Abstract
Colletotrichum gloeosporioides is the common causal agent of anthracnose in papaya (Carica papaya L.) fruits, and infection by this fungal pathogen results in severe post-harvest losses. In the Yucatán peninsula (Mexico) a different Colletotrichum species was isolated from papaya fruits with atypical anthracnose lesions. The DNAs from a variety of Colletotrichum isolates producing typical and atypical lesions, respectively, were amplified by PCR with C.gloeosporioides-specific primers. All isolates from typical anthracnose lesions yielded a 450 bp PCR product, but DNAs from isolates with atypical lesions failed to produce an amplification product. For further characterization, the rDNA 5.8S-ITS region was amplified by PCR and processed for sequencing and RFLP analysis, respectively, to verify the identity of the papaya anthracnose pathogens. The results revealed unequivocally the existence of two Colletotrichum species causing anthracnose lesions on papaya fruits: C. gloeosporioides and C. capsici. PCR-RFLP using the restriction endonuclease MspI reliably reproduced restriction patterns specific for C. capsici or C. gloeosporioides. The generation of RFLP patterns by MspI (or AluI or RsaI) is a rapid, accurate, and unequivocal method for the detection and differentiation of these two Colletotrichum species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colletotrichum is the most important and most common fungal genus causing anthracnose in temperate, subtropical, and tropical areas, with an extremely wide host range including vegetable, forage crops, fruit trees, and ornamentals [1]. Numerous cases have been reported in which several Colletotrichum species or biotypes are associated with a single host [2–5], making their identification by morphological and physiological methods more difficult. The use of molecular marker techniques has improved the accuracy and speed of identification and classification of phytopathogenic fungi [6, 7]. Among these molecular techniques, DNA fragment analysis [e.g. randomly amplified polymorphic DNA (RAPD), and arbitrarily primed (AP)-PCR] has been extensively used to investigate relationships among isolates of many fungal genera including Colletotrichum spp [8, 9]. Similarly, nucleotide sequence information for the 5.8S gene and the internal transcribed spacer [ITS] region of ribosomal DNA (rDNA) has been used to design Colletotrichum species-specific primers for diagnostic purposes and for phylogenetic analysis [5, 10].
Anthracnose causes significant economic losses in papaya (Carica papaya L.) fruits, and Colletotrichum gloeosporioides is the primary causal agent of this disease [11, 12] which is characterized by the typical lesions shown in Fig. 1b. In addition, however, in papaya orchards in the Yucatan peninsula of Mexico, atypical anthracnose lesions were observed. Therefore, the objective of this study was to use PCR-based molecular tools for the rapid and reliable identification and characterization of the pathogen(s) involved in atypical lesion development.
Materials and Methods
Fungal Isolates
The Colletotrichum isolates were taken at different locations in the state of Yucatan and obtained from naturally infected papaya fruits showing atypical or typical anthracnose lesions (Table 1). The fungi were isolated and cultured on potato dextrose agar (PDA) at 28°C. For DNA extraction, 150 ml of nutrient broth was inoculated with two plugs of 5-mm diameter for each isolate, and cultures were incubated in an orbital shaker at 100 rpm at 28°C. The mycelial mass of each isolate was harvested with a glass hook and deposited on conical corning sterile tubes.
Isolation of DNA
Total genomic DNA was extracted according to a method developed in the GeMBio laboratory and this was used in all tests performed [13].
Diagnostic PCR with Species-Specific Primers
The primer CgInt (5′-GGCCTCCCGCCTCCGGGCGG-3′) specific for C. gloeosporioides [10, 14] was used in conjunction with the conserved primer ITS4 [10, 15] for rDNA amplification. Amplification reactions without DNA template were used as negative controls as well as the strain Cgb1 (NCBI accession no. EU056738) of Colletotrichum capsici. The PCR reaction (25 μl final volume) contained 25 ng of DNA, 1× PCR buffer (10×: 200 mM Tris-HCl, 500 mM KCl, pH 8.4; Invitrogen), 0.20 mM of each dNTP (Invitrogen), 1.5 mM MgCl2, 1 μM primers and 1U Taq polymerase (Invitrogen). DNA amplification was performed in a GeneAmp 9700 DNA Thermal Cycler (Perkin-Elmer), and consisted of an initial denaturing step at 95°C for 5 min, followed by 25 cycles of 30 s at 94°C, 2 min at 62°C, and 2 min at 72°C, and a final extension step of 5 min at 72°C [10]. PCR products were visualized by electrophoresis in 1.5% (wt/vol) agarose gels run 1× Tris-Borate-EDTA [TBE] buffer and stained with ethidium bromide.
PCR Amplification and Sequencing of 5.8S-ITS of rDNA
The Colletotrichum isolates were further characterized by nucleotide sequence analysis. For this, the 5.8S-ITS regions were amplified with the universal primers ITS1 and ITS4 [15], and the strain Cgb1 of C. capsici was used as positive control. PCR reactions were performed in reaction volumes of 50 μl containing 25 ng of genomic DNA, 1× PCR buffer (Invitrogen), 0.20 mM of each dNTP (Invitrogen), 1.5 mM MgCl2, 1 μM primers, and 1U Taq polymerase (Invitrogen). DNA amplification was performed in a GeneAmp 9700 DNA Thermal Cycler (Perkin-Elmer), and the program consisted of an initial denaturing step at 94°C for 1 min, followed by 30 cycles of 60 s at 94°C, 2 min at 58°C, and 60 s at 72°C; and a final extension step of 5 min at 72°C [16]. PCR products (10 μl aliquots) were separated by electrophoresis in 1.5% (wt/vol) agarose gels and visualized by ethidium bromide staining.
DNA sequencing was performed with two primers (ITS1 and ITS4) in both directions to ensure that there was no misreading. PCR products were purified and sequenced by Macrogen Inc, Korea. Alignment and edition were carried out with the BioEdit Program v 7.0.5 [17] and visually corrected. Sequences were then compared against those available in the GenBank database.
PCR-RFLP Analysis of rDNA
The 5.8S-ITS amplification products (10 μl aliquots) of 12 selected Colletotrichum strains (including the Cbg1 control strain) were digested with the restriction enzymes AluI, HaeIII, Hind III, MseI, MspI, RsaI, and TaqI, respectively, in a volume of 20 μl. Digestion products were separated on a 2% agarose gel (NuSieves 3:1) using 0.5× TBE buffer, and stained with ethidium bromide. Digestion patterns were visualized under a UV transilluminator and images were taken with a UVP Biolmaging Systems.
Results
Thirty-four Colletotrichum isolates were obtained from papaya fruits with atypical and typical anthracnose lesions, respectively (Fig. 1). Typical lesions produced by C. gloeosporioides are round, water soaked, and sunken spots with pinkish-orange areas are formed by the conidial masses, that cover the lesion center and sometimes produce a concentric rings pattern (Fig. 1b). In the case of atypical lesions, the main difference with typical ones is that the lesion areas formed are totally covered by brownish-black conidial masses (Fig. 1c).
PCR Amplification and Sequencing of 5.8S-ITS of rDNA
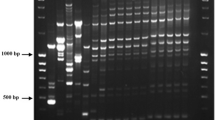
In first PCR tests with the C. gloeosporioides-specific primer CgInt used in conjunction with the conserved primer ITS4, eight isolates (all from typical anthracnose lesions) yielded the expected 450-bp product, while PCR products were not obtained for the other isolates from atypical lesions (Fig. 2). The DNAs of fungal isolates with typical and atypical anthracnose lesions were then used in PCR with the general primers ITS1 and ITS4 for the amplification of the rDNA region comprising the two non-coding internal transcribed spacers ITS1 and ITS2 and the 5.8S rRNA gene. All isolates amplified a PCR product of approximately 600 bp. Sequences were determined of each isolate, and blast analyses were carried out for eight isolates, previously identified with species-specific primers as C. gloeosporioides. The results showed a 100% homology with DNA sequences from other C. gloeosporioides strains deposited in the GenBank, while the blast analysis of the remaining isolates (atypical symptoms) produced a 99% homology with DNA sequences from C. capsici strains registered in the same database (data not shown). Some of these DNA sequences were registered in NCBI GenBank database.
Amplification products obtained in the species-specific PCR test (a selection of isolates is shown). All test isolates were analyzed using primer specific for C. gloeosporioides. M: molecular marker (100 bp DNA ladder), lane 1: Cgb1 (accession no. EU056738), lane 2: gb2, lane 3: gb9, lane 4: gb12, lane 5: gb15, lane 6:gb16, lane 7: gb21, lane 8: gb22, lane 9: gb32, lane10: gb23, lane 11: gb30, lane12: gb34, lane 13: negative control
PCR-RFLP Analysis of rDNA
The PCR amplification products were then processed for RFLP analysis and digested with seven different endonucleases each (Table 2). With the exception of HindIII and MseI that did not cut the DNA, the digestions resulted in clear and reproducible restriction patterns as depicted in Fig. 3. The enzyme AluI (Fig. 3a) produced fragments around 190 and 380 bp specific for all C. gloeosporioides isolates, and fragments around 200 and 380 bp specific for all C. capsici. While HaeIII digestion generated specific bands around 140 and 280 bp for C. gloeosporioides and 145 and 300 bp for C. capsici (Fig. 3b). In the case of MspI specific bands at 130 and 300 bp for C. gloeosporioides and 135 and 450 bp for C. capsici were visualized (Fig. 3c). RsaI has no recognition site for this region of the C. capsici rDNA, while specific bands around 180 and 380 bp were obtained for C. gloeosporioides (Fig. 3d). In the cases of TaqI and HaeIII only slight differences among the banding patterns for C. capsici and C. gloeosporioides were discernible.
PCR-RFLP profiles of rDNA obtained with restriction enzymes: AluI (a), HaeIII (b), MspI (c), and RsaI (d). M: molecular marker (100 bp DNA ladder for a, c, and d; 50 bp DNA ladder for b), lane 1: Cgb1 (accession no. EU056738), lane 2: gb2, lane 3: gb6, lane 4: gb9, lane 5: gb12, lane 6: gb15, lane 7: gb16, lane 8: gb22, lane 9: gb32, lane 10: gb21, lane11: gb23, lane 12: gb30
Discussion
The main aim of this research was to use PCR-based methods to identify rapidly and accurately the species of Colletotrichum responsible for typical and atypical anthracnose lesions on fruits in papaya fields in Yucatán. This article presents the first report of molecular detection of C. capsici affecting C. papaya fruits in Yucatan, Mexico.
For several years molecular techniques have been widely used to differentiate the Colletotrichum genus at species or race level [5, 14, 18, 19]. In this study and based on PCR amplification with species-specific primers, all isolates from typical anthracnose lesions were identified as C. gloeosporioides. The size of the amplification product (450 bp) confirmed other reports for the same species [10], and subsequent rDNA sequence analysis corroborated these results.
PCR with species-specific primers as well as PCR amplification of the 5.8S-ITS region of DNA, subsequent sequence analysis and PCR-RFLP analysis of the rDNA product revealed unequivocally the existence of two species causing anthracnose lesions on papaya fruits: C. gloeosporioides and C. capsici. The species C. capsici is found throughout the world causing anthracnose in Habanero pepper (Capsicum chinense Jacquin) and pepper (Capsicum annuum L.) [20–22]. C. capsici has also been reported as a pathogen for several other hosts such as cowpea (Vigna unguiculata), bean (Phaseolus vulgaris), and betle vine (Piper betle) [23]. In the Yucatan peninsula it is common to find papaya orchards side by side to Habanero pepper fields. This together with the broad range of host plants that are infected by Colletotrichum species [1] could account for the infection of papaya by C. capsici. RFLP patterns generated from the rDNA region spanning the ITS and the 5.8S, made it possible to differentiate between C. gloeosporioides and C. capsici, since each one has a characteristic banding pattern. The ITS region has already shown before to be useful for Colletotrichum species identification [5, 18, 24]. The C. gloeosporioides restriction patterns obtained with AluI, HaeIII, MspI, and RsaI were similar to those reported previously for the same species [25].
It is interesting to note that, with the use of one endonuclease MspI, a reliable and reproducible distinction between C. capsici and C. gloeosporioides was obtained. Sequence data undoubtedly offer more precise information than the restriction analysis, but the procedure is time-consuming and cost-intensive. PCR-RFLP in contrast with any of the three enzymes AluI, MspI, or RsaI achieves a rapid and unequivocal identification of Colletotrichum isolates derived from papaya fruits and their assignation to C. capsici and C. gloeosporioides species. This technique will thus facilitate routine work in phytopathogen diagnostics. In any laboratory providing the services of phytopathogen detection, it is essential to have the support of these kind of techniques which will facilitate the correct and rapid identification of the agents causing the diseases, and to be able to provide the crop producers with this response so that they can take the necessary steps towards the control of these diseases and reduce crop yield losses.
References
Freeman, S. (2000). Genetic diversity and host specificity of Colletotrichum species on various fruits. In D. Prousky, S. Freeman, & M. B. Dickman (Eds.), Colletotrichum. Host specificity, pathology and host-pathogen interaction. (pp. 131–144). St Paul Minnesota USA: APS Press.
Smith, B. J., & Black, L. L. (1990). Morphological, cultural and pathogenic variation among Colletotrichum species isolated from strawberry. Plant Disease, 74, 69–76. doi:10.1094/PD-74-0069.
Brown, A. E., Sreenivasaprasad, S., & Timmer, L. W. (1996). Molecular characterization of slow-growing-orange and key lime anthracnose strains of Colletotrichum from citrus as C. acutatum. Phytopathology, 86, 523–527. doi:10.1094/Phyto-86-523.
Freeman, S., Katan, T., & Shabi, E. (1998). Characterization of Colletotrichum species responsible for anthracnose diseases of various fruits. Plant Disease, 82, 596–605. doi:10.1094/PDIS.1998.82.6.596.
Peres, N. A. R., Kuramae, E. E., Dias, M. S. C., & de Sousa, N. L. (2002). Identification and characterization of Colletotrichum spp affecting fruit after harvest in Brazil. Journal of Phytopathology, 150, 128–134. doi:10.1046/j.1439-0434.2002.00732.x.
McDonald, B. A., & Mc Dermott, J. M. (1993). Population genetics of plant pathogenic fungi. Bioscience, 43, 311–319. doi:10.2307/1312063.
Samuels, G. J., & Siefert, K. A. (1995). The impact of molecular characteristics on systematics of filamentous ascomycetes. Annual Review of Phytopathology, 33, 37–67. doi:10.1146/annurev.py.33.090195.000345.
Frederick, R. D., Snyder, C. L., Peterson, G. L., & Bonde, M. R. (2002). Polymerase chain reaction assays for the detection and discrimination of the soybean rust pathogens Phakopsora pachyrhizi and Phakopsora meibomiae. Phytopathology, 92, 217–227. doi:10.1094/PHYTO.2002.92.2.217.
Saha, T., Kumar, A., Ravindran, M., Jacob, C. K., Roy, B., & Nazeer, M. A. (2002). Identification of Colletotrichum acutatum from rubber using random amplified polymorphic DNAs and ribosomal DNA polymorphisms. Mycological Research, 106, 215–221. doi:10.1017/S0953756201005342.
Talhinhas, P., Sreenivasaprasad, S., Neves-Martin, J., & Oliveira, H. (2002). Genetic and morphological characterization of Colletotrichum acutatum causing anthracnose of lupins. Phytopathology, 92, 986–996. doi:10.1094/PHYTO.2002.92.9.986.
Gonzalez-Chavira, M. M., Cardenas-Soriano, E., Nieto-Angel, D., Guevara-Gonzalez, R. G., Cruz-Hernandez, A., & Casarrubias-Carrillo, U. (2003). Genetic variability of Colletotrichum gloeosporioides (Penz.) Penz and Sacc. isolated from papaya (Carica papaya L.) fruits using RAPD molecular markers. Revista Mexicana de Fitopatologia, 21, 338–345.
Cia, P., Pascholati, S. F., Benato, E. A., Camili, E. C., & Dantos, C. A. (2007). Effects of gamma and UV-C irradiation on the post harvest control of papaya anthracnose. Postharvest Biology and Technology, 43, 366–373. doi:10.1016/j.postharvbio.2006.10.004.
Tapia-Tussell, R., Lappe, P., Ulloa, M., Quijano-Ramayo, A., Cáceres-Farfán, M., Larque-Saavedra, A., et al. (2006). A rapid and simple method for DNA extraction from yeast and fungi isolated from Agave fourcroydes. Molecular Biotechnology, 33, 67–70.
Mills, P. R., Sreenivasaprasad, S., & Brown, A. E. (1992). Detection and differentiation of Colletotrichum gloeosporioides isolates using PCR. FEMS Microbiology Letters, 98, 137–144. doi:10.1111/j.1574-6968.1992.tb05503.x.
White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. Innis, D. Gelfand, J. Sninsky, & T. White (Eds.), PCR protocols, a guide to methods and applications (pp. 315–322). San Diego CA USA: Academic Press.
Naumova, E. S., Sukhotina, N. N., & Naumov, G. I. (2004). Molecular-genetic differentiation of the dairy yeast Kluyveromyces lactis and its closest wild relatives. FEMS Yeast Res, 5, 263–269. doi:10.1016/j.femsyr.2004.08.006.
Hall, T. (2005). BioEdit v 7.0.5. http://www.mbio.ncsu.edu/BioEdit/.
Chen, L. S., Chu, C., Liu, C. D., Chen, R. S., & Tsay, J. G. (2006). PCR-based detection and differentiation of anthracnose pathogens, Colletotrichum gloeosporioides and C. truncatum, from vegetable soybean in Taiwan. Journal of Phytopathology, 154, 654–662. doi:10.1111/j.1439-0434.2006.01163.x.
Bardas, G. A., Koutita, O., & Tzavella-Klonari, K. (2007). Geographical distribution, pathotype characterization, and molecular diversity of Colletotrichum lindemuthianum in Greece and resistance of Greek bean cultivars. Plant Disease, 91, 1379–1385. doi:10.1094/PDIS-91-11-1379.
Voorrips, R. E., Finkers, R., Sanjaya, L., & Groenwold, R. (2004). QTL mapping of anthracnose (Colletotrichum spp) resistance in a cross between Capsicum annuum and C chinense. Theoretical and Applied Genetics, 109, 1275–1282. doi:10.1007/s00122-004-1738-1.
Sharma, P. N., Kaur, M., Sharma, O. P., Sharma, P., & Pathania, A. (2005). Morphological, pathological and molecular variability in Colletotrichum capsici, the cause of fruits rot of chillies in the subtropical region of north-western India. Journal of Phytopathology, 153, 232–237. doi:10.1111/j.1439-0434.2005.00959.x.
Than, P. P., Jeewon, R., Hyde, K. D., Pongsupasamit, S., Mongkolporn, O., Taylor, P. W. J. (2008). Characterization and pathogenicity of Colletotrichum species associated with anthracnose on chilli (Capsicum spp) in Thailand. Plant Pathology. doi: 10.1111/j.1365-3059.2007.01782.x.
Pring, R. J., Nash, C., Zakaria, M., & Bailey, J. A. (1995). Infection process and host range of Colletotrichum capsici. Physiological and Molecular Plant Pathology, 46, 137–152. doi:10.1006/pmpp. 1995.1011.
Martinez-Culebras, P. V., Barrio, E., Garcia, M. D., & Querol, A. (2000). Identification of Colletotrichum species responsible for anthracnose of strawberry based on the internal transcribed spacers of the ribosomal region. FEMS Microbiology Letters, 189, 97–101.
Abang, M. M., Winter, S., Green, K. R., Hoffmann, P., Mignouna, H. D., & Wolf, G. A. (2002). Molecular identification of Colletotrichum gloeosporioides causing yam anthracnose in Nigeria. Plant Pathology, 51, 63–71. doi:10.1046/j.0032-0862.2001.00655.x.
Acknowledgements
The authors are grateful to Dr. Jairo Alejo for providing some of the samples of papaya fruits with anthracnose used in this study; and especially to Prof. Dr. Wolfgang Rohde, from Max Planck Institute for Plant Breeding Research, for his complete review of this manuscript and his time and effort in providing comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tapia-Tussell, R., Quijano-Ramayo, A., Cortes-Velazquez, A. et al. PCR-Based Detection and Characterization of the Fungal Pathogens Colletotrichum gloeosporioides and Colletotrichum capsici Causing Anthracnose in Papaya (Carica papaya L.) in the Yucatan Peninsula. Mol Biotechnol 40, 293–298 (2008). https://doi.org/10.1007/s12033-008-9093-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-008-9093-0