Abstract
The wheat dehydrin DHN-5 has been previously shown to exhibit heat protecting effect on enzymatic activities. In order to understand the molecular mechanism by which DHN-5 exerts its protective function, we performed an approach to dissect the functional domains of DHN-5 responsible for this feature. In two distinct enzymatic assays, we found that the truncated forms of DHN-5 containing only one K- or two K-segments are able to protect albeit to less extent than the wild type protein, lactate dehydrogenase and β-glucosidase against damage induced by various stresses in vitro. However, the YS- and Φ-segments alone have no protective effects on these enzymes. Therefore, our study provides the evidence that the protective function of DHN-5 seems to be directly linked to its K-segments which through their amphipatic α-helical structure, may act to prevent protein aggregation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Group 2 late embryogenesis abundant proteins or dehydrins (DHN) are believed to play a fundamental role in plant drought stress tolerance [1]. DHN have been divided into five subclasses based on their conserved amino acid sequences. In the most N-terminal part, the Y-segment (V/TDEYGNP, sometimes referred to as DEYGNP), can be found in one to three copies [2, 3]. Downstream of the Y-repeat, five to seven serine residues followed by three acidic amino acids form the S-segment. Finally, the K-segment (EKKGIMDKIKEKLPG) is a lysine-rich sequence localized near the C-terminus. The K-segment which is the most conserved region (found in all DHNs in one or multiple copies) has a tendency to form an amphipathic α-helix [4]. In addition, most DHNs lack cysteine and tryptophan residues and show high percentage of charged and polar amino acids, allowing them to remain soluble after boiling, a feature shared with intrinsically disordered proteins [4]. It was suggested that the K-segments play relevant role in protecting cell macromolecules [5]. Cryoprotective activity has been reported for several DHNs, such as COR85, WCS120, and PCA60 from spinach, wheat and peach, respectively [6–8]. DHNs might also prevent heat inactivation and recently, Brini et al. [9] showed that the wheat dehydrin DHN-5 improved the activity and/or thermostability of the fungal β-glucosidase (bglG) and glucose oxidase (GOD/POD) enzymes in vitro. It is therefore plausible to imagine that DHNs can act as chaperones on other proteins and help them to fold properly and/or prevent their aggregation under heat or cold stress [10]. However, classical chaperones not only prevent inappropriate protein aggregation but also form specific complexes with target proteins through interaction of hydrophobic patches [11]. So far, no specific interactions between DHNs and other proteins have been reported and it was hence suggested that DHNs may rather behave as ′molecular shields′ preventing denatured proteins from interacting with one another as was previously suggested [12].
In the current study, we report the implication of the structural domains of DHN-5 in the protection of LDH and bglG enzymes against distinct stresses in vitro. Our data provide the evidence that the K-segments are indispensable for the enhanced stability of both enzymes under various stress conditions.
Materials and Methods
Cloning, Production and Purification of DHN-5 and Its Truncated Derivatives
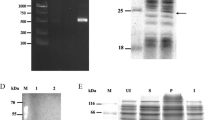
The full-length DHN-5 open reading frame (ORF) was amplified with PfuTurbo DNA polymerase (Stratagene; La Jolla, CA, USA) using VL3/VL4 primers corresponding to the 5′ and 3′ ends and containing EcoRI restriction sites at their ends VL3: 5′-GAATTCATGGAGTTCCAAGGGCAG-3′ and VL4: 5′-GAATTCTCAGTGCTG-GCCTGGG-3′). The DHN-5 ORF was cloned into the EcoRI site of the Escherichia coli expression vector pGEX-4T-1, resulting in a fusion with GST. To construct truncated versions of DHN-5 containing either ‘YS’ segments (DL1), ‘K1ΦK2’ segments (DL2), ‘ΦK2’ segment (DL3), or ‘Φ’ segment (DL4), PCR was performed using four sets of primers (Fig. 2a). The DL1 variant was amplified using forward primer PR1: 5′-GCGAATTCTTTCCCCTAGCCGGCG-3′ and reverse primer PR4: 5′-GCGAATTCTCACATGCCCTTCTTTCT-3′. The DL2 variant was generated by PCR using forward primer PR2: 5′-GCGAATTCGAGGACGACGGCATGGGC-3′ and reverse primer VL4. The DL3 variant was generated by PCR using forward primer PR3: 5′-GCGAATTCGGCCACGGTGTGACCAGCAG-3′ and reverse primer VL4. The DL4 variant was generated by PCR using forward primer PR3 and reverse primer PR5: 5′-GCGAATTCTCACATGATGCCCTTCTT-3′. All PCR products were also cloned into the EcoRI site of the E. coli expression vector pGEX-4T-1.
Recombinant proteins were produced and purified from E. coli cells as described previously by Brini et al. [9].
LDH Protective Assay
A solution of the freeze-labile LDH enzyme (EC1.1.1.27, rabbit muscle lactate dehydrogenase) from Sigma (Tokyo, Japan) was prepared (10 μg/ml, 20 μl) in 10 mM sodium phosphate, pH 7.4. LDH samples were mixed with an equal volume of buffer containing 20 μg/ml of BSA, GST, DHN-5 or its truncated variants, then submitted to various stress treatments as indicated. Before measuring the LDH activity, the samples were diluted (to reach an enzyme concentration of 0.5 μg/ml) into 750 μl of reaction mix (10 mM sodium phosphate, pH 7.4, 2 mM NADH, and 10 mM pyruvic acid). NADH oxidation was monitored by measuring the absorbance A340 over 3 min, during which the reaction rate was linear. The rate of absorbance decrease was then used to calculate activity ∆DO/min × 8095 = U/l (Biomaghreb kit). All samples were assayed in triplicates.
Purification and Enzymatic Assays of bglG
The bglG enzyme was purified from Stachybotrys microspora as described by Saibi and Gargouri [13]. BglG activity using para-nitrophenyl-β-d-glucopyranoside (pNPG) as a substrate was monitored as described previously [13].
Statistical Analysis
Data interpretation of relative activities was performed by analysis of variance using the software ′STATITCF′ version 4, followed by a comparison of means with Fisher’s test at 5 % level.
Results
Monitoring the Effect of DHN-5 on LDH Activity Under Various Stresses
First, we examined the effect of DHN-5 on prolonged storage of enzymes. We have chosen LDH as substrate since it appeared to lose its activity during extended storage on ice, under heat or after dehydration stress. LDH (10 μg/ml) was mixed with an equal volume of buffer containing 20 μg/ml of sucrose, BSA, GST or DHN-5 and a time-dependent loss of activity was measured as described previously (see “Materials and methods” section). The BSA was chosen as a positive control since it protects LDH against freeze–thaw induced denaturation of LDH [14, 15]. When incubated on ice alone, LDH lost more than 20 % of its activity after 2 h and this loss reached more than 80 % after 24 h (Fig. 1a). Similar activity loss rates were also recorded in the presence of sucrose and GST suggesting that both molecules did not exhibit any protective effect on LDH (Fig. 1a). In contrast, LDH activity remained unaffected after 24 h of ice incubation in the presence of BSA or DHN-5 (with which even a higher activity is observed) (Fig. 1a). These results indicated that DHN-5 like BSA efficiently protected LDH from gradual inactivation on ice.
DHN-5 protected LDH activity from various stresses in vitro. a LDH solutions were kept on ice in the presence of sucrose, BSA, GST and recombinant DHN-5. A small aliquot was taken at each of several time points, and LDH activity was measured. LDH activity before the treatment was taken as 100. b LDH solutions were 70 % dehydrated using a speed-vac centrifuge. The sample was then re-hydrated to the original volume with water, and LDH activity was measured. Relative activity was as described in panel A. c LDH solutions were incubated at 43 °C for the specified time. After incubation, LDH activity was measured. Relative activity was as described in panel A. Values are mean ± SD (n = 4)
As cold reduces water availability, we checked the protective effect of DHN-5 on LDH under dehydration stress. It is also well known that highly hydrophilic proteins (including DHNs) protect enzymatic activity from dehydration stress [16]. Samples containing equal concentrations of enzyme (10 μg/ml) mixed with the indicated molecules (20 μg/ml) were dehydrated to 70 %, and then rehydrated to the original volume, after which the LDH activity was measured. After this dehydration treatment, the LDH activity was significantly impaired in the presence of the buffer, GST or sucrose solution. In all cases, the activity was about 80 % lower than that under standard conditions (Fig. 1b). Interestingly, DHN-5 exhibited a higher protection on LDH and more than 85 % of its activity was preserved. The effect of DHN-5 seemed to be even better than the one observed with BSA where the residual LDH activity was ~80 % (Fig. 1b). These data indicate that DHN-5 has the ability to protect LDH activity against enzymatic inactivation caused by the dehydration stress.
We have also evaluated the effects of DHN-5 on LDH activity during heat stress. When incubated at 43 °C, LDH activity decreased rapidly and only 20 % of this activity was still detected after 30 min (Fig. 1c). The LDH activity decreased with similar rates, when the enzyme was heated in the presence of GST or sucrose solutions at the concentration of 20 μg/ml (Fig. 1c). However, in the presence of DHN-5 (20 μg/ml), the inactivation of LDH was greatly attenuated and 90 % of the activity was preserved after 30 min of heat stress. Similar rates of LDH decline following heat stress were recorded with BSA (Fig. 1c). All together, our data showed unambiguously that DHN-5 can protect LDH in vitro against various adverse treatments including heat, dehydration and cold stresses.
The K-Segments of DHN-5 are Required for the Protective Effect of LDH Activity Against Various Stresses
The ability of DHN-5 to protect LDH from stress induced-inactivation incited us to investigate further the mechanistic basis of this feature by mapping the structural domains of DHN-5 required for the protective effect. For this purpose, we generated a series of truncated forms of the recombinant protein. The truncated variants DL1, DL2, DL3 and DL4 contain deletions of K1, Φ and K2; Y and S; Y, S and K1; and Y, S, K1 and K2 segments, respectively (Fig. 2a). The ORFs of all forms together with the wild-type one were cloned in frame with the 3′ end of the coding sequence of GST using pGEX-4T-1 expression vector. After IPTG induction, the resulting recombinant proteins accumulated to high amounts in E. coli cells (Fig. 2b). After digestion with thrombin, the eluted DHN-5 protein and its truncated variants migrated at a little higher position instead of the calculated molecular mass (Fig. 2b). Similar results were observed frequently in the SDS-PAGE analysis of DHN [6]. When we performed the same assays as above to monitor the LDH activity using these truncated forms, we found that the K-segment(s), and not Φ- or YS-segments, were responsible for the protective effect on LDH against various stresses (Fig. 3). In fact, when LDH was incubated at 0 °C in the presence of K1ΦK2 segments, the activity rates were similar to those registered with the full-length protein and reached even 120 % after 24 h of cold stress. Although to a less extent, the ΦK2-segment also exhibited protective effect. However, Φ- or YS-segments did not exhibit any protective effect on LDH and the activity decline rates were similar to control sample (with buffer) (Fig. 3a). Similar results were obtained when LDH was subjected to heat and dehydration stresses (Fig. 3b, c). These data indicate that the K-segments seem to be indispensable for the protective effect of DHN-5 on LDH activity, under various stress treatments.
Production of recombinant forms of DHN-5 and its truncated derivatives in E. coli. a Schematic representation of the distinct forms of DHN-5 used in this study. Positions of the structural domains Y, S and K (on DHN-5, DL1, DL2, DL3 and DL4 truncated derivatives) as well as the PCR primers are indicated. b Protein analysis by SDS-PAGE using coomasie blue. Panel 1 protein extract from IPTG induced cells expressing GST alone (lane 1), purified GST (lane 2), protein extract from IPTG induced cells expressing GST::DHN-5 (lane 3), after its purification (lane 4) and elution from thrombin digested protein (lane 5). Panel 2 protein extract from IPTG induced cells expressing the DL1 derivative, GST::YS (lane 1) and after its purification/elution from thrombin digested protein. Panel 3 protein extract from IPTG induced cells expressing the DL2 derivative GST:: K1ΦK2 (lane 1), after its purification (lane 2) and elution from thrombin digested protein (lane 3). Panel 4 protein extract from IPTG induced cells expressing the DL3 derivative, GST:: ΦK2 (lane 1) after its purification (lane 2) and elution from thrombin digested protein (lane 3). Panel 5 protein extract from IPTG induced cells expressing the DL4 derivative, GST:: Φ (lane 1) after its purification (lane 2) and elution from thrombin digested protein (lane 3). Positions of the recombinant proteins are indicated by arrows. Protein markers in kDa are shown on the left of each panel
DHN-5 truncated variants protect LDH activity from various stresses in vitro. LDH solutions were kept on ice (a), dehydrated to 70 % (b) or heated at 43 °C (c) for the specified time in the absence or the presence of recombinant DHN-5, YS, K1ΦK2, ΦK2 and Φ derivatives. A small aliquot was taken at each of several time points, and LDH activity was measured. The dehydration stress was achieved using a speed-vac centrifuge, after which the sample was rehydrated to the original volume with water, and LDH activity was then measured. Data are presented as relative activity (%) respective to the LDH activity registered before treatment. Three independent assays have been performed and standard errors are included. Values are mean ± SD (n = 5)
Monitoring the Effect of DHN-5 and Its Truncated Variants on bglG Activity Under Various Stresses
As we have previously found that DHN-5 has a protective effect against the heat induced damage of the fungal enzyme bglG [7], we address here the question whether the truncated forms display also this protective activity on bglG submitted to cold and heat stresses. DHN-5 and its truncated variants (DL1, DL2, DL3 and DL4) were then tested for their capacity to protect bglG at 50 (optimal temperature), 0 and 70 °C, temperatures at which bglG loses drastically its catalytic activity (40 and 37.5 % at 0 and 70 °C, respectively). As shown in Table 1, the full-length DHN-5 exhibited a protective effect on bglG not only at 50 °C but also at high (70 °C) and low (0 °C) temperatures, but with distinct efficiencies. DHN-5 was more effective under heat stress (with an activity maintained at 80.62 % corresponding to more than twofold increase in comparison with the activity registered in control sample (bglG alone 37.5 %). Under cold stress, DHN-5 exerted only a modest protection on the bglG activity which was estimated as 44.8 % instead of 40 % in control sample. When incubated with the DL1 or DL4 forms (containing only YS-segments or Φ-segment) the bglG activities were similar to those registered in the control sample regardless the temperature tested. This result indicates that YS- and Φ-segments had no protective effect on bglG under standard and stress conditions. Whereas, the DL2 (K1ΦK2-segments) and to a less degree DL3 (ΦK2-segment) forms displayed protective effects on bglG under heat and cold stresses. Under cold conditions, both truncated forms were able to increase slightly bglG activity almost with the same efficiency as the wild-type protein did. Under heat stress, the incubation with DL2 and DL3 forms resulted into an enhanced bglG activity (75 and 64.2 %), but lower than that registered with DHN-5.
Furthermore, the effect of the K-segment containing variants (DL2 and DL3) on the thermostability of bglG was also assessed by performing a time course activity of this enzyme incubated at 70 °C (Fig. 4a). The activity of bglG in the presence of DL2 or DL3 variants followed rates similar to that of the full-length DHN-5. In all cases, the maximum reached after ~18 min was followed by a relatively rapid decay dropping the bglG activity to comparable levels (between 0.4 and 0.6 units/ml) after 60 min of heat stress. All together these data point out that the K-segments of DHN-5 play relevant role in protecting bglG against heat stresses.
Effect of DHN-5 and its truncated variants on the thermostability of the purified bglG. a bglG was incubated without and with dehydrin at 70 °C. Aliquots were taken at various times and the relative activity was determined. Three independent experiments have been performed and standard errors are included. b Enzymatic recovery of bglG after a heat stress, in the absence or the presence of DHN-5 and its truncated derivatives. The bglG enzyme was incubated alone at 70 °C. Samples were taken at 0, 10, 20 and 30 min and incubated for 15 min at 37 °C without or with recombinant forms of DHN-5 or its truncated derivatives. The relative activity was determined as described in enzyme assay section. Data presented are means (with standard errors) of four independent experiments. Values are mean ± SD (n = 4)
Involvement of Dehydrin K-Segments in the Recovery of Heat Inactivated bglG
Beside the involvement of K-segments in enhancing the heat stability of bglG, we examined whether these segments were also able to help in the refolding of this enzyme following heat stress as the wild-type protein did [9]. To this end, we setup an assay where bglG was first incubated at 70 °C for increasing times (0, 10, 20 and 30 min) before a shift to 37 °C for 15 min in the presence or the absence of DHN-5, DL2 or DL3 truncated forms. As shown in Fig. 4b, when incubated alone, bglG activity decreased dramatically at 70 °C to lose up to 80 % of its initial activity after 30 min. This indicates that during the recovery step (at 37 °C); most of the heat denatured bglG was unable to refold properly, which results in a decrease in the enzymatic activity. In the presence of DHN-5, DL2 or DL3 variants, a decrease in bglG activity was also observed, but at a lower rate. The bglG activities with DHN-5, DL2 or DL3 remained 20 and 15 % higher than the control sample (bglG alone). This enhancement in the activity might have been simply due to a higher proportion of a properly refolded enzyme. Therefore, we propose that DHN-5 and K-segments (especially K1ΦK2 together) may exhibit a chaperone-like effect on bglG, perhaps by helping its proper refolding after a heat stress.
Discussion
Dehydrins are ubiquitous proteins in plants that accumulate in response to water stresses such as drought, freezing and salinity [2, 17]. The most conserved motif shared by all DHNs is the lysine-rich motif known as the K-segment which exists as a single or multiple copies. Under reduced hydration, the K-segment was shown to form an amphipathic α-helix that is encountered in biologically active peptides and proteins such as apolipoproteins or peptide hormones [18]. In addition, DHNs contain high proportions of hydrophilic amino acids and appear in aqueous solutions as intrinsically unstructured proteins. It is believed that the intrinsically unstructured character of DHNs allow them to adopt multiple conformations (according to the changes in their ambient microenvironment), resulting in multiple functions [5]. Such feature is common for the intrinsically unstructured/disordered proteins which behave as moonlight proteins since they have the capacity to possess multiple functions according to their conformation [4, 19]. Accordingly, the structural flexibility of IUP offers them the capacity to bind to and display opposing (inhibiting or activating) actions on different partners or even on the same partner molecule. In eukaryotes, the moonlighting proteins fulfill important functions that are often associated with signal transduction, gene expression and chaperone action. For example, the fully disordered cyclin-dependent kinase (Cdk) inhibitor p21Cip1 binds to both the cyclin and kinase subunits of activated Cdk and inhibits kinase action [20].
Many arguments are in favour to suggest that the DHNs behave as moonlight proteins. DHNs were indeed reported to exhibit myriad of functions (e.g. chaperone, cryoprotective, antifreeze, radical-scavenging and ion-binding functions) in vivo (during plant response to stress factors) and in vitro (for review see [5]). In this study, we showed that DHN-5 and its truncated variants K1ΦK2 and ΦK2 have protective activities not only on LDH but also on bglG. We provide here the evidence that the K-segment containing truncated forms exhibit on these enzymes cold- and heat-protective activities. The cold-protective effect was however more significant on LDH than on bglG. This difference could be explained by the fact that cold stress induced a more harmful damage on LDH, in comparison with bglG. As both cold and heat cause water loss, it is plausible that the protective effect of DHN-5 is linked to a better water availability. This assumption was confirmed when we found that DHN-5 provides a better protection of LDH under dehydration stress during which the enzymatic activity was only 20 % lower. In addition, regardless the enzymatic assay, it seems that the association of both K-segments (K1ΦK2) gave better effects in comparison with those observed with ΦK2-segment alone. The mechanism by which the K-segments exhibit their protective effects remains unknown. Using nuclear magnetic resonance (NMR), Hughes and Graether [21], demonstrated that there is no direct binding interaction between K2 and LDH. The lack of a direct interaction suggests that the K-segments present in one DHN, are able when present in α-helical conformation to act as a molecular shield that prevents denatured LDH from associating with other LDH molecules [21]. It is worth to note that the protective effects exerted by the K-segments of DHN-5 are less pronounced than those of the full-length protein. This difference can be due to the higher size of DHN-5 (26 kDa) compared to the truncated forms (20 and 18 kDa for K1ΦK2 and ΦK2, respectively). Therefore, if we assume that DHN-5 acts as a molecular shield on LDH or bglG, it is plausible to think that the full-length protein DHN-5 is able to offer a larger shield. This assumption is supported by the cryprotection studies using the YSK2 dehydrin from Vitis riparia (13.9 kDa), which is more effective than the K2 molecules (5.4 kDa) at protecting LDH from freeze–thaw denaturation [21]. The conserved K-segments in DHNs are highly positively charged due to the presence of several Lys residues. Electrostatic interactions between the positively charged K-segments and the negatively charged LDH surface help keep the two proteins in close proximity without binding. The loss of activity after various treatments has been attributed a loss of its tetrameric state [22], aggregation [23] and/or changes in its three dimensional structure [24, 25]. Reyes et al. [26] showed that the removal of the K-segments from several DHNs lowered the recovery of LDH activity after freezing and thawing the enzyme, whereas the deletion of a Φ-like segment had no such effect. The same study stated however, that a K-segment peptide alone did not show any protective effect suggesting that the Φ-region still may also contribute to this protective effect. The assays performed by Hughes and Graether [21] revealed also that the K-segments and the Φ-region were both required to ensure cryoprotection of LDH. In the case of DHN-5, although our assays showed that the Φ-region has no protective activity, this flexible peptide may still be of assistance for the two K-segments to prevent a stress induced denaturation of the enzymes and hence ensure a better enzymatic stability. It is plausible that the Φ-segment might help the two K-segments of DHN-5 to fold properly into an α-helical conformation thus enhancing their amphipathic character in protein–protein interactions.
In conclusion, our data confirm that DHN-5 via its K-segments offer higher protection of LDH and bglG under heat, dehydration and cold stress. Our finding reinforces the idea that the DHN are attractive molecules which can be exploited as molecular chaperones for engineering novel thermo- or cryo-resistant enzymes.
References
Wise, M. J. (2003). LEAping to conclusions: A computational reanalysis of late embryogenesis abundant proteins and their possible roles. BMC Bioinformatics, 4, 52.
Close, T. J. (1996). Dehydrins: Emergence of a biochemical role of a family of plant dehydration proteins. Physiologia Plantarum, 97, 795–803.
Close, T. J. (1997). Dehydrins: A commonality in the presence of plants to dehydration and low temperature. Physiologia Plantarum, 100, 291–296.
Tompa, P., Szász, C., & Buday, L. (2005). Structural disorder throws new light on moonlighting. Trends in Biochemical Sciences, 30, 484–489.
Hanin, M., Brini, F., Ebel, Ch., Toda, Y., Takeda, Sh., & Masmoudi, K. (2011). Plant dehydrins and stress tolerance: Versatile proteins for complex mechanisms. Plant Signaling & Behavior, 6, 1–7.
Kazuoka, T., & Oeda, K. (1994). Purification and characterization of COR85-oligomeric complex from cold acclimated spinach. Plant and Cell Physiology, 35, 601–611.
Houde, M., Daniel, C., Lachapelle, M., Allard, F., Laliberté, S., & Sarhan, F. (1995). Immunolocalization of freezing-tolerance associated proteins in the cytoplasm and nucleoplasm of wheat crown tissues. The Plant Journal, 8, 583–593.
Wisniewski, M., Webb, R., Balsamo, R., Close, T. J., Yu, X. M., & Griffith, M. (1999). Purification, immunolocalization, cryoprotective, and antifreeze activity of PCA60: A dehydrin from peach (Prunus persica). Physiologia Plantarum, 105, 600–608.
Brini, F., Saibi, W., Amara, I., Gargouri, A., Masmoudi, K., & Hanin, M. (2010). The wheat dehydrin DHN-5 exerts a heat-protective effect on β-glucosidase and glucose oxidase activities. Bioscience, Biotechnology, and Biochemistry, 74, 1050–1054.
Hara, M., Terashima, S., & Kuboi, T. (2001). Characterization and cryoprotective activity of cold responsive dehydrin from Citrus unshiu. Journal of Plant Physiology, 158, 1333–1339.
Ellis, R. J. (2004). From chloroplasts to chaperones: How one thing led to another. Photosynthesis Research, 80, 333–343.
Tunnacliffe, A., & Wise, M. J. (2007). The continuing conundrum of the LEA proteins. Naturwissenschaften, 94, 791–812.
Saibi, W., & Gargouri, A. (2011). Purification and biochemical characterization of an atypical β-glucosidase from Stachybotrys microspora. Journal of Molecular Catalysis B-Enzymatic, 72, 107–115.
Tamya, T., Okahashi, N., Sakuma, R., Aoyama, T., Akahane, T., & Matsumoto, J. J. (1985). Freeze denaturation of enzymes and its prevention with additives. Cryobiology, 22, 446–456.
Carpenter, J. F., & Crowe, J. H. (1988). The mechanism of cryoprotection of proteins by solute. Cryobiology, 25, 244–255.
Reyes, J. L., Rodrigo, M. J., Colmenero-Flores, J. M., Gil, J. V., Garay-Arroyo, A., Campos, F., et al. (2005). Hydrophilins from distant organisms can protect enzymatic activities from water limitation effects in vitro. Plant, Cell and Environment, 28, 709–718.
Ingram, J., & Bartels, D. (1996). The molecular basis of dehydration tolerance in plants. Annual Review of Plant Physiology and Plant Molecular Biology, 47, 377–403.
Segrest, J. P., De Jones, M. K., Loof, H., Brouillette, C. G., Venkatachalapathi, Y. V., & Anantharamaiah, G. M. (1992). The amphipathic helix in the exchangeable apolipoproteins: A review of secondary structure and function. Journal of Lipid Research, 33, 141–166.
Tompa, P. (2002). Intrinsically unstructured proteins. Trends in Biochemical Sciences, 27, 527–533.
Kriwacki, R. W., Hengst, L., Tennant, L., Reed, S. I., & Wright, P. E. (1996). Structural studies of p21Waf1/Cip1/Sdi1 in the free and Cdk2-bound state: conformational disorder mediates binding diversity. Proceedings of the National Academy of Sciences USA, 93, 11504–11509.
Hughes, S., & Graether, S. P. (2011). Cryoprotective mechanism of a small intrinsically disordered dehydrin protein. Protein Science, 20, 42–50.
Zheng, Y. B., Meng, F. G., Chen, B. Y., & Wang, X. C. (2002). Inactivation and conformational changes of lactate dehydrogenase from porcine heart in sodium dodecyl sulphate solutions. International Journal of Biological Macromolecules, 31, 97–102.
Goyal, K., Walton, L. J., & Tunnacliffe, A. (2005). LEA proteins prevent protein aggregation due to water stress. Biochemical Journal, 388, 151–157.
Bai, J. H., Wang, H. J., & Zhou, H. M. (1998). Alkaline-induced unfolding and salt-induced folding of pig heart lactate dehydrogenase under high pH conditions. International Journal of Biological Macromolecules, 23, 127–133.
Gabellieri, E., & Strambini, G. B. (2006). ANS fluorescence detects widespread perturbations of protein tertiary structure in ice. Biophysical Journal, 90, 3239–3245.
Reyes, J. L., Campos, F., Wei, H., Arora, R., Yang, Y., Karlson, D. T., et al. (2008). Functional dissection of hydrophilins during in vitro freeze protection. Plant, Cell and Environment, 31, 1781–1790.
Acknowledgments
This study was supported by grants from the Ministry of Higher Education and Scientific Research, Tunisia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drira, M., Saibi, W., Brini, F. et al. The K-Segments of the Wheat Dehydrin DHN-5 are Essential for the Protection of Lactate Dehydrogenase and β-Glucosidase Activities In Vitro. Mol Biotechnol 54, 643–650 (2013). https://doi.org/10.1007/s12033-012-9606-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-012-9606-8