (Table 6), while the expression of MtATP6 in wild genotypes (especially Ae. crassa) was lower than in the cultivated genotypes before stress (Table 6).
The MtATP6 expression profile at different times before NaCl treatment in each wheat genotype showed significant (P ≤ 0.05) differences between the four genotypes (Table 6). Additionally, there was a significant difference between the MtATP6 levels of T. boeoticum and those of other genotypes before NaCl treatment.
In Silico Promoter Analysis and Protein Structure Prediction
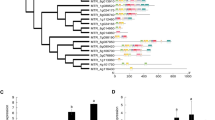
The MtATP6 promoter analysis showed the existence of the elements MYB/MYC, W-boxes, ABRE, and GTLs (Fig. 2). This suggested that the 6-kDa subunit might induce under different stress conditions (Fig. 2) (Supplementary data 2) (Table 7). A mitochondrial signal peptide, an N-terminal cleavage site, phosphorylation sites, and a transmembrane domain were founded in RMtATP6 [33].
The sequence (2000 bp) of the putative promoter region of the RMtATP6 (HM173320) in rice; the ATG in the translation initiation codon (Adenine) was regarded as +1. The putative CAAT and TATA boxes are shaded and underlined. The ABRE, MYB, MYC, GTLs, and W-boxes as early responsive elements that are potentially involved in stress responsiveness are shown, shaded, and boxed. Based our hypothesis, the ABA hormone correlates the mitochondria and nucleus communications based on activation of ABA responsive elements of RMtATP6 promoter
Discussion
The central role of mitochondria in the adaptation to environmental stresses at the subcellular level has become evident [28]. The electron transport chain in the plant mitochondrion is one of the aspects underlying the so-called energetic flexibility [9]. The mitochondrial F1F0-ATP synthase is the terminal complex of the electron transport chain that catalyzes the final step of ATP production in the cell [6]. One of the newly found subunits of the mitochondrial F1F0-ATP synthase is MtATP6, whose overexpression in transgenic plants and yeast confers a considerable resistance to various abiotic stresses [12, 60, 61]. However, its role in response to environmental stresses is still unclear. More importantly, it is unknown which role the nucleus plays in the energy production that occurs through the F1F0-ATP synthase complex in mitochondria. Additionally, it will be interesting to know whether this salt tolerance pathway based on energy management confers a selective advantage to wild genotypes.
We presumed that MtATP6, as a nuclear regulatory element, regulates mitochondrial F1F0-ATP synthase activity to produce more ATP, especially in stress-tolerant wheat genotypes and in particular in Aegilops genotype DD, when the cell needs additional energy to deal with an abiotic stress. Stress conditions have a great impact on the cellular ATP levels. Ensuing energy deficiency signal triggers downstream transcriptional responses that are shared by different types of stresses [3]. In fact, energy support can be seen as a crosstalk between different stresses system responses this can happen because of activation of other resistance mechanisms in wheat to deal with salinity or because sudden salt stress shock increases in the expression of other salt-responsive genes, for example, metallothionein-like protein [8, 41], HKT (high affinity potassium transporter) [58], SOS1 (salt overly sensitive), and SOS4 [40] are observed after salt treatment; therefore, the first response (increased ATP production) seems to be stronger. At first it may be argued that Ae. crassa, T. boeoticum, and T. aestivum have different-sized genomes and that the absence of 66 % of the genome of T. aestivum in Ae. crassa and T. boeoticum would predict a lower level of tissue expression of MtATP6. Our findings of MtATP6 expression in wild genotypes contradict this expectation. The high expression level of MtATP6 in wild genotypes may indicate that MtATP6 is an important mechanism for salt tolerance in the leaves of this wild wheat.
In this study, qRT-PCR confirmed that MtATP6 in the leaves of all four genotypes suddenly increased around 3 h after the salt stress and peaked at 6–10 h (Fig. 1; Table 2). Possibly, MtATP6, an early responsive gene, may play a key role in the early stages of the response to salt stress (Fig. 1). Similar to our results, Zhang et al. [60] showed that AtMtATP6 peaks shortly after the introduction of different NaHCO3, drought, H2O2 and low temperature stresses.
Our result indicated that MtATP6 behaves as an early stress-response gene (Fig. 1).
In agreement with this result, promoter analysis demonstrated that RMtATP6 and AtMtATP6 include early stress-responsive cis-acting elements such as ABRE, MYB/MYC, GTLs, and W-boxes in the upstream region (Fig. 2). The existence of these elements (Table 7) in the promoter (Fig. 2) candidate MtATP6 as an early stress-related energy-regulating gene (Fig. 2), a similar result was reported by Heise et al. [23]. The promoter of a drought-, high salinity-, and cold-inducible gene contains major cis-acting elements, ABRE, MYB/MYC, and DRE, which are involved in stress-inducible gene expression [55].
ABRE is a major cis-acting element in ABA-responsive gene expression [44] because ABA plays a pivotal role in adaptation to drought, high salinity, and cold (Table 7) [55]. MYB/MYC regulatory element involves in early response to osmotic stress and ABA induction (Table 7) [45]. WRKY (tryptophan, arginine, lysine, tyrosine domain) transcription factors act as major regulatory proteins by binding to the W-box [30, 53]. The WRKYs has proven significant induction under salt and drought stresses, and pathogens, implying that those response to various biotic and abiotic stresses [30]. Other important elements on the MtATP6 promoters are GT-element-binding proteins (GTLs) [48]. It has been documented that promoters of genes carrying GTL rapidly induce their target genes after encountering pathogen attack or NaCl stress [36]. Presence of GTLs and rapid response to NaCl are evidences showing that MtATP6 is a novel early stress responsive gene (Figs. 1, 2). For example, GTL1 overexpression enhances drought stress tolerance in plants [56]. Moreover, these elements are involved in repression or activation of different plant photosynthetic genes [54]. Furthermore, their known downstream target genes encode proteins functioning in the mitochondria [11] and chloroplast, such as Rubisco, ATP synthase, and ribosomal proteins [62], as well as other non-chloroplastic genes, and consequently are involved in the regulation of carbon metabolism and energy balance [14]. It seems that 6-kDa subunit not only has important roles in mitochondria but also has unknown roles (or similar roles) in photosynthetic pathways in chloroplasts because the F1F0-ATP synthase complex exists in both of these organelles. It is possible GTLs in MtATP6 promoter are other related factors of organelle communications that act in ABA-dependent pathways [39] in abiotic or biotic stress conditions. All of these results showed that possibly the MtATP6 is an early ABA-responsive gene under stress conditions.
Interestingly, these results can explain why the overexpression of AtMtATP6 and RMtATP6 in transgenic yeast and Arabidopsis plants [60, 61] or JcMtATP6 in the salt-hypersensitive mutant of yeast [12] increases the tolerance to various stresses such as: salt, drought, oxidative, and cold. For example, under salt stress to reduce Na+ toxicity and maintain ion homeostasis, plants need extra energy, mainly in the form of ATP, whose production depends on phosphorylation efficiency [52].
The pattern of MtATP6 transcript accumulation differed in Mahuti and Alamut (Fig. 1; Table 2 part A). We observed more MtATP6 upregulation, especially in salt-tolerant Mahuti and the wild genotypes, than in salt-sensitive Alamut (Fig. 1; Table 2 part A). Alamut did not adapt to high NaCl concentrations (Fig. 1). The increased level of MtATP6 transcripts at 10 h (especially in 100 and 200 mM NaCl) in Alamut may be viewed as a final attempt to avoid death (Fig. 1; Table 2 part A).
In Mahuti, after an increase in the level of MtATP6 from 3 to 10 h in response to salinity, there was an ascending trend of transcript accumulation from 24 to 72 h in all NaCl concentrations, while in Alamut this expression pattern was seen only in 50 mM NaCl. In Alamut, MtATP6 expression generally decreased in 100 and 200 mM NaCl and only slightly increased at 10 h after the beginning of the stress. Apparently, Alamut is unable to cope with the toxicity of high concentrations of sodium (see 100 and 200 mM in Fig. 1; Table 2 part A) because salt-sensitive genotypes have lower ATP production than salt-tolerant genotypes [21, 25]. Alamut was able to manage only the low NaCl concentrations, so MtATP6 expression was induced only at 50 mM NaCl in this genotype (Fig. 1).
However, Mahuti (salt-tolerant wheat) and the wild wheat genotypes coped with the toxicity of accumulated sodium in the cytosol of the high-NaCl treatments because salt-tolerant genotypes had higher ATP production than salt-sensitive genotypes (Fig. 1; Table 2 part A). Ghavami et al. [16] reported that Mahuti uses different mechanisms comparing with the other Iranian wheats under high salt stress and therefore performs better than other salt-tolerant genotypes. This difference between Mahuti and Alamut could also be due to the different efficiency of Na+ efflux and vacuolar compartmentation of Na+ (for more information, see results of Hamilton et al. [21] and Kong et al. [25]). For these reasons, Mahuti leaves accumulate MtATP6 transcript over time to upregulate ATP production whenever the Na+ and Cl− concentrations increase, especially at 100 and 200 mM NaCl (Fig. 1; Table 2 part A).
Hamilton et al. [21] suggested that the differential salt sensitivity between contrasting wheat genotypes is in part conferred by higher F1F0-ATP synthase activity (possibly from upregulated MtATP6 expression, according to our hypothesis) in salt-tolerant genotypes. Based on these results, we propose that MtATP6 is an important regulator of ATP production by the mitochondrial F1F0-ATP synthase, especially under stress conditions (Fig. 1; Table 2 part A). The higher MtATP6 expression under salt stress induces higher F1F0-ATP synthase activity to produce ATP needed for energy balance in both tolerant and sensitive genotypes [60, 61]. According to our results and the results of Kong et al. [25] and Hamilton et al. [21], more sustained MtATP6 expression is induced under high NaCl concentrations, especially in salt-tolerant genotypes.
Ae. crassa (genome D) had the highest MtATP6 expression in this study. Apparently, our expression results are related to the type of genome and polyploidy level. In wheat (hexaploid), the 4D chromosome, derived from the wild grass Aegilops, is responsible for salt tolerance and K+/Na+ discrimination [43]. Gorham et al. [19] tested several accessions of Ae. crassa and showed they have much lower Na+ concentrations and higher K+/Na+ ratios in leaves than durum wheat. They suggested that genome D imparts the Na+ ‘exclusion’ and enhances the K+/Na+ discrimination to bread wheat. T. urartu (AA), the species that probably gave rise to the genome As of durum and bread wheat, shows greater Na+ ‘exclusion’ and K+/Na+ discrimination than durum wheat (AABB), as do the closely related A-genome species T. monococcum ssp. monococcum and T. monococcum ssp. aegilopoides (syn. T. boeoticum) [19].
In this work, cultivated genotypes had lower MtATP6 expression than wild genotypes (Fig. 1; Tables 2 part A, 3, 4, 5, 6). This might have been due to the traits conferred by genome A [7], whereas the wild genotypes of Ae. crassa, which lacks the A and B genomes, and T. boeoticum, which lacks the B genome (some of the salt tolerance genes are located on genomes A and B) showed more extensive MtATP6 upregulation after salt treatment (Fig. 1).
The cultivated genotypes significantly consume more MtATP6 than the wild genotypes before NaCl treatment (Table 6). This result suggests that energy management in the wild genotypes is more effective. As shown in Table 6, T. boeoticum showed similar MtATP6 expression profiles as cultivated genotypes. These results confirm that T. boeoticum has a closer affinity with cultivated genotypes, and consequently, genome D in Aegilops can be considered a better genomic source transferring these energy-based abiotic tolerance genes (Tables 5, 6). The wild genotypes showed greater MtATP6 expression fold changes than cultivated genotypes after stress. T. boeoticum (AA) showed similar MtATP6 expression fold changes to the cultivated genotypes at all NaCl concentrations, but it was significantly different from Ae. crassa at 100 and 200 mM after salt stress compared to before salt stress (Table 6). In other words, Ae. crassa has an efficient regulation of F1F0-ATP synthase complex via MtATP6 and activates the pump when it is really require fold change in Ae. crassa (genome D) was significantly larger under high NaCl concentrations than before stress (Table 6). Many salt tolerant genes are on genomes A and D and Aegilops, the source of genome D in bread wheat, exhibits high discrimination between K+ and Na+ due to traits conferred by genome D [19, 20, 50]. Many resistance genes from wild ancestors of wheat are inherited [34], but it seems that energy management mechanisms have not been transferred to the cultivated genotypes. Resistance to fungal pathogens that cause severe crop damage by infecting the spikes, leaves, and roots is another important trait that is not inherited in wheat [38]. Resistance to fungal pathogens is closely associated with genome A, and wild species are valuable sources of resistance to the main fungal diseases and resistance to root rot [57]. Another overlooked but important trait is energy homeostasis under stress, possibly related to genome D, which has not transferred to cultivated wheat. In fact, one under-studied genetic pathway in plants is the electron transport chain in mitochondria, which is central to the energy metabolism [9]. The encoding genes of this pathway play important roles in plant acclimation and response to stress. These genes are novel, important targets for genetic engineering to enhance stress tolerance in crop plants [9]. It seems that the higher stress tolerance of the wild genotypes include unknown mechanism that has not transferred to cultivated genotypes and better energy management is one mechanism by which wild wheat relatives can overcome stresses.
Wild genotypes had lower MtATP6 expression before stress and had higher MtATP6 expression after stress (Table 6). Therefore, wild genotypes can produce more ATP in stressed conditions, while they save energy when this is not required (Table 6). This trait enables higher K+ maintenance and lower Na+ accumulation and explains the NaCl tolerance in tolerant wheat genotypes as compared to NaCl-sensitive ones [25].
The wild-genotype T. boeoticum (harboring A genome), based on the results presented in this investigate, has lower MtATP6 expression than Ae. crassa (harboring D genome). Wild genotypes, especially Ae. crassa, manage energy better than cultivated genotypes, a characteristic that is possibly related to genome D and has not been transferred to cultivated genotypes, opening a new avenues for plant breeding.
All together, under stress conditions, energy management and cell homeostasis need close communications between the nucleus and mitochondria [42]. These processes require the expression of a specific collection of nuclear genes which their products need to be targeted to the organelle, a process which involves sensing of disorders in mitochondrial functions and retrograde signaling to the nucleus [42]. In fact, mitochondrial response to nuclear signaling is a key component of the overall plant responses [51]. It has become evident that a variety of nuclear genes that code for mitochondrial proteins are responsive to a wide range of stress conditions which confirms the role of mitochondria as a significant target and regulator of stress responses [51]. For example, oxygen species (ROS) are common productions of many stress responses in mitochondria [15] causing oxidative damage to proteins, lipids, and nucleic acids. Alternative oxidase (AOX) has the ability to suppress ROS production which is a component of the alternative mitochondrial electron transport chain [51]. Almost in all plants, AOX is encoded by a small gene family [51]. These observations make AOX a one of the best-described target genes for mitochondrial retrograde signaling in plants [51]. Therefore, AOX lack of activity or activity leads to a radical alteration of the defence equilibrium at cellular level playing a key role in programming the stress response [51]. Another example in nuclear-organelle communications is ABI4 (abscisic acid insensitive 4) which regulates mitochondrial retrogrades signaling and provides point of convergence in the plastid and mitochondrial retrograde signaling pathways [18]. In addition ABI4, as an AP2 (Apetala 2)-type transcription factor is a component of the plastid retrograde signaling pathway [18]. ACGTL (GT element) significantly over-represented in the promoter of ABI4 [39]. Indeed, in retrograde signaling, ACGT is the core sequence of ABA response elements, implicating components of the ABA response pathway [55]. The MtATP6 promoter analysis interestingly demonstrated that MtATP6 also contains GTLs (Fig. 2) that possibly responds to the stress hormone ABA and similar to ABI4, plays roles in ABA-mediated signaling and the associated sugar, energy metabolism, and organelle communications under stress. The identification of 6-kDa subunit, ABI4, and AOX as components of the mitochondria or chloroplasts providing an additional way in which internal hormonal and developmental signals can be integrated with stress signal (through ABA) to affect a high-order regulation of organelle function [18]. More importantly, crosstalk between chloroplast and mitochondria seems to contribute to chloroplast-to-nucleus retrograde signaling [17]. We suggest 6-kDa subunit is another communicating protein between the nucleus and these two organelles in regulation of cell energy under stress and roles in ABA-mediated signaling (Supplementary data 1, Fig. 1). Although ABA is not synthesized in plastids or mitochondria, it may represent an important intermediate or signaling molecules (such as 6-kDa subunit, ABI4, and AOX) to mediate and integrate plastid and mitochondrial retrograde signals [17].
It seems that MtATP6 as an early stress-ABA-energy responsive gene involves in the similar communication mechanisms (Supplementary data 1, Fig. 1). Dual targeting of plastids and mitochondria is an interesting phenomenon [5] so a specific targeting signal peptide was found in chloroplastic and mitochondrial proteins [47]. The reason for dual targeting of 6-kDa subunit is that may comprise as a mean of inter-organelle communication [51] and can save energy for the cell. Sending the same proteins to both organelles at the same time ensures that they are both at least capable of carrying out these functions in a coordinated manner [51].
In conclusion, our data and previous data indicate that MtATP6 behaves as an early stress-response gene, suggesting multiple roles for MtATP6. Although MtATP6 is a subunit of F0, it is also a nuclear protein with alpha-helix structures and phosphorylation sites, suggesting its role as a regulatory factor activating the expression of F1F0-ATP synthase subunits.
Further studies are required to shed light on this interesting hypothesis. Apparently, MtATP6 suddenly accumulates in early hours after stress in ABA interacting manner, and it induces or completes the expression of other subunits of F1F0-ATP synthase. Therefore, in response to certain stresses, F1F0-ATP synthase will be more active, producing more ATP for ion balance and energy management in cells. Soontharapirakkul et al. [46] postulated that this complex maintains the ATP levels of cells by electron-transfer chain in the stress conditions. All together, these results suggest that the induction of F1F0-ATP synthase probably plays critical roles in stress tolerance.
Abbreviations
- MtATP6:
-
Mitochondrial F1F0-ATP synthase 6-kDa subunit
- qRT-PCR:
-
Quantitative reverse transcriptase polymerase chain reaction
- ABA:
-
Abscisic acid
- AREB/ABF:
-
ABA-responsive element binding/ABA-responsive element binding factor
- ABRE:
-
Abscisic acid response element
- MYC:
-
Myelocytomatosis oncogene cellular homolog
- MYB:
-
Myeloblastosis viral oncogene homolog
- W-boxes:
-
WRKY-binding sites
- CT :
-
Cycle threshold
- HKT:
-
High affinity potassium transporter
- SOS:
-
Salt overly sensitive
- DRE:
-
Dehydration-responsive element
- GTLs:
-
GT-element-binding proteins
- CBF/DREB:
-
C-repeat binding factor/Dehydration-responsive element binding protein
- AP2:
-
Apetala 2
- ROS:
-
Reactive oxygen species
- AOX:
-
Alternative oxidase
- ABI4:
-
Abscisic acid insensitive 4
References
Agarwal, B. (2011). A role for anions in ATP synthesis and its molecular mechanistic interpretation. Journal of Bioenergetics and Biomembranes, 43, 299–310.
Alkhani, H., & Ghorbani, M. (1993). A contribution to the halophytic vegetation and flora of Iran. In H. Lieth & A. Al Masoom (Eds.), Towards the Rational Use of High Salinity Tolerance Plants (Vol. 1, pp. 35–44). Dordrecht: Kluwer Academic Publishers.
Baena-González, E. (2010). Energy signaling in the regulation of gene expression during stress. Molecular Plant, 3, 300–313.
Bayer, J. S. (1982). Plant productivity and environment. Science, 218, 443–448.
Carrie, C., Giraud, E., & Whelan, J. (2009). Protein transport in organelles: dual targeting of proteins to mitochondria and chloroplasts. The Federation of European Biochemical Societies Journal, 276, 1187–1195.
Chandra, S. B., & Manatt, M. (2011). The effects of mitochondrial dysfunction in schizophrenia. The Journal of Medical Genetics and Genomics, 3, 84–89.
Colmer, T. D., Flowers, T. J., & Munns, R. (2006). Use of wild relatives to improve salt tolerance in wheat. The Journal of Experimental Botany, 57, 1059–1078.
Deihimi, T., Niazi, A., Ebrahimi, M., Kajbaf, K., Fanaee, S., Bakhtiarizadeh, M. R., et al. (2012). Finding the undiscovered roles of genes: an approach using mutual ranking of coexpressed 4 genes and promoter architecture-case study: Dual roles of thaumatin like proteins in biotic and abiotic stresses. SpringerPlus, 1, 30.
Dobrota, C. (2006). Energy dependant plant stress acclimation. Reviews in Environmental Science & Biotechnology, 5, 243–251.
Doğan, M. (2011). Antioxidative and proline potentials as a protective mechanism in soybean plants under salinity stress. African Journal of Biotechnology, 10, 5972–5978.
Escobar, M. A., Franklin, K. A., Svensson, A. S., Salter, M. G., hitelam, G. C., & Rasmusson, A. G. (2004). Light regulation of the Arabidopsis respiratory chain: multiple discrete photoreceptor responses contribute to induction of type II NAD(P)H dehydrogenase genes. Plant Physiology, 136, 2710–2721.
Eswaran, N., Parameswaran, S., Sathram, B., Anantharaman, B., Kumar, G. R. K., & Tangirala, S. J. (2010). Yeast functional screen to identify genetic determinants capable of conferring abiotic stress tolerance in Jatropha curcas. BMC Biotechnology, 10, 23.
Flowers, T. J. (2004). Improving crop salt tolerance. The Journal of Experimental Botany, 55, 307–319.
Galon, Y., Finkler, A., & Fromm, H. (2010). Calcium-regulated transcription in plants. Molecular Plant, 3, 653–669.
Gechev, T. S., Van Breusegem, F., Stone, J. M., Denev, I., & Laloi, C. (2006). Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays, 28, 1091–1101.
Ghavami, F., Malboobi, M. A., Ghannadha, M. R., Yazdi-Samadi, B., Mozaffari, J., & Jafar-Aghaei, M. (2004). Evaluation of salt tolerance of Iranian wheat genotypes at germination and seedling stages. Iranian Journal of Agricultural Sciences, 35, 453–464.
Giraud, E., Ho, L. H. M., Clifton, R., et al. (2008). The absence of alternative oxidase 1a in Arabidopsis thaliana results in acute sensitivity to combined light and drought stress. Plant Physiology, 147, 595–610.
Giraud, E., Van Aken, O., Ho, L. H. M., & Whelan, J. (2009). The transcription factor ABI4 is a regulator of mitochondrial retrograde expression of alternative oxidase 1a. Plant Physiology, 150, 1286–1296.
Gorham, J., Bristol, A., Young, E. M., & Wyn Jones, R. G. (1991). The presence of the enhanced K/Na discrimination trait in diploid Triticum species. Theoretical and Applied Genetics, 82, 729–736.
Gorham, L., Hardy, C., Wyn Jones, R. G., Joppa, L. R., & Law, C. N. (1987). Chromosomal location of a K+/Na+ discrimination character in the genome D of wheat. Theoretical and Applied Genetics, 74, 584–588.
Hamilton, C. A., Allin, G. G., & Gregory, J. T. (2001). Induction of vacuolar ATP synthase and mitochondrial ATP synthase by aluminum in an aluminum-resistant genotype of wheat. Plant Physiology, 125, 2068–2077.
Hasegawa, P. M., Bressan, R. A., Zhu, J. K., & Bohnert, H. J. (2000). Plant cellular and molecular responses to high salinity. Annual Review of Plant Physiology and Plant Molecular Biology, 51, 463–499.
Heise, A., Lippok, B., Kirsch, C., & Hahlbrock, K. (2002). Two immediate-early pathogen-responsive members of the AtCMPG gene family in Arabidopsis thaliana and the W-box-containing elicitor-response element of AtCMPG1. Proceedings of the National Academy of Sciences of the United States of America, 99, 9049–9054.
Jain, M., Nijhawan, A., Tyagi, A. K., & Khurana, J. P. (2006). Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochemical and Biophysical Research, 345, 646–651.
Kong, Y., Zhou, G., & Wang, Y. (2001). Physiological characteristics and alternative respiratory pathway under salt stress in two wheat genotypes differing in salt tolerance. The Russian Journal of Plant Physiology, 48, 595–600.
Langridge, P., Paltridge, N., & Fincher, G. (2006). Functional genomics of abiotic stress tolerance in cereals. Briefings in Functional Genomics and Proteomics, 4, 34–354.
Larionov, A., Krause, A., & Miller, W. (2005). A standard curve based method for relative qRT-PCR data processing. BMC Bioinformatics, 6, 62.
Laus, M. N., Flagella, Z., Trono, D., Soccio, M., Fonzo, N. D., & Pastore, D. (2007). Sea water stress affects mitochondrial proline oxidation but not alternative oxidase activity in durum wheat germinating seedlings, Water Saving in Mediterranean Agriculture and Future Research Needs proceedings of the International Conference. Valenzano (Italy), 2, 98–108.
Lescot, M., Déhais, P., Thijs, G., et al. (2002). PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Research, 30, 325–327.
Li, S., Fu, Q., Chen, L., Huang, W., & Yu, D. (2011). Arabidopsis thaliana WRKY25, WRKY26, and WRKY33 coordinate induction of plant thermo tolerance. Planta, 233, 1237–1252.
Meyer, E. H., Taylor, N. L., & Millar, A. H. (2008). Resolving and identifying protein components of plant mitochondrial respiratory complexes using three dimensions of gel electrophoresis. Journal of Proteome Research, 7, 786–794.
Michalecka, A. M., Svensson, A. S., Johansson, F. I., et al. (2003). Arabidopsis genes encoding mitochondrial type II NAD(P)H dehydrogenases have different evolutionary origin and show distinct responses to light. Plant Physiology, 133, 642–652.
Moghadam, A. A., Ebrahimie, E., Taghavi, S. M., Niazi, A., & Djavaheri, M. (2012). Isolation and in silico functional analysis of MtATP6, a 6-kDa subunit of mitochondrial F1F0-ATP synthase, in response to abiotic stress. Genetic and Molecular, 11, 3547–3567.
Munns, R., & Richards, R. A. (2007). Recent advances in breeding wheat for drought and salt stresses. In M. A. Jenks, et al. (Eds.), Advances in molecular breeding toward drought and salt tolerant crops (pp. 565–585). Berlin: Springer.
Naghavi, M. R., Aghaei, M. J., Taleei, A. R., Omidi, M., Mozafari, J., & Hassani, M. E. (2009). Genetic diversity of the D-genome in T. aestivum and Aegilops species using SSR markers. Genetic Resources and Crop Evolution, 56, 499–506.
Park, H. C., Kim, M. L., Kang, Y. H., et al. (2004). Pathogen- and NaCl-induced expression of the SCaM-4 promoter is mediated in part by a GT-1 box that interacts with a GT-1-like transcription factor. Plant Physiology, 135, 2150–2161.
Pastore, D., Trono, D., Laus, M. N., Di Fonzo, N., & Flagella, Z. (2007). Possible plant mitochondria involvement in cell adaptation to drought stress. A case study: Durum wheat mitochondria. The Journal of Experimental Botany, 58, 195–210.
Patnaik, D., & Khurana, P. (2001). Wheat biotechnology: A mini review. Electronic Journal of Biotechnology, 4, 0717–3458.
Priest, H. D., Filichkin, S. A., & Mockler, T. C. (2009). cis-Acting elements in plant cell signaling. Current Opinion in Plant Biology, 12, 643–649.
Ramezani, A., Niazi, A., Moghadam, A. A., Zamani, B. M., Deihimi, T., Ebrahimi, M., Akhtardanesh, H., & Ebrahimie, E. (2012) Quantitative expression analysis of TaSOS1 and TaSOS4 genes in cultivated and wild wheat plants under salt stress. Molecular Biotechnology. doi:10.1007/s12033-012-9513-z.
Raven, J. A. (1985). Regulation of pH and generation of osmolarity in vascular plants: A cost-benefit analysis in relation to efficiency of use of energy, nitrogen and water. New Phytologist, 101, 25–77.
Ryan, M. T., & Hoogenraad, N. J. (2007). Mitochondrial–nuclear communications. Annual Review Biochemistry, 76, 701–722.
Shah, S., Gorham, I., Forster, B. P., & Wyn Jones, R. G. (1987). Salt tolerance in the Triticeae: The contribution of the genome D to cation selectivity in hexaploid wheat. The Journal of Experimental Botany, 38, 254–269.
Shen, Q., Zhang, P., & Ho, T. H. (1996). Modular nature of abscisic acid (ABA) response complexes: composite promoter units that are necessary and sufficient for ABA induction of gene expression in barley. Plant Cell, 8, 1107–1119.
Shinozaki, K., & Yamaguchi-Shinozaki, K. (2007). Gene networks involved in drought stress response and tolerance. The Journal of Experimental Botany, 58, 221–227.
Soontharapirakkul, K., Promden, W., Yamada, N., et al. (2011). Halotolerant cyanobacterium aphanothece halophytica contains a Na+-dependent F1F0-ATP synthase with potential role in salt-stress tolerance. The Journal of Biological Chemistry, 286, 10169–10176.
Tahmasebi, A., Aram, F., Ebrahimi, M., Mohammadi-Dehcheshmeh, M., & Ebrahimie, E. (2012). Genome-wide analysis of cytosolic and chloroplastic isoforms of glutathione reductase in plant cells. Plant Omics Journal, 5, 94–102.
Tariq, M., Nazar, N., Haider, A. B., & Saqlan, N. S. M. (2010). Comparative analysis of regulatory elements in different germin-like protein gene promoters. African Journal of Biotechnology, 9, 1871–1881.
Türkoglu, N., Erez, M. E., & Battal, P. (2011). Determination of physiological responses on hyacinth (Hyacinthus orientalis) plant exposed to different salt concentrations. African Journal of Biotechnology, 10, 6045–6051.
Valkoun, J. J. (2001). Wheat pre-breeding using wild progenitors. Euphytica, 119, 17–23.
Van Aken, O., Giraud, E., Clifton, R., & Whelan, J. (2009). Alternative oxidase: A target and regulator of stress responses. Physiology Plant, 137, 354–361.
Veen, B. W. (1980). Energy cost on ion transport. Genetic engineering of osmoregulation. In D. W. Rains, R. C. Valentine, & A. Hollaender (Eds.), Impact on plant productivity for food, chemicals and energy (pp. 187–195). New York: Plenum.
Voigt, C., Oster, U., Börnke, F., et al. (2010). In-depth analysis of the distinctive effects of norflurazon implies that tetrapyrrole biosynthesis, organellar gene expression and ABA cooperate in the gun-type of plastid signaling. Physiology Plant, 138, 503–519.
Xie, Z., Zou, H., Lei, G., et al. (2009). Soybean trihelix transcription factors GmGT-2A and GmGT-2B improve plant tolerance to abiotic stresses in transgenic Arabidopsis. PLoS ONE, 4, e6898.
Yamaguchi-Shinozaki, K., & Shinozaki, K. (2005). Organization of cis-acting regulatory elements in osmotic- and cold-stress responsive promoters. Trends in Plant Science, 10, 88–94.
Yoo, C. Y., Jin, J. B., Miura, K., Jin, Y. H., Gosney, M., Mickelbart, M. V., Bressan, R. A., & Hasegawa, P. M. (2007). Ca2+/CaM signaling through AtGTL1 mediates drought stress adaptation. Botany and Plant Biology Joint Congress, July 7–11, Chicago, Illinois, USA.
Yu, B. S., & Sun, G. R. (1995). Preliminary study of several spring wheat varieties for resistance to Septoria diseases (Chinese). Crop Genetic Resources, 1, 27–29.
Zamani Babgohari, M., Niazi, A., Moghadam, A. A., Deihimi, T., & Ebrahimie, E. (2012). Genome-wide analysis of key salinity-tolerance transporters (HKTs) in wheat and wild wheat relatives (A and D genomes). In Vitro Cellular and Developmental Biology-Plant. doi:10.1007/s11627-012-9478-4.
Zhang, X. X., & Liu, S. K. (2003). Identification and characterization of mitochondrial ATP synthase small subunit gene in rice (Oryza sativa L.). Molecular Plant Breeding, 1, 605–612.
Zhang, X., Liu, S., & Takano, T. (2008). Overexpression of a mitochondrial ATP synthase small subunit gene (AtMtATP6) confers tolerance to several abiotic stresses in Saccharomyces cerevisiae and Arabidopsis thaliana. Biotechnology Letters, 30, 1289–1294.
Zhang, X., Takano, T., & Liu, S. K. (2006). Identification of a mitochondrial ATP synthase small subunit gene (RMtATP6) expressed in response to salts and osmotic stresses in rice (Oryza sativa L.). The Journal of Experimental Botany, 57, 193–200.
Zhou, D. X. (1999). Regulatory mechanism of plant gene transcription by GTLs and GT-factors. Trends Plant Science, 4, 210–214.
Acknowledgments
The authors would like to thank the Institute of Biotechnology for supporting this research and the Bioinformatics Research Group in the College of Agriculture (Shiraz University). We thank Dr. Mehrabi (Ilam University) for kindly supplying seeds of wild genotypes for this study. We thank Dr. Manijeh Mohammadi Dehcheshmah for his help in performing the qRT-PCR experiments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moghadam, A.A., Ebrahimie, E., Taghavi, S.M. et al. How the Nucleus and Mitochondria Communicate in Energy Production During Stress: Nuclear MtATP6, an Early-Stress Responsive Gene, Regulates the Mitochondrial F1F0-ATP Synthase Complex. Mol Biotechnol 54, 756–769 (2013). https://doi.org/10.1007/s12033-012-9624-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-012-9624-6