Abstract
While heparin has been shown to eliminate cell aggregation in suspension adaptations of insect and HEK293 cells for virus-based cell cultures, the role of heparin in long period serum-free suspension adaptation of the anchorage-dependent Chinese hamster ovary (CHO) cell lines remains inconclusive. In this paper, we explore the potential application of heparin in suspension adaptation of CHO cell line which produces an anti-human chimeric antibody cHAb18. Heparin showed a concentration-dependent inhibition of CHO–TS28 cell-to-cell adhesion, with a significant inhibitory effect occurring when the concentration exceeded 250 μg/ml (P < 0.001). Heparin also exhibited a cell aggregation elimination role at all concentrations (P < 0.001). Furthermore, heparin promoted cell growth and antibody secretion, with the highest cell density ((99.83 ± 12.21) × 104 cells/ml, P = 0.034) and maximum antibody yield ((9.46 ± 0.94) mg/l, P < 0.001) both occurring at 250 μg/ml heparin. When agitated, cell aggregates were effectively dispersed by 250 μg/ml heparin and a single-cell suspension culture process was promoted. In suspension adapted CHO–TS28 cells, cell growth rates and specific antibody productivity were maintained; while antigen-binding activity improved slightly. Together, our results show that heparin may promote suspension adaptation of anchorage-depended CHO cells by resisting cell aggregation without reducing cell growth, antibody secretion, and antigen-binding activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
HAb18 is an anti-human monoclonal antibody used against HAb18G/CD147 for hepatocellular carcinoma (HCC) treatment. Based on HAb18, our lab developed an Iodine (131I) Metuximab Injection (RS20050039) for radioimmunotherapy [1, 2] and established large-scale antibody production technology to support its clinical trial [3, 4]. To further improve therapeutic efficacy and reduce human antimouse antibody (HAMA) reaction, a chimeric antibody cHAb18 was constructed and expressed in a Chinese hamster ovary (CHO) cell line, namely CHO–TS28. Now, the industrial production technology for cHAb18 targeting HAb18G/CD147 is urgently needed.
In industrial production, a serum-free suspension process is the preferred mode due to the well-understood principles of scaling parameters and the ease of process control [5]. Although commercially available media could transit anchorage-dependent CHO cells into suspension mode in a suspension adaptation process, it is still a time-consuming and costly process [6] and, more importantly, cells in suspension have a tendency to aggregate into large and uncontrolled cell clumps, which may lead to cell death, altered cell metabolism, and reduced product secretion [7]. Several methods including combining media development with gradual weaning, clonal selection, mechanical agitation, trypsin, and calcium complex disodium salt (EDTA) application have been used to address the problem of cell aggregation, but these methods dislodge cells at the cost of growth inhibition, productivity lost and product’s glycosylation alteration [8, 9]. Thus, a dispersing agent that competitively binds to the adhesion receptor to block aggregation, while not impacting cell proliferation and product secretion would be a more reasonable anti-aggregation solution. Success of this process would speed up the rate of adaptation and provide a valuable tool for industrial and research applications.
Recently, anti-aggregation agents with high sulfate group density including dextran sulfate, suramin, and heparin were introduced into serum-free suspension culture, which resulted in reduced cell conglobation and improved suspension adaptation. But the usage of dextran was limited to the insect cell BTI-TN 5B-1-4 and inhibited cell growth [10, 11]; and the exact effect of suramin on cell growth was inconsistent [12, 13]. In contrast, heparin has been successfully applied in suspension cultures of TN 5B-1-4 insect cells [14] and HEK293 cell [15, 16] in virus-cell culture systems that have culture phases short in duration and on a small scale. Herein, heparin plays important roles of anti-aggregation by inhibiting cell-to-cell adhesion [17, 18]. Until recently, there was limited data about its application in CHO cell suspension adaptation. So, it would be interesting to explore its role in CHO cell suspension adaptation while avoiding its negative effect on protein secretion.
In this study, we assessed and selected the optimal heparin dosage to resist cell aggregation without affecting cell growth and product secretion. Next, a suspended CHO cell line for industrial production was obtained in an agitation bioreactor by combining three parameters: serum-free condition, heparin, and agitation. Finally, we evaluated the product quality which produced from the suspended CHO cell line and then explored the possible molecular mechanism involved. Our results may provide a simple but feasible model for suspension adaptation of anchorage-dependent cells for a long-period cell culture process.
Materials and Methods
Cell Line and Medium
The engineered cell line CHO–TS28 producing anti-human HCC chimeric antibody cHAb18 was used throughout this study. CHO–TS28 cells were cultured in Dulbecco’s Modified Eagle’s Medium/Ham’s Nutrient Mixture F-12 (DMEM/F12) (Hyclone, Logan, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, USA). The SFM28 medium for suspension adaptation was serum-free and based on DMEM/F12 (HyQ® Lot No. RRC040057), which was supplemented with insulin, transferrin, sodium selenite, ethanolamine, and 2-mercaptoethanol (Sigma, St. Louis, USA).
Effect of Heparin on Cell-to-Cell Adhesion and Cell Aggregation
To explore the potential role of heparin on inhibiting cell-to-cell adhesion, the CHO–TS28 cell suspension (2 × 104 cells/well) in 10% FBS was distributed into a 2 μg fibronectin-coated 24 well plate and cultured at 37°C, 5% CO2 overnight to achieve adhesion. Next, the CHO–TS28 cell suspension (5 × 104 cells/well) in 10% FBS/DMEM containing different concentrations of heparin (Sigma, St. Louis, USA), ranging from 0 to 2000 μg/ml, were seeded and incubated for 6 h. Then, the adhered cells were dissociated with 0.1 ml 0.4% EDTA/phosphate buffered saline (PBS) (Sigma, St. Louis, USA) for 30 min and then counted. The cell adhesion inhibition ratio of heparin was determined by the follow relationship: (adhered cell numbercontrol − adhered cell numberheparin)/(adhered cell numbercontrol) × 100%. The role of heparin in eliminating cell aggregation was investigated by cultivating a CHO–TS28 cell suspension (3 × 105 cells/ml) in SFM28 containing heparin, as described above, in a 25 cm2 T-flasks for 48 h. The dissociated single cells were collected and counted, and the dissociation ratio was determined as follows: (dissociated cell number/total cell number) × 100%.
Effect of Heparin on Cell Growth, Antibody Secretion, and Antigen-Binding Activity
CHO–TS28 cells in the mid-exponential growth phase were collected by centrifugation at 190 g for 5 min. The cells were then transferred and cultured in a SFM28 medium containing seven different concentrations of heparin, ranging from 0 to 2000 μg/ml, for 48 h at 37°C in a 5% CO2 atmosphere. Static cultures were set up in duplicate in 75 cm2 T-flasks with a cell density of 3 × 105 cells/ml. Cells were washed with a warm PBS and dissociated with the 10 ml nonenzymatic cell dissociation solution. At 1 min intervals, samples were taken in triplicate to determine cell growth, antibody production and antigen-binding activity. All results were expressed as mean and standard error.
Cell Growth Assay
The aggregated cells were dissociated using 0.1 ml 0.4% EDTA/PBS at 37°C for 30 min, and were then counted. Total cell number and viability were determined by trypan blue (Invitrogen, Carlsbad, USA) exclusion and manual cell counting using a haemocytometer. The specific growth rate (μ) was calculated using the equation \( \mu = {\frac{{{\text{LN}}\chi_{{{\text{v}}2}} - {\text{LN}}\chi_{{{\text{v}}1}} }}{{t_{2} - t_{1} }}} \) (LN refers to natural logarithm which is the logarithm to the base e, χ v is viable cell density, t is culture time).
Antibody Secretion Assay
The antibody yield assay was performed using a sandwich enzyme-linked immunoassay (ELISA). The plate was coated with a goat anti-human immunoglobulin G (IgG) (Sigma, St. Louis, USA) at a concentration of 10 μg/ml and then blocked with 5% non-fat milk (Bio-Rad, Hercules, USA) in PBS. The primary antibody was a diluted supernatant of CHO–TS28 cells, and horseradish peroxidase (HRP) conjugated goat anti-human IgG (Thermo, Waltham, USA) of 1:10000 was used as the detection antibody. And, 3,3′5,5′-tetramethyl benzidine (TMB) (Sigma, St. Louis, USA) was used as a substrate to develop color. A standard curve was graphed using purified hHAb18 IgG as a standard. And, the purity of hHAb18 IgG was above 95%, which was confirmed by high performance liquid chromatography (HPLC) and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Specific antibody productivity (q MAb) was calculated using an expression level based on ELISA results and measured viable cell densities using the equation \( q_{\text{MAb}} = {\frac{{{\text{Ab}}_{2} - {\text{Ab}}_{1} }}{{{\frac{{\chi_{{{\text{v}}2}} - \chi_{{{\text{v}}1}} }}{2}}(t_{2} - t_{1} )}}} \) (Ab is antibody yield).
Antigen-Binding Activity Assay
ELISA and flow cytometry (FCM) were used to measure the binding activity of cHAb18 IgG to the target antigen HAb18G/CD147 in a molecular pattern of full length and extracellular domain which were constructed and expressed in CHO. Here, full length of antigen CD147 was fused to human IgG–Fc fragment and purified by protein A affinity column, while extracellular domain of antigen CD147 was obtained by enzyme digestion and remove of human IgG–Fc fragment from full length of antigen CD147. In ELISA assay, HAb18G/CD147 with purity above 95% was coated at 10 μg/ml. Then the plates were incubated first with 1% bovine serum albumin (BSA) (Sigma, St. Louis, USA) at room temperature for 2 h and then with a diluted supernatant of CHO–TS28 cells at 4°C overnight, followed by incubation with HRP-conjugated goat anti-human IgG (Sigma, St. Louis, USA) at 37°C for 1 h. To complete the process, TMB was added to develop color. As for FCM, HAb18G/CD147 highly expressed target cells, FHCC98 (1 × 106 cells) [19], were blocked with 1% BSA in PBS on ice for 30 min. The cells were then incubated on ice with supernatant at a concentration of 5 μg/ml for 1 h. After washing twice with PBS, fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgG (Thermo, Waltham, USA) at a dilution of 1:500 were incubated for another 30 min. Finally, FHCC98 cells were suspended in PBS containing 2% FBS, and analyzed by FCM.
Suspension Adaptation Culture
The cell suspension prepared from the adhered cells whose specific growth rate of 0.022–0.025/h was inoculated at 5 × 105 viable cells/ml in SFM28 medium, supplemented with 0.1% PF-68 (Pluronic F-68) (Sigma, St. Louis, USA) and 250 μg/ml heparin, and then transferred to a sterile Superspinner and agitated at a speed of 75–95 rpm. When viable cell density reached 1 × 106 cells/ml, the continuously cultivated cells were remove and passaged again with the inoculated density of 5 × 105 cells/ml again. Once the cell density reached 1 × 106 cells/ml with a viability of at least 90% for three consecutive passages, we may assume the cells have adapted to a suspension culture of a certain serum level. At periodic points in the adaptation process, samples were taken for comparison of cell growth, antibody production and antigen-binding activity.
Flow Cytometry Analysis of E-Cadherin Expression
CHO–TS28 cells were cultured in a 10% FBS/DMEM with or without 250 μg/ml heparin addition. After 48 h, 5 × 105 cells were washed with a warm PBS and dissociated with the 10 ml nonenzymatic cell dissociation solution. Then cells were washed twice in staining buffer (PBS, 2% FCS, pH 7.5) and blocked with 5% normal goat serum (Invitrogen, Carlsbad, USA) on ice for 30 min. Cells were incubated on ice for 1 h with 50 μl of staining buffer containing a 5 μl mouse monoclonal antibody against mouse E-cadherin (RD, Minneapolis, USA). Specificity of staining was confirmed by incubation of cells with an isotype-matched control. Cells were washed twice in staining buffer and then incubated for 30 min in the dark with 50 μl of staining buffer containing a 1/500 dilution of FITC-conjugated goat anti-mouse IgG. Cells were washed a further three times, resuspended in 0.5 ml of staining buffer and stored on ice in the dark prior to analysis by flow cytometry. Propidium iodide (PI; Sigma, St Louis, USA) was added at a final concentration of 1 μg/ml to each tube 5 min prior to cell acquisition for the identification of live cells and for analysis of cell viability. Values are given as percentages of positive cells, which is an indication of the level of expression.
Statistics Analysis
All numerical data are presented as means ± SD, figured by Graphpad Prism, Version 4.01, and analyzed by SPSS10.0 software. Significance was assigned at the P < 0.05 level.
Results
Heparin Showed a Dose-Dependent Inhibition of CHO–TS28 Cell Aggregation
To explore the potential anti-aggregation role of heparin in serum-free suspension adaptation, the inhibitory effect of heparin on cell-to-cell adhesion was first investigated in serum-containing condition (Fig. 1a). Heparin showed a concentration-dependent inhibition of cell-to-cell adhesion with a significant inhibitory effect occurring when heparin concentration exceeded 250 μg/ml. Compared with an untreated control, the number of adhered cells in the 250 μg/ml heparin-treated group was significantly reduced (50.00 ± 2.50 × 103 vs. 35.03 ± 3.19 × 103 cells/ml, P < 0.001). The adhesion inhibitory ratio was 0.3 ± 0.050 in the group treated with 250 μg/ml heparin and reached a maximum of 0.49 ± 0.024 in the group treated with 2000 μg/ml heparin. For the CHO–TS28 serum-free culture, heparin effectively dissociated cell clumps into single-cell suspensions (Fig. 1b). The percentage of dissociated single cells was significantly higher in groups treated with heparin, ranging from 26.25 ± 1.64 to 52.96 ± 1.08%, than in untreated control groups (10.37 ± 0.82%) in a concentration-dependent manner. These results indicate that heparin effectively dispersed cell aggregates through inhibition of cell-to-cell adhesion.
Heparin showed a dose-dependent inhibition of CHO–TS28 cell-to-cell adhesion. a The influence of heparin concentration on CHO–TS28 cell-to-cell adhesion. b The eliminating role of heparin on cell aggregates at different concentration (* indicated P < 0.05; ** indicated P < 0.01; *** indicated P < 0.001)
Heparin Improved CHO–TS28 Cell Growth and Antibody Secretion
As shown in Fig. 2a, heparin incubation resulted in a visible change in cell shape, from fibroblast-like into round. It also significantly increased the viability of cells in the groups treated with 125 μg/ml (figure not shown), 250 μg/ml and 500 μg/ml heparin (P = 0.008, P = 0.004, and P = 0.016, respectively). Compared to control groups without added heparin, a significantly higher cell density was found in groups treated with 125 and 250 μg/ml heparin (P = 0.047 and P = 0.034, respectively; Fig. 2b), and a significantly higher μ was found in groups treated with 250 μg/ml heparin (P = 0.039; Fig. 2c, d). The maximal cell density of (99.83 ± 12.21) × 104 cells/ml and the maximal specific growth rate (μmax) of (0.60 ± 0.06)/day were both reached at 250 μg/ml heparin; thereafter cell density and μ decreased with increased heparin concentration from 250 to 2000 μg/ml. A similar trend also presented for antibody secretion, with the maximal antibody yield of 9.46 ± 0.94 mg/l and the maximal antibody productivity (q MAb/max) of 5.85 ± 1.30 pg/cell/day obtained at 250 and 500 μg/ml heparin, respectively (Fig. 2c, d). Heparin concentrations of 250, 500, 1000, and 2000 μg/ml all significantly increased antibody yield (P < 0.001, P = 0.001, P = 0.038, and P = 0.045 accordingly), but only 250 and 500 μg/ml heparin-treated groups have a significant q MAb (P = 0.029 and P = 0.006, respectively). These results indicate that heparin promoted cell growth and antibody secretion in a concentration-dependent manner, and that 250 μg/ml was the optimal concentration for enhancing both cell density and antibody production simultaneously.
Heparin improved CHO–TS28 cell growth and antibody production. a The comparison of cell growth pattern and morphology at different heparin concentration. b The influence of heparin concentration on cell growth and antibody secretion. c, d The curve of specific cell growth rate (μ) and specific antibody productivity (q MAb) at different heparin concentration (* indicated P < 0.05; ** indicated P < 0.01; *** indicated P < 0.001)
Heparin Promoted CHO–TS28 Cell Serum-Free Suspension Adaptation Process
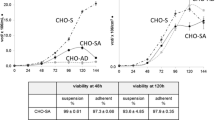
When CHO–TS28 cells were directly transferred from serum-containing static culture conditions into a serum-free superspinner agitated culture, large cell clumps formed rapidly, and then many cells gradually died due to loss of their natural anchorage-dependent growth characteristics. To reduce the clumps under serum-free condition, we combined the addition of 250 μg/ml heparin with agitation during suspension process. And suspended single cells with relatively high viability were obtained (Fig. 3a). During the suspension adaptation process, μ first decreased to 0.408/day with viability reducing to 65% at the eight passage, and then μ recovered to 0.511–1.248/day with viability around 90% after the cultivation of 11 passages; μ maintained at this level for 2 months (Fig. 3b).
Heparin promoted CHO–TS28 serum-free suspension adaptation process. a Cell growth pattern and morphology under different conditions including serum-containing (10% FBS), serum-free (SFM28), and serum-free suspension (Sp). b Change of cell density, μ and viability during serum-free suspension adaptation. c Comparison of μ and q MAb under culture condition of 10% FBS, SFM28, and Sp (* indicated P < 0.05; ** indicated P < 0.01; *** indicated P < 0.001)
After suspension adaptation (Sp), q MAb increased slightly compared to serum-containing cultures (10% FBS) and serum-free cultures (SFM28), exhibiting a value of 17.76 ± 5.04 pg/cell/day compared to 15.65 ± 2.88 and 15.84 ± 8.81 pg/cell/day, respectively. Compared to serum-containing culture conditions, μ first decreased in serum-free culture conditions (0.389 ± 0.034/day vs. 0.758 ± 0.265/day, P = 0.072), but then recovered gradually after a 2-week suspension adaptation culture process (0.715 ± 0.24/day vs. 0.758 ± 0.265/day, P = 0.807) (Fig. 3c). These results revealed that heparin promoted a serum-free suspension adaptation process for CHO–TS28 cells without reducing cell growth rate and antibody productivity.
In addition to antibody yield, we also monitored the alteration of cHAb18 IgG binding activity to its target antigen HAb18G/CD147 during suspension adaptation. As shown in FCM (Fig. 4a), the fluorescence intensity of cHAb18 IgG binding to the HAb18G/CD147 highly expressed cell line FHCC98 increased in serum-free cultures (SFM 28, #16: 722.62) while decreasing in serum-free suspension conditions (Sp, #15: 487.54), as compared to serum-containing conditions (10% FBS, #17: 509.08). However, the percentage of positive cells remains unchanged (97.77–99.77%) among three conditions. Furthermore, ELISA was used to detect antigen-binding activity in the molecular pattern of both extracellular domain and full length (Fig. 4b). In detail, the A490nm detected from the extracellular domain maintained among 0.33 ± 0.21, 0.45 ± 0.22, and 0.54 ± 0.27 when culture conditions changed from serum-containing, serum-free to serum-free suspension (P = 0.555, P = 0.318 for SFM28 and Sp, respectively). A similar trend was also found in the binding activity of cHAb18 IgG to full length target antigen HAb18G/CD147 (P = 0.640, P = 0.236 for SFM28 and Sp, respectively). These results revealed that heparin promoted a CHO–TS28 cell serum-free suspension adaptation process without influencing antigen-binding activity.
Antigen-binding activity profiles of cHAb18 IgG during serum-free suspension adaptation. a The positive percentage analysis of target antigen in HAb18G/CD147 highly expressed cell line FHCC98 detected by cHAb18 IgG. b The binding activity of cHAb18 IgG to target antigen HAb18G/CD147 in molecule pattern of both extracellular domain and full length detected by ELISA. NC negative control (PBS), 10% FBS serum-containing, SFM28 serum-free, Sp serum-free suspension
Heparin Repressed Cell–Cell Adhesion by Inhibiting E-Cadherin Expression
E-cadherin is a well characterized adhesion molecule that plays a major role in epithelial cell adhesion. Based our findings that cell–cell adhesion was inhibited by heparin, we assumed that heparin may exert its anti-adhesion role by way of decreasing E-cadherin expression. To explore the potential mechanism of heparin inhibiting cell–cell adhesion, E-cadherin expression in CHO–TS28 cells with or without heparin treatment for 48 h was detected by flow cytometry. Our result showed that 250 μg/ml heparin reduced the positive rate of E-cadherin from 51.5 to 26.4%. Thus, it may indicted that heparin exert its role of anti-aggregation by reducing E-cadherin expression. However, more studies needed to be done to confirm this presumption.
Discussion
Heparin is a multifunctional, highly sulfated polysaccharide consisting of alternating uronic acid and d-glucosamine residues, and it carries a negative charge. Although heparin is best known for its anticoagulant properties, it could also mediate cell-to-matrix and cell-to-cell adhesion in a variety of physiological processes such as endothelial cell and smooth muscle cell growth, angiogenesis, inflammation, and tumor metastasis [20]. Through increased agitation and the addition of an optimal dosage of heparin, we successfully adapted CHO–TS28 cells into single-cell suspension, indicating heparin plays a significant role in mammalian cell culture and adaptation. This single-cell suspension can be maintained for 2 months and may theoretically facilitate nutrient and oxygen transport, improve cell growth, recombinant protein production, and post-translational processing, which still need to be validated further. Also, the elimination of necrotic centers that form in large aggregates enhanced cell viability. Now, we have successfully scaled this process from flask, 100 ml/500 ml/1l superspinner to 5l/20l bioreactors. However, protein modifications such as glycosylation and oxidation frequently occur during up-scaling process which resulted from varied process parameters including medium supplements changing (such as heparin addition). So, the detailed antibody structural analysis remains to be determined among suspension adaption process for product equivalence.
Similar results have been reported using HEK293 cells and TN 5B-1-4 insect cells in virus-cell culture systems for gene therapy. In the HEK293 cell serum-free suspension culture, heparin (100 μg/ml) not only reduced the diameter of cell clumping by 50% but also decreased μ and maximal cell density [15]. However, contrary results have also been reported. For example, Zhang reported that 100 μg/ml heparin increased μ and cell density of single-suspended HEK293 cells, while decreasing virus yield by 46 times [16]. Similarly, heparin significantly eliminated cell aggregates of TN 5B-1-4 insect cell and greatly improved cell growth from 0.5 × 106 to 3 × 106 cells/ml in 48 h, but decreased the virus yield [14]. In order to mitigate the negative effects of heparin on product secretion, we selected the optimal dosage on the basis of balancing cell growth and antibody secretion. For these reasons, we chose the heparin concentration of 250 μg/ml as the optimal level; cell concentration ((99.83 ± 12.21) × 104 cells/ml) and antibody yield (9.46 ± 0.94 mg/l) reached their highest levels while q MAb maintained a relatively high level (5.85 ± 1.30 pg/cell/day). Overall, our studies exhibit the role of heparin in CHO–TS28 serum-free suspension adaptation as promoting cell growth and product secretion, maintaining original μ and q MAb, and enhancing antigen-binding activity.
Heparin inhibited cell-to-cell adhesion in a dose-dependent manner, but its optimal dosage differed according to different cell lines and products. For TN 5B-1-4 insect cells and HEK293 cells, the most significant anti-aggregation effect occurred at concentrations of 100 IU/ml (667 μg/ml) and 100 μg/ml, respectively. But for CHO–TS28 cells producing a chimeric antibody cHAb18, the optimal level of heparin for inhibiting cell-to-cell adhesion is 250 μg/ml, which is between the optimal heparin concentrations for TN 5B-1-4 and HEK293.
The ability of heparin to induce single-cell formations may be a generic property of negatively charged polymers, or it could arise from specific interactions between the cells and the sulfate groups, the carboxylate groups, or the sugar backbone itself; or it also probably arise from interfering with the function of cell adhesion molecules. Here, our result of FCM showed that 250 μg/ml of heparin reduced the positive rate of E-cadherin expression from 51.5 to 26.4% (Fig. 5). This result indicates that heparin possibly exerts its role in anti-aggregation by reducing E-cadherin expression; however, further research is needed to confirm this phenomenon. Additional research is also needed to explore the optimal heparin concentration and its combination with other strategy in some other individual cell lines. Nevertheless, this paper may offer an example for optimization of large-scale cell culture technology for recombinant protein production by way of culturing a widely used host CHO cell line in a suspended mode.
References
Xu, J., Shen, Z. Y., Chen, X. G., Zhang, Q., Bian, H. J., Zhu, P., et al. (2007). A randomized controlled trial of LICARTIN for preventing hepatoma recurrence after liver transplantation. Hepatology, 45, 269–276.
Chen, Z. N., Mi, L., Xu, J., Song, F., Zhang, Q., Zhang, Z., et al. (2006). Targeting radioimmunotherapy of hepatocellular carcinoma with iodine (131I) metuximab injection: Clinical phase I/II trails. International Journal of Radiation Oncology, Biology, Physics, 65, 435–444.
Feng, Q., Mi, L., Li, L., Liu, R., Xie, L., Tang, H., et al. (2006). Application of “oxygen uptake rate—amino acids” associated mode in controlled-fed perfusion culture. Journal of Biotechnology, 122, 422–430.
Li, L., Mi, L., Feng, Q., Liu, R., Tang, H., Xie, L., et al. (2006). Stability validations of seed cell quality control parameters in enlarge scale hybridoma cell culture. Applied Microbiology and Biotechnology, 70, 34–39.
Wurm, F. M. (2004). Production of recombinant protein therapeutics in cultivated mammalian cells. Nature Biotechnology, 22, 1393–1398.
Astley, K., Naciri, M., Racher, A., & Al-Rubeai, M. (2007). The role of p21cip1 in adaptation of CHO cells to suspension and protein-free culture. Journal of Biotechnology, 130, 282–290.
Rory, M. (2002). Adaptation of Chinese hamster ovary cells in culture: A literature survey of adhesion proteins. CHEE4006, Individual Inquiry, A, 2–3.
Sinacore, N., Charlebois, T., Harrison, S., Brennan, S., Richards, T., Hamilton, M., et al. (1996). CHO DUKX cell lineages preadapted to growth in serum-free suspension culture enable rapid development of cell culture processes for the manufacture of recombinant proteins. Biotechnology and Bioengineering, 52, 518–528.
Cruz, H. J., Dias, E. M., Moreira, J. L., & Carrondo, M. J. T. (1997). Cell-dislodging methods under serum-free conditions. Applied Microbiology and Biotechnology, 47, 482–488.
Dee, K., Shuler, M. L., & Wood, H. A. (1997). Inducing single-cell suspension of BTI-TN5B1-4 insect cells: I. The use of sulfated polyanions to prevent cell aggregation and enhance recombinant protein production. Biotechnology and Bioengineering, 54, 191–205.
Dee, K., Wood, H. A., & Shuler, M. L. (1997). Inducing single-cell suspension of BTI-TN5B1-4 insect cells: II. The effect of sulfated polyanions on baculovirusinfection. Biotechnology and Bioengineering, 54, 206–220.
Nakata, H. (2004). Stimulation of extracellular signal-regulated kinase pathway by suramin with concomitant activation of DNA synthesis in cultured cells. Journal of Pharmacology and Experimental Therapeutics, 308, 744–753.
Karam, G. A., Rasaee, M. J., Mahmoodi, M., & Khaksari, M. (2005). Inhibition of proteinase 3 (PR3) by suramin and fetal calf serum (FCS): Effect of PR3 and suramin on Chinese hamster ovary cells (CHO-cells). Pakistan Journal of Pharmaceutical Sciences, 18, 46–48.
Taticek, R. A., McKennac, K. A., Granadosc, R. R., & Shuler, M. L. (1997). Rapid initiation of suspension cultures of Trichoplusia ni insect cells (TN 5B-1-4) using heparin. Biotechnological Technique, 11, 237–240.
Tsao, Y. S., Condon, R., Schaefer, E., Lio, P., & Liu, Z. (2001). Development and improvement of a serum-free suspension process for the production of recombinant adenoviral vectors using HEK293 cells. Cytotechnology, 37, 189–198.
Zhang, S. Y., Thwin, C., Wu, Z., Cho, T. (2001). Method for the production and purification of adenoviral vectors. US Patent, 6194191.
Harvima, Ilkka. T., Lappalainen, Katriina., Hirvonen, Maija.-Riitta., Mättö, Mikko., Kivinen, Petri. K., Hyttinen, Mika., et al. (2004). Heparin modulates the growth and adherence and augments the growth-inhibitory action of TNF-α on cultured human keratinocytes. Journal of Cellular Biochemistry, 92, 372–386.
Presta, M., Leali, D., Stabile, H., Ronca, R., Camozzi, M., Coco, L., et al. (2003). Heparin derivatives as angiogenesis inhibitors. Current Pharmaceutical Design, 9, 553–566.
Lou, C. Y., Feng, Y. M., Qian, A. R., Li, Y., Tang, H., Shang, P., et al. (2004). Establishment and characterization of human hepatocellular carcinoma cell line FHCC-98. World Journal of Gastroenterology, 10, 1462–1465.
Yoshitomia, Y., Nakanishib, Ho., Kusanoc, Y., Munesuea, S., Oguric, K., Tatematsub, M., et al. (2004). Inhibition of experimental lung metastases of Lewis lung carcinoma cells by chemically modified heparin with reduced anticoagulant activity. Cancer Letters, 207, 165–174.
Acknowledgments
This research is supported by the National High Technology Research and Development Program of China (No. 2007AA021701). Special thanks go to Ms. Rachel Thompson for her careful reading and editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ling Li and Jun Qin are co-first authors.
Rights and permissions
About this article
Cite this article
Li, L., Qin, J., Feng, Q. et al. Heparin Promotes Suspension Adaptation Process of CHO–TS28 Cells by Eliminating Cell Aggregation. Mol Biotechnol 47, 9–17 (2011). https://doi.org/10.1007/s12033-010-9306-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-010-9306-1