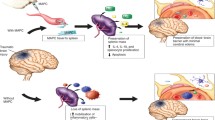
Abstract
Cell therapy plays an important role in multidisciplinary management of the two major forms of central nervous system (CNS) injury, traumatic brain injury and spinal cord injury, which are caused by external physical trauma. Cell therapy for CNS disorders involves the use of cells of neural or non-neural origin to replace, repair, or enhance the function of the damaged nervous system and is usually achieved by transplantation of the cells, which are isolated and may be modified, e.g., by genetic engineering, when it may be referred to as gene therapy. Because the adult brain cells have a limited capacity to migrate to and regenerate at sites of injury, the use of embryonic stem cells that can be differentiated into various cell types as well as the use of neural stem cells has been explored. Preclinical studies and clinical trials are reviewed. Advantages as well as limitations are discussed. Cell therapy is promising for the treatment of CNS injury because it targets multiple mechanisms in a sustained manner. It can provide repair and regeneration of damaged tissues as well as prolonged release of neuroprotective and other therapeutic substances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
This review will cover the role of cell therapy in the two major forms of central nervous system (CNS) injury—traumatic brain injury (TBI) and spinal cord injury (SCI)—which are caused by external physical trauma. Cell therapy is defined as the prevention or treatment of human disease by the administration of cells that have been selected, multiplied, and pharmacologically treated or altered ex vivo, i.e., outside the body. The scope of cell therapy can be broadened to include methods, pharmacological as well as non-pharmacological, in order to modify the function of intrinsic cells of the body for therapeutic purposes [1].
An important reason for interest in cell therapy for CNS injuries is that, in comparison to most other tissues and organs, adult CNS exhibits less ability to divide or generate new neural tissue for replacing the one that is lost by injury. The aim of cell therapy is to replace, repair, or enhance the function of damaged tissues or organs. The cells used can originate from the patient or from a donor or cell lines or from another species. Apart from secreting therapeutic substances, transplanted cells may also form connection with host tissues, e.g., formation of neuronal connections in case of the brain. Because endogenous progenitor cells make an effort to repair the CNS injury, cell therapy strategies also include enhancement of endogenous stem cell regeneration. Currently, most of the interest in cell therapy is focused on stem cells.
Cells Used for Therapy of CNS Disorders
Cell therapy for CNS disorders involves the use of cells of neural or non-neural origin to replace, repair or enhance the function of the damaged nervous system and is usually achieved by transplantation of the cells, which are isolated and may be modified, e.g., by genetic engineering. Tissue engineering in the nervous system is the science of designing, creating and realizing systems where neural cells are organized in a controlled manner, to perform appropriate diagnostic, palliative, and therapeutic tasks in the nervous system.
Non-neural cells used for CNS injury include autologous macrophages, activated T lymphocytes and stem cells. The proposed mechanism of action of implanted autologous macrophages is simulation of the events that occur naturally in spontaneously regenerating systems. Key factors in the recovery of injured tissues, but missing or deficient in the CNS, are the processes of recruitment and activation of immune cells. This is the basis of the proposal for the development of immune cell therapies in which the injured CNS is exogenously provided with an adequate number of appropriately activated immune cells such as T lymphocytes, controlled in such a way as to derive maximal benefit with minimal risk of disease. It is expected that these self-adjusting cells can communicate with the damaged tissue, monitor tissue needs, and control the dynamic course of CNS healing.
Adult brain cells have a limited capacity to migrate to and regenerate at sites of injury. Embryonic stem cells (ESCs) are able to differentiate in vitro into various cell types. Other stem cells such as hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), and umbilical cord blood stem cells can be differentiated into neuronal cells. Because spontaneous development of neuronal cells from ESCs is rather limited, specific protocols are required to increase the differentiation of neuronal cells. Transplantation of human neural stem cells (NSCs) may offer a method of circumventing these limitations, since these cells are both able to migrate and have the potential to become the cellular components of the nervous system. NSCs migrate through the parenchyma along non-stereotypical routes in a precisely directed manner across great distances to injury sites in the CNS, where they might engage niches harboring local transiently expressed reparative signals.
Cell Therapy of TBI
TBI has long been thought to evoke immediate and irreversible damage to the brain. This is followed by delayed and progressive pathobiological changes. Current treatment consists of surgical evacuation of hematomas and management of cerebral edema. A number of neuroprotective agents are being evaluated [2]. No therapeutic intervention has been found to be effective so far in repairing the damage following TBI. Experimental studies have explored cell therapy as treatment for TBI.
Preclinical Studies of Cell Therapy for TBI
Stem cell-based cellular replacement strategies have potential therapeutic role following TBI but the mechanism by which stem cells produce their effect, e.g., via integration into surviving neuronal circuits, local neurotrophic support, or modification of the local microenvironment to enhance endogenous regeneration and neuroprotection remains to be assessed further [3]. Transplantation of bone marrow-derived MSCs into the injured brain has potential therapeutic benefit. Although it has been suggested that differentiation of MSCs into cells of neural lineage may occur, this is unlikely to be a major factor in functional recovery after TBI, but other mechanisms that may play a role include neuroprotection, creation of a favorable environment for regeneration, expression of growth factors, or cytokines [4].
Cell Therapy for Repair of Blood–Brain Barrier Damage in TBI
Damage to blood–brain barrier (BBB) aggravates the pathology of TBI and passage of drugs across it is unreliable. Therefore, it is desirable to reduce increased permeability of BBB following TBI. Stem cells are known to migrate to areas of damage and are potential agents for repair of BBB.
NSCs are capable of inducing BBB properties by interactions with brain microvascular endothelial cells (BMECs) and have been used to devise a rudimentary BBB in the laboratory [5]. Those authors demonstrated that NSCs grown as neurospheres can coax BMECs to form a tighter, more dense barrier to small molecules that would otherwise diffuse through the blood vessel cells. This might provide clues about how to mend the barrier when it is damaged in TBI.
Cell/Gene Therapy for TBI
Neural stem cells have been retrovirally transduced to produce NGF and transplanted into the injured brain with marked improvement of cognitive and neuromotor function and rescue of hippocampal CA3 neurons during the acute posttraumatic period. The clinical implication of this is that NGF gene transfer can provide neuroprotection. Human Ntera-2 neurons genetically modified to express NGF significantly attenuate cognitive dysfunction following TBI in mice indicating that this is a practical and effective method for stable ex vivo gene delivery into the CNS [6].
Undifferentiated NT2 cells, when transduced in vitro with a lentiviral vector to release NGF, differentiate into NT2N neurons by exposure to retinoic acid. NGF gene therapy using transduced NT2 N neurons (as a source of delivery) may selectively improve cognitive function following TBI [7].
A review of the currently available information on preclinical studies reveals that there are several gene targets with therapeutic potentials and vectors that can be used to deliver the candidate genes [8]. In spite of obstacles in translating these techniques into effective gene therapy in humans, they provide new strategies for neuroprotection in TBI.
Clinical Trials of Autologous HSC Therapy for TBI
A unique clinical trial to gauge the safety and potential of treating children suffering TBI with HSCs derived from their own bone marrow is in progress [9]. This trial is the first to apply stem cells to treat TBI, but does not involve ESCs. Approved by the FDA, the trial is based on laboratory and animal research indicating that bone marrow-derived HSCs can migrate to an injured area of the brain, differentiate into new neurons and support cells, and induce brain repair.
Limitations of Stem Cell Therapy for Acute TBI
Although ESC transplantation is being investigated for the treatment of TBI, characteristic of massive loss of multiple cell types due to primary insult and secondary sequelae need to be considered. The local cerebral environment in the first 48 h after TBI is highly proinflammatory. The proinflammatory cytokines interleukin (IL)-1α, IL-1β, IL-6, and tumor necrosis factor-α are significantly elevated after controlled cortical impact in the injury as well as the surrounding region in experimental animals [10]. The local, acute proinflammatory response after TBI may serve as a therapeutic target of early cell therapy or, conversely, may create an unfavorable local environment, limiting the efficacy of early cell therapy.
In one study, green fluorescent protein (GFP)-transfected murine ESCs were implanted into the brain on the side of injury or cortex of the opposite hemisphere in male Sprague-Dawley rats 72 h after fluid-percussion injury [11]. After seven weeks, only a few GFP-positive cells were found, indicating an extensive loss of stem cells during this time period. The observed cell loss was mediated via phagocytosis of implanted cells by activated macrophages. Cerebral trauma, induced 3 days prior to implantation, had activated the inflammatory potential of otherwise immunologically privileged brain tissue. Subsequent cell implantation was accompanied by reactive astrogliosis, activation of microglia, as well as a massive invasion of macrophages into transplantation sites even when the grafts were placed into opposite healthy hemispheres. These results demonstrate a significant posttraumatic inflammatory response, which impairs survival and integration of implanted stem cells and has generally not been taken into account in designs of earlier transplantation studies.
Combination of Cell Therapy for TBI with other Approaches
Because of multiple pathways involved in the pathomechanism of secondary damage in TBI, no single approach may be adequate, and hence integration of several approaches would be required. Selection of these methods would vary according to severity and location of injury as well as other factors taken into consideration for personalizing therapy to attain optimal efficacy and safety in an individual patient. Cell therapy is expected to play an important role in this approach.
Improving the Microenvironments of Transplanted Cells in TBI
For repair of TBI, it is important to ensure that cells are transplanted into an environment that is favorable for extended survival and integration within the host tissue. Features such as three-dimensionality of the graft and adhesive support for donor cells facilitate integration. Extracellular matrix proteins such as fibronectin and laminin are involved in neural development and may mediate subsequent cell signaling events. Enhanced cell survival was demonstrated following transplantion of a NSC construct containing laminin-based scaffold into the traumatically injured mouse brain [12]. Furthermore, behavioral analyses showed that mice receiving NSCs within the laminin-based scaffold performed significantly better than untreated mice on a spatial learning task, indicating that functional recovery correlates positively with donor cell survival. These results suggest that appropriate extracellular matrix-based scaffolds can be exploited to improve cell transplantation therapy.
Nanomaterials and Stem Cell Therapy of TBI
The peptide nanofiber scaffold is an effective technology for tissue repair and restoration, and is a promising candidate in the treatment of TBI. This peptide nanofiber scaffold has several advantages over currently available polymer biomaterials. The network of nanofibers is similar in scale to the native extracellular matrix and thus provides an environment for cell growth, migration, and differentiation. This peptide disintegrates and is immunologically inert.
Nanoparticles are used as markers to follow the fate of cells transplanted in TBI. Magnetic resonance imaging (MRI) of grafted adult- as well as ESCs-labeled with iron oxide nanoparticles is a useful method for evaluating cellular migration toward a lesion site [13].
Cell Therapy for Spinal Cord Injury
The SCI can lead to serious neurological disability and the most serious form of it is paraplegia or quadriplegia. Local spinal cord lesions are often greatly enlarged by secondary damage, which is accompanied by additional massive cell death that involves neurons, microglia, and macroglia and is virtually complete at 12 h. Immediate care involves stabilization of the patient’s general condition by supportive measures. Surgery is carried out in some cases for removal of compressing lesions and stabilization of spinal fractures. A number of neuroprotective strategies are under investigation. There is no therapeutic measure available currently, which enhances functional recovery significantly. Experimental studies as well as clinical trials have explored the use of cell therapy for SCI.
Preclinical Studies of Cell Therapy for SCI
Autoimmune T cells against CNS myelin-associated peptide have a neuroprotective effect in experimental models, i.e., they reduce the spread of damage and promote recovery in injured rat spinal cord. Some research groups are trying to promote surviving axons to grow across the damaged area and reconnect with others, using combinations of different growth factors. Considerable advances have also been made in the last decade in devising and evaluating cell transplantation strategies for enhancing axon regeneration for patients with spinal cord injuries and other lesions. Thus, a number of studies have established that transplantation of glial cells can have beneficial consequences in experimental models of spinal cord trauma and demyelination.
NSCs present in the adult spinal cord can be expanded in vitro and improve recovery when transplanted to the injured spinal cord, demonstrating the presence of cells that can promote regeneration but that normally fail to do so efficiently. Use of genetic fate mapping has shown that close to all in vitro NSC potential in the adult spinal cord resides within the population of ependymal cells lining the central canal, which are recruited by SCI and produce not only scar-forming glial cells, but also, to a lesser degree, oligodendrocytes [14]. Modulating the fate of ependymal progeny after SCI may offer an alternative to cell transplantation for cell replacement therapies in SCI.
Fetal Neural Grafts for SCI
Studies of intraspinal transplantation in experimental animals reveal that solid and suspension grafts of fetal tissues from several areas of neuraxis survive after placement into the spinal cord. Moreover, these grafts mature, integrate with host’s CNS, enhance the growth of specific systems from the host, and enhance recovery of function. It is questionable whether fetal cell transplantation would become an important method for the treatment of human SCI. Ethical and procedural problems associated with procuring human fetal tissues may be formidable.
Olfactory-Ensheathing Cells for SCI
Olfactory-ensheathing cells (OECs) are found along the full length of the olfactory nerve, from the basal lamina of the epithelium to the olfactory bulb, crossing the peripheral nervous system–CNS junction. In vitro, these cells promote robust axonal growth, in part, through cell adhesion molecules and possibly by secretion of neurotrophic growth factors that support axonal elongation and extension. In animal models of SCI, transplantation of ensheathing cells supports axonal remyelination and extensive migration throughout the length of the spinal cord. Potential therapeutic superiority of olfactory glia over Schwann cells has been demonstrated by experiments in which the number of regenerating axons crossing a transection site was dramatically increased when olfactory-ensheathing glia were placed at the interface between a Schwann-cell-filled guidance tube and the damaged spinal cord.
The OECs, transplanted into the injured spinal cord of laboratory rats, have a remarkable capacity to integrate into damaged pathways, laying a ‘bridge’ over the gap in the nerve fibers caused by injury [15]. The cells can be obtained from tissue samples taken from the adult nasal lining by a technique that does no permanent damage, since the system, such as skin, contains adult stem cells and is in a state of continuous self renewal. If this technique can be transferred to humans, the patient can be his/her own cell donor. This will avoid the need to use hESCs, to find donor individuals, foreign stem cells, or to use powerful drugs with unknown side effects. Thus, the repair of the injured spinal cord is now a possibility although the findings of another study indicate that OEC transplants alone are not sufficient for neural repair and functional recovery after SCI [16]. In addition, OECs can induce abnormal axonal growth, making further studies necessary before considering their clinical use. A clinical trial of autologous transplantation of OECs into the spinal cord in six SCI patients with complete, thoracic paraplegia showed that this procedure is feasible and is safe up to 3 years of post-implantation; however, this conclusion should be considered preliminary because of the small number of trial patients [17].
Oligodendrocyte Precursor Cells for Treatment of SCI
Isolated rat adipose tissue-derived stromal cells (rATSCs) contain pluripotent cells that can be differentiated into a variety of cell lineages, including neural cells. Recent work has shown that ATSCs can make neurosphere-like clumps and differentiate into neuron-like cells expressing neuronal markers, but their therapeutic effect is unclear. One study has reported that intravenous infusion of oligodendrocyte precursor cells (OPCs) derived from rATSC autograft cells sources improve motor function in rat models of SCI [18]. Following intravenous injection, rATSC-OPC cells survived and migrated into the injured region of SCI very efficiently (30–35%), and migrated cells were partially differentiated into neurons and oligodendrocytes. Behavioral analysis revealed that the locomotor functions of OPC-autografted SCI rats were significantly restored. Efficient migration of intravenously engrafted rATSC-OPCs cells into SCI lesion suggests that SCI-induced chemotaxic factors facilitate migration of rATSC-OPCs. Engrafted rATSCs and SCI-induced chemotaxic factors play an important role in proliferation, migration, and differentiation of endogenous spinal cord-derived neural progenitor cells in the injured region. In transplantation paradigms, the interaction between engrafted rATSC-OPCs and endogeneous spinal cord-derived neuronal progenitor cells will be important in promoting healing resulting in coordinated induction of cell migration and differentiation.
Demyelination contributes to loss of function after SCI, and thus a potential therapeutic strategy involves replacing myelin-forming cells. Transplantation of hESC-derived OPCs into adult rat SCIs enhances remyelination and promotes improvement of motor function [19]. Transplanted OPCs survived, redistributed over short distances, and differentiated into oligodendrocytes. Animals that received OPCs 7 days after injury exhibited enhanced remyelination and substantially improved locomotor ability. In contrast, when OPCs were transplanted 10 months after injury, there was no enhanced remyelination or locomotor recovery. These studies document the feasibility of predifferentiating hESCs into functional OPCs and demonstrate their therapeutic potential at early time points after SCI.
Schwann Cell Transplants for SCI
To promote growth and remyelination, Schwann cells (the peripheral nervous system equivalent of oligodendrocytes) have been transplanted. Unfortunately, Schwann cells and oligodendrocytes erect an inhibitory barrier when they encounter each other. Grafting of Schwann cells modified to overexpress human nerve growth factor significantly supports extensive axonal growth in models of SCI.
Transplantation of Glial Cells for SCI
Following acute damage to the CNS by trauma or ischemia, the glial cells as well as the neurons die leading to a breakdown in the integrity of the glial environment and this limits regeneration. Glia-depleted areas of the CNS can be reconstituted by the introduction of cultured glial cells. When introduced into infarcted white matter in the spinal cord, progenitor-derived astrocytes fill the damaged area more effectively than tissue-culture astrocytes although their axons do not regenerate into the reconstituted areas. However, they promote revascularization and attenuate scarring which is an impediment to regeneration.
Transplantation of astrocytes derived from embryonic glial-restricted precursors (GRPs) has been shown to promote robust axon growth and restoration of locomotor function after acute transection injuries of the adult rat spinal cord. Predifferentiation of glial precursors into GDAs before transplantation into spinal cord injuries leads to significantly improved outcomes over precursor cell transplantation, providing both a novel strategy and a highly effective new cell type for repairing CNS injuries [20].
Embryonic Stem Cells for SCI
Several experimental studies in rats have investigated the implantation of ESCs for SCI. The oligodendrocytes myelinated both in vitro and in vivo in the spinal cord. Although recovery of some spinal cord function has been demonstrated, it is not yet clear exactly how cell transplantation works. The newly formed cells may secrete growth factors that induce regeneration of native oligodendrocytes. Ongoing studies are trying to increase the migration of cells to achieve a more uniform distribution at the site of injury.
Transplantation of MSCs for SCI
A study has demonstrated that genetically modified hMSC lines can survive in healthy rat spinal cord over at least 3 weeks by using adequate immune suppression and can serve as vehicles for transgene expression [21]. However, before genetically modified hMSC can potentially be used in a clinical setting to treat SCI, more research on standardization of hMSC culture and genetic modification needs to be done in order to prevent tumor formation and transgene silencing in vivo.
Transplantation of NSCs for SCI
Isolated, human NSCs grown as neurospheres (hCNS-SCns) survive, migrate, and express differentiation markers for neurons and oligodendrocytes after long-term engraftment in spinal cord-injured NOD-SCID mice [22]. Remyelination occurred and electron microscopy demonstrated synapse formation between hCNS-SCns and mouse host neurons. Locomotor recovery following engraftment was abolished by selective ablation of engrafted cells by diphtheria toxin. Glial fibrillary acidic protein-positive astrocytic differentiation was rare, and hCNS-SCns did not appear to contribute to the scar. These data suggest that hCNS-SCns may possess therapeutic potential for CNS injury and disease.
However, additional animal studies are necessary both to establish the mechanism of recovery and to evaluate the potential of these cells for possible therapeutic use. Transplantation of human NSCs into the lumbar cord of normal or injured adult nude rats have been shown to undergo large-scale differentiation into neurons that formed axons and synapses and established extensive contacts with host motor neurons [23]. Spinal cord microenvironment appeared to influence the fate of NSCs, with centrally located cells taking on a predominant neuronal path, and cells located under the pia membrane persisting as NSCs or presenting with astrocytic phenotypes. Slightly fewer than one-tenth of grafted neurons differentiated into oligodendrocytes. The presence of lesions increased the frequency of astrocytic phenotypes in the white matter. In view of recent similar findings from other studies, the extent of neuronal differentiation observed in this study disputes the belief that the spinal cord is constitutively unfavorable to neuronal repair. Restoration of spinal cord circuitry in traumatic and degenerative diseases may be more realistic than previously thought, although major challenges remain, especially with respect to the establishment of neuromuscular connections.
The NSC grafting in rats with SCI improves motor recovery. Although differentiation of engrafted NSCs is restricted exclusively toward the astrocytic phenotype, the NSC-derived astrocytes show features that are typical of the early phase after SCI when the glial scar still allows regeneration of axons [24]. Modifying the glial scar with NSCs might enhance axonal regeneration in the injured area. The use of genetically engineered NSCs that express trophic factors appears to be an attractive tool in SCI research.
Combined Approaches for Regeneration in SCI
In a combinatorial approach, a preconditioning stimulus to sensory neuronal cell bodies was delivered by injecting cAMP into the lumbar dorsal root ganglion, and a postinjury stimulus to the injured axon was administered by injecting neurotrophin-3 (NT-3) within and beyond a cervical spinal cord lesion site grafted with autologous bone marrow stromal cells [25]. One to 3 months later, long-projecting dorsal-column sensory axons regenerated into and beyond the lesion. Regeneration beyond the lesion did not occur after treatment with cAMP or NT-3 alone. Thus, clear axonal regeneration beyond SCI sites can be achieved by combinatorial approaches that stimulate both the neuronal soma and the axon, representing a major advance in strategies to enhance spinal cord repair.
Cell Therapy Combined with Neurogenein-2
Although the transplantation of stem cells improves recovery from SCI in rats, a painful condition can also develop, which can be prevented if the stem cells are supplemented with neurogenin-2, a gene that controls their maturing process [26]. The aggravated sensitivity to pain was thought to be the result of the fact that many stem cells developed into astrocytes that encourage the growth of pain axons in the spinal cord by secreting substances that stimulate neuronal development. The presence of neurogenin-2, a transcription factor that regulates the activity of other genes during the stem cell maturing process, inhibited the development of astrocytes and encouraged the formation of oligodendrocytes. The small number of astrocytes that developed from the neurogenin-2-bearing stem cells corresponded to the lack of growth of pain axons. The greater number of oligodendrocytes that were produced by the neurogenin-2-bearing stem cells also corresponded to a greater volume of white substance, i.e., myelin-coated nerve fibers, in the damaged area. The use of functional Magnetic Resonance Imaging (fMRI) demonstrated the return of sensory function following spinal injury. fMRI can be used to compare results from animal and human studies if and when new therapies for the treatment of SCI can be tested on patients.
Stem Cell Therapy Combined with T Cell-based Vaccination
T cell-based vaccination of mice with a myelin-derived peptide, when combined with transplantation of adult neural stem/progenitor cells (aNPCs) into the CSF, synergistically promotes functional recovery after SCI [27]. The synergistic effect is correlated with modulation of the nature and intensity of the local T cell and microglial response, expression of BDNF and noggin protein, and appearance of newly formed neurons from endogenous precursor-cell pools. These results substantiate the contention that the local immune response plays a crucial role in recruitment of aNPCs to the lesion site, and suggest that similar immunological manipulations might also serve as a therapeutic means for controlled migration of stem/progenitor cells to other acutely injured CNS sites.
Combination of Stem Cells with Scaffolds for Repair of SCI
To better direct repair following SCI, scientists have designed an implant modeled after the intact spinal cord consisting of a multicomponent polymer scaffold seeded with neural stem cells [28]. Implantation of the scaffold-neural stem cells unit into an adult rat hemisection model of SCI promoted long-term improvement in function (persistent for 1 year in some animals) relative to a lesion-control group. At 70 days postinjury, animals implanted with scaffold-plus-cells exhibited coordinated, weight-bearing hindlimb stepping. Histology and immunocytochemical analysis suggested that this recovery might be attributable partly to a reduction in tissue loss from secondary injury processes as well as in diminished glial scarring. Tract tracing demonstrated corticospinal tract fibers passing through the injury epicenter to the caudal cord, a phenomenon not present in untreated groups. Together with evidence of enhanced local GAP-43 expression not seen in controls, these findings suggest a possible regeneration component. These results may suggest a new approach to SCI and, more broadly, may serve as a prototype for multidisciplinary strategies against complex neurological problems.
Combined Cell/Gene Therapy for SCI
Demyelination contributes to the neurological deficits after contusive SCI. Therefore, remyelination may be an important strategy to facilitate repair after SCI. Combining partially differentiated stem cells with gene therapy can promote the growth of new “insulation” around nerve fibers in the damaged spinal cords of rats [29]. Glial-restricted precursor cells (GRPs), which differentiate into both oligodendrocytes and astrocytes, formed normal-appearing central myelin around axons of cultured neurons, and had enhanced proliferation and survival in the presence of neurotrophin 3 (NT3) and brain-derived neurotrophin factor (BDNF). GRPs, transfected with retroviruses carrying D15A gene (expressing both BDNF and NT3), were transplanted then into the damaged spinal cord at 9 days after injury. Six weeks after transplantation, the grafted GRPs differentiated into mature oligodendrocytes expressing both myelin basic proteins, and there was recovery of motor function. Therefore, combined treatment with neurotrophins and GRP grafts can facilitate functional recovery after traumatic SCI and may prove to be a useful therapeutic strategy to repair the injured spinal cord. The researchers are now investigating ways to improve this type of therapy with additional genetic modifications to the transplanted cells, and they plan to test similar techniques that start with undifferentiated ESCs instead of glial-restricted precursor cells. ESCs would be better for human studies than glial-restricted precursors because ESCs can be more readily obtained.
Delivery of Cells in SCI
Neural precursor cells (NPCs) are promising grafts for treatment of traumatic CNS injury and neurodegenerative disorders because of their potential to differentiate into neurons and glial cells. When designing clinical protocols for NPC transplantation, it is important to develop alternatives to direct parenchymal injection, particularly at the injury site. Since it is minimally invasive, intrathecal delivery of NPCs at lumbar spinal cord by lumbar puncture represents an important and clinically applicable strategy. Similar to direct parenchymal injections, transplanted NRP and glial-restricted precursors cells survive at the injury cavity for at least 5 weeks post-engraftment, migrate into intact spinal cord along white matter tracts and differentiate into all the three mature CNS cell types: neurons, astrocytes, and oligodendrocytes [30]. Furthermore, very few graft-derived cells localize to areas outside the injury site, including intact spinal cord and brain. These results demonstrate the potential of delivering lineage-restricted NPCs using the minimally invasive lumbar puncture method for the treatment of SCI.
Intrathecal Injection of Cells Labeled with Magnetic Nanoparticles
Autologous bone marrow CD34+ cells labeled with magnetic nanoparticles have been delivered into the spinal cord via lumbar puncture in a study on patients with chronic SCI [31]. One group received their own labeled-CD34+ cells whereas the others received an injection containing only magnetic nanoparticles without stem cells to serve as controls. CD34+ cells were labeled with magnetic nanoparticles coated with a monoclonal antibody specific for the CD34 cell membrane antigen. MRI showed that magnetically labeled CD34+ cells were visible at the lesion site as hypointense signals following transplantation, but these signals were not visible in any patient in the control group. This study shows that autologous bone marrow CD34+ cells labeled with magnetic nanoparticles, when delivered intrathecally migrate into the site of injury in patients with chronic SCI and can be tracked by MRI. It also supports the feasibility of treatment of SCI with intrathecal cell therapy.
Intravenous Injection of Stem Cells for Spinal Cord Repair
Transplantation of NPCs has been reported recently to promote regeneration of the injured spinal cord. In the majority of these reports, cell transplantation was performed by local injection with a needle. However, direct injection might be too invasive for clinical use; therefore, a new method of delivering NPCs for the treatment of SCI has been investigated [32]. In this study, NPCs were obtained from E15 fetal hippocampus of transgenic rats expressing green fluorescent protein and 100,000 cells were transplanted intravenously into each animal 24 h after contusion injury. The injected NPCs migrated to the lesion site widely and demonstrated nestin at an early phase after transplantation. These NPCs differentiated into neurons, astrocytes, and oligodendrocytes, and survived at least for 56 days. These results indicated that intravenously injected neural stem cells migrated into the spinal cord lesion while preserving their potential as NPCs, and that this procedure is a potential method of delivering cells into the lesion for the treatment of SCI.
Clinical Trials of Cell Therapy for SCI
There are several anectodal reports of use of stem cells to repair the spinal cord in paraplegic patients. The procedure has been performed in China, Russia, South Korea, and in Portugal. Variable results have been reported with some claims of recovery. Various clinical trials of cell therapy for SCI are listed in Table 1.
Autologous Bone Marrow Cell Transplantation for SCI
Based on the findings that transplanted bone marrow (BM) cells improve neurologic disease in CNS injury models by generating neural cells or myelin-producing cells, a study has evaluated nine patients with chronic complete SCI who were treated with autologous BM-derived hematopoietic progenitor stem cell transplantation without any serious complications [33]. Autologous hematopoietic progenitor stem cell avoids the problems associated with immunologic rejection and GvHD, which are frequently caused by allografts. Another advantage of this type of cell therapy is that it is not associated with carcinogenesis, which can occur with ESC therapy. Results of this study show that autologous BM stem cell therapy is effective and safe for the treatment of chronic SCI.
The therapeutic effects of autologous bone marrow cell transplantation (BMT) in conjunction with the administration of granulocyte macrophage-colony stimulating factor (GM-CSF) have been investigated in six complete SCI patients [34]. BMT in the injury site and subcutaneous GM-CSF administration were performed on five patients. One patient was treated with GM-CSF only. The follow-up periods were from 6 to 18 months. Sensory improvements were noted over a period from 3 weeks to 7 months postoperatively whereas significant motor improvements were noted 3–7 months postoperatively. Four patients showed neurologic improvements in their American Spinal Injury Association Impairment Scale (AIS) grades (from A to C). One patient improved to AIS grade B from A and the last patient remained in AIS grade A. No immediate worsening of neurologic symptoms was found. Follow-up study with MRI 4–6 months after injury showed slight enhancement within the zone of BMT. No syrinx formation had occurred in the treated patients. The authors concluded that BMT and GM-CSF administration represent a safe protocol to efficiently manage SCI patients, especially those with acute complete injury. To demonstrate the full therapeutic value of this protocol, long-term and more comprehensive controlled clinical trials are required.
Clinical Trials of hESCs for SCI
On January 22, 2009, the FDA approved the first clinical trial using hESCs derived from cell lines at NIH (previously forbidden for human use) for the treatment of SCI. The trial, planned for the summer of 2009, will be conducted by Geron Corporation. A special device will be used to inject hESCs into the spinal cords of patients with complete paraplegia due to SCI to determine whether procedure is safe and also whether there will be any signs of recovery of function.
Concluding Remarks on the Cell Therapy of CNS Injury
Cell therapy is promising for the treatment of CNS injury because it targets multiple mechanisms in a sustained manner. It can provide repair and regeneration of damaged tissues as well as prolonged release of neuroprotective and other therapeutic substances. Microenvironments of cell transplants can be improved by incorporation of substances to enhance cell survival. Transplantation of ESCs along with nanofiber scaffolds may emerge as an important component of multidisciplinary approach toward management of severe TBI along with use of neuroprotective therapies. Although several types of cells have been investigated for this purpose, there is increasing use of ESCs in experimental studies; however, usefulness of this procedure remains to be proven by human clinical trials.
Cell therapy alone may not suffice and may need to be combined with other methods. To improve the efficacy of cell therapy for CNS injury, one has to consider and combine four main approaches: (i) tissue or cell transplantation; (ii) neurotrophic factors; (iii) blocking of inhibitors of neural regeneration; and (iv) modulation of inflammatory response following SCI [35].
Overall TBI, which is more frequent than SCI and results in multiple serious disabilities, is a top priority for improvement in management in the United States, and some of the projects involve cell therapy. However, SCI with obvious physical disability such as paraplegia is currently receiving more research support than TBI for projects involving cell therapy and several clinical trials are in progress.
References
Jain, K. K. (2009). Cell therapy: Technologies, markets & companies. Basel, Switzerland: Jain PharmaBiotech Publications.
Jain, K. K. (2008). Neuroprotection in traumatic brain injury. Drug Discovery Today, 13, 1082–1089. doi:10.1016/j.drudis.2008.09.006.
Maegele, M., & Schaefer, U. (2008). Stem cell-based cellular replacement strategies following traumatic brain injury (TBI). Minimally Invasive Therapy and Allied Technologies, 17, 119–131. doi:10.1080/13645700801970087.
Parr, A. M., Tator, C. H., & Keating, A. (2007). Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplantation, 40, 609–619. doi:10.1038/sj.bmt.1705757.
Weidenfeller, C., Svendsen, C. N., & Shusta, E. V. (2007). Differentiating embryonic neural progenitor cells induce blood–brain barrier properties. Journal of Neurochemistry, 101, 555–565. doi:10.1111/j.1471-4159.2006.04394.x.
Watson, D. J., Longhi, L., Lee, E. B., et al. (2003). Genetically modified NT2 N human neuronal cells mediate long-term gene expression as CNS grafts in vivo and improve functional cognitive outcome following experimental traumatic brain injury. Journal of Neuropathology and Experimental Neurology, 62, 368–380.
Longhi, L., Watson, D. J., Saatman, K. E., et al. (2004). Ex vivo gene therapy using targeted engraftment of NGF expressing human NT2 N neurons attenuates cognitive deficits following traumatic brain injury in mice. Journal of Neurotrauma, 21, 1723–1736.
Shen, F., Wen, L., Yang, X., & Liu, W. (2007). The potential application of gene therapy in the treatment of traumatic brain injury. Neurosurgical Review, 30, 291–298. doi:10.1007/s10143-007-0094-4.
Harting, M. T., Baumgartner, J. E., Worth, L. L., et al. (2008). Cell therapies for traumatic brain injury. Neurosurgical Focus, 24, E18. doi:10.3171/FOC/2008/24/3-4/E17.
Harting, M. T., Jimenez, F., Adams, S. D., et al. (2008). Acute, regional inflammatory response after traumatic brain injury: Implications for cellular therapy. Surgery, 144, 803–813. doi:10.1016/j.surg.2008.05.017.
Molcanyi, M., Riess, P., Bentz, K., et al. (2007). Trauma-associated inflammatory response impairs embryonic stem cell survival and integration after implantation into injured rat brain. Journal of Neurotrauma, 24, 625–637. doi:10.1089/neu.2006.0180.
Tate, C. C., Shear, D. A., Tate, M. C., et al. (2009). Laminin and fibronectin scaffolds enhance neural stem cell transplantation into the injured brain. Journal of Tissue Engineering and Regenerative Medicine. doi:10.1002/term.154.
Sykova, E., & Jendelova, P. (2007). In vivo tracking of stem cells in brain and spinal cord injury. Progress in Brain Research, 161C, 367–383. doi:10.1016/S0079-6123(06)61026-1.
Meletis, K., Barnabé-Heider, F., Carlén, M., et al. (2008). Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biology, 6(7), e182. doi:10.1371/journal.pbio.0060182.
Li, Y., Carlstedt, T., Berthold, C. H., & Raisman, G. (2004). Interaction of transplanted olfactory-ensheathing cells and host astrocytic processes provides a bridge for axons to regenerate across the dorsal root entry zone. Experimental Neurology, 188, 300–308. doi:10.1016/j.expneurol.2004.04.021.
Collazos-Castro, J., Muneton-Gomez, V., & Nieto-Sampedro, M. (2005). Olfactory glia transplantation into cervical spinal cord contusion injuries. Journal of Neurosurgery Spine, 3, 308–317.
Mackay-Sim, A., Féron, F., Cochrane, J., et al. (2008). Autologous olfactory ensheathing cell transplantation in human paraplegia: A 3-year clinical trial. Brain, 131, 2376–2386. doi:10.1093/brain/awn173.
Kang, S. K., Shin, M. J., & Jung, J. S. (2006). Autologous adipose tissue-derived stromal cells for treatment of spinal cord injury. Stem Cells and Development, 15, 583–594. doi:10.1089/scd.2006.15.583.
Keirstead, H. S., Nistor, G., Berna, G., et al. (2005). Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. The Journal of Neuroscience, 25, 4694–4705. doi:10.1523/JNEUROSCI.0311-05.2005.
Davies, J. E., Huang, C., Proschel, C., et al. (2006). Astrocytes derived from glial-restricted precursors promote spinal cord repair. Journal of Biology (Online), 5, 7. doi:10.1186/jbiol35.
Ronsyn, M. W., Daans, J., Spaepen, G., et al. (2007). Plasmid-based genetic modification of human bone marrow-derived stromal cells: Analysis of cell survival and transgene expression after transplantation in rat spinal cord. BMC Biotechnology, 7, 90. doi:10.1186/1472-6750-7-90.
Cummings, B. J., Uchida, N., Tamaki, S. J., et al. (2005). Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proceedings of the National Academy of Sciences of the United States of America, 102, 14069–14074. doi:10.1073/pnas.0507063102.
Yan, J., Xu, L., Welsh, A. M., et al. (2007). Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Medicine, 4, e39. doi:10.1371/journal.pmed.0040039.
Pallini, R., Vitiani, L., Bez, A., et al. (2005). Homologous transplantation of neural stem cells to the injured spinal cord of mice. Neurosurgery, 57, 1014–1025. doi:10.1227/01.NEU.0000180058.58372.4c.
Lu, P., Yang, H., Jones, L. L., et al. (2004). Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. The Journal of Neuroscience, 24, 6402–6409. doi:10.1523/JNEUROSCI.1492-04.2004.
Hofstetter, C., Holmstrom, N., Lilja, J., et al. (2005). Allodynia limits the usefulness of intraspinal neural stem cell grafts and directed differentiation improves outcome. Nature Neuroscience, 8, 346–353. doi:10.1038/nn1405.
Ziv, Y., Avidan, H., Pluchino, S., et al. (2006). Synergy between immune cells and adult neural stem/progenitor cells promotes functional recovery from spinal cord injury. Proceedings of the National Academy of Sciences of the United States of America, 103, 13174–13179. doi:10.1073/pnas.0603747103.
Teng, Y. D., Lavik, E. B., Qu, X., et al. (2002). Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proceedings of the National Academy of Sciences of the United States of America, 99, 3024–3029. doi:10.1073/pnas.052678899.
Cao, Q., Xu, X. M., Devries, W. H., et al. (2005). Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. The Journal of Neuroscience, 25, 6947–6957. doi:10.1523/JNEUROSCI.1065-05.2005.
Lepore, A. C., Bakshi, A., Swanger, S. A., et al. (2005). Neural precursor cells can be delivered into the injured cervical spinal cord by intrathecal injection at the lumbar cord. Brain Research, 1045, 206–216.
Callera, F., & de Melo, C. (2007). Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells’ migration into the injured site. Stem Cells and Development, 16, 461–466. doi:10.1089/scd.2007.0083.
Fujiwara, Y., Tanaka, N., Ishida, O., et al. (2004). Intravenously injected neural progenitor cells of transgenic rats can migrate to the injured spinal cord and differentiate into neurons, astrocytes and oligodendrocytes. Neuroscience Letters, 366, 287–291. doi:10.1016/j.neulet.2004.05.080.
Deda, H., Inci, M., Kurekci, A., et al. (2008). Treatment of chronic spinal cord injured patients with autologous bone marrow-derived hematopoietic stem cell transplantation: 1-year follow-up. Cytotherapy, 10, 565–574. doi:10.1080/14653240802241797.
Park, H. C., Shim, Y. S., Ha, Y., et al. (2005). Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Engineering, 11, 913–922. doi:10.1089/ten.2005.11.913.
Ronsyn, M. W., Berneman, Z. N., Van Tendeloo, V. F., et al. (2008). Can cell therapy heal a spinal cord injury? Spinal Cord, 46, 532–539. doi:10.1038/sc.2008.13.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jain, K.K. Cell Therapy for CNS Trauma. Mol Biotechnol 42, 367–376 (2009). https://doi.org/10.1007/s12033-009-9166-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-009-9166-8