Abstract
Gliomas are one of the most aggressive brain tumors with a poor prognosis in the central nervous system. Bexarotene is a third-generation retinoid X receptor agonist that is promising in the treatment of both cancer and neurodegenerative diseases. In this study, we aimed to investigate the cytotoxic and anti-proliferative effects of bexarotene in C6 glioma cells through the PPARγ/NF-κB pathway. In the study, first cytotoxic bexarotene concentrations for C6 cells were detected, and then apoptosis profile, reactive oxygen species (ROS), total antioxidant (TAS), 8-hydroxy-2′-deoxyguanosine (8-OHdG) and nuclear factor-κB (NF-κB) levels in the cells were determined. In addition, peroxisome proliferator-activated receptor γ (PPARγ) mRNA expression analysis was carried out. As a result, we detected concentration- and time-dependent antiproliferative effects of bexarotene on C6 cells. We found that bexarotene treatment decreased NF-κB and TAS levels and increased PPARγ and 8-OHdG levels in C6 cells. Bexarotene enhanced PPARγ expression in a dose-dependent manner when compared to the control group (P < 0.01). Furthermore, we determined that bexarotene-induced apoptotic C6 cells enhanced through Annexin V-FITC/PI staining and caspase-3/-7 activation analyses since phosphatidylserine level on the outer surface of the cell membrane and caspase-3/-7 activities were increased in the cells treated with bexarotene. In conclusion, bexarotene treatment in C6 glioma cells could modulate apoptosis profile, DNA damage, ROS production, and reduction of TAS levels through inhibition of NF-κB by enhancing PPARγ expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma is a primary malignant brain tumor that can occur in the brain or spinal cord originating from glial cells [1]. Gliomas are the most common tumors of the central nervous system (CNS) with their high proliferation and vascularization abilities [2]. Although revealing the current molecular mechanisms of gliomas led to the proposal of various innovative treatment strategies, there has not been discovered any successful clinical methodology [3]. However, thanks to molecular and genetic technological advances and understanding of the physiological and biochemical pathways of the disease, patient survival and quality of life can be maintained high with a more effective treatment. In particular, rat glioma cell lines provide an experimental model system for examining gliomas by simulating the basic cellular properties of human neoplastic gliomas. Rat C6 cell lines widely used in neuro-oncology exhibit the same histological characteristics as spindle-like cells simulating human glioblastoma with a high mitotic index and up-regulation of oncogenes [4].
Bexarotene [Targretin; (4- {1- (3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl) ethenyl} benzoic acid] is a synthetic compound that slightly resembles natural retinoids except for its isoprene backbone. Bexarotene has a lipophilic character with a molecular weight of 348.48 kD. Bexarotene is a synthetic retinoid analog and anti-neoplastic agent that acts through retinoid receptors (as RXR agonist) to regulate genes involved in cellular differentiation and growth [5]. Bexarotene was approved for use in cutaneous T-cell lymphoma (CTCL) by the Food and Drug Administration (FDA) and continues to be the second-line treatment for the cutaneous symptoms of CTCL. In addition to stopping the cell cycle in CTCL cell lines, bexarotene also inhibits cell proliferation through the induction of apoptosis accompanied by caspase activation [6].
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that play a role in various metabolic processes and regulate genes for cellular functions [7]. PPARs contain a DNA binding region at the N terminus, a flexible DNA binding region that specifically recognizes peroxisome proliferator response elements (PPRE), and a ligand-binding region at the C terminus. Following interaction with specific ligands, PPARs are transported to the nucleus where they change their structure and regulate gene transcription and heterodimerize with another nuclear receptor, the retinoid X receptor (RXR) [8]. PPARγ tightly regulates the expression of many genes, including G-protein coupled receptors, growth factors, antioxidant enzymes, stem cell genes, kinases, cytokines/chemokines, pro-inflammatory factors, ion channels, and transporters. The regulated genes can be divided into 3 main categories: (1) fatty acid/glucose metabolism, (2) inflammation/oxidative stress/apoptosis, and (3) cancer [9]. PPARγ protects neurons and glial cells against oxidative damage, mitochondrial dysfunction, and apoptosis. PPARγ activates both the antioxidant response element (ARE) and antioxidant genes that are critical for the maintenance of redox homeostasis with PPRE regions [10].
Cumulative changes in cancer cells induce genetic changes that result in proto-oncogene activation and loss of tumor suppressors through the production of proinflammatory cytokines, chemokines, and reactive oxygen species (ROS). PPARγ, which is expressed in various cell types and can manipulate cell growth and apoptosis, is important in the regulation of nuclear factor -κB (NF-κB) activation [11]. NF-κB activation is a key regulator of signaling pathways that can stimulate cell proliferation, growth, and migration [12]. A previous study reported that PPAR overexpression in cancer cells induced apoptosis significantly by inhibiting the expression of anti-apoptotic proteins by suppressing the activity of NF-κB [13]. NF-κB promotes cancer cell proliferation and survival by inducing the production of ROS that causes DNA chain damage, oncogenic mutations, and disruption of cell cycle control mechanisms [14]. Also, NF-κB can trigger epigenetic changes as well as chromosomal abnormalities, leading to nuclear DNA double-strand breaks [15]. It was reported that ROS production or a decrease in antioxidant defense mechanisms caused DNA damage-stimulated NF-κB upregulation [16].
Clinical trials of bexarotene are currently ongoing; however, its therapeutic responses have not been entirely unfolded. Additionally, although therapeutic approaches have been suggested for various molecular signaling pathways in gliomas, there is no successful treatment method in clinical studies yet. Therefore, in our study, we chose the C6 cell line as it would provide a basis for in vivo models that are more suitable and predictive for new therapeutic agents and treatment strategies in the treatment of glioma tumors. This study aims at investigating the effects of bexarotene treatment on cell proliferation via the PPARγ/NF-κB signaling pathway in C6 glioma cells. We hypothesize that bexarotene inhibits the viability of C6 cells by increasing ROS production, caspase activity, and PPARγ expression via regulating the activation of NF‑κB and antioxidant mechanisms. In the current study, PPARγ levels, NF‑κB levels, ROS levels, caspase 3/7 levels, total antioxidant levels (TAS), and 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels were measured to determine the effects of bexarotene on these signaling pathways. Our results could provide new insights into new cancer therapeutic strategies by indicating the anti-proliferative and anti-cancer effects of bexarotene on gliomas.
Materials and methods
Cell culture and bexarotene treatment
Rat C6 glioma cell line from the American Type Culture Collection (ATCC, Rockville, MD, USA) was incubated in Dulbecco's Modified Eagle Medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA) containing 10% (v/v) fetal bovine serum (FBS; Gibco, Grand Island, NY) plus 1% (v/v) penicillin–streptomycin (100 U/ml–100 µg/ml respectively) (Sigma-Aldrich) at 37 °C in a humidified atmosphere containing 95% air and 5% CO2. The cells grew at 80–90% confluency in 75 cm2 cell culture flasks and separated with 0.25% trypsin-1 mM ethylenediaminetetraacetic acid (EDTA) solution (Thermo Fisher Scientific, Waltham, MA, USA).
100 mM stock of bexarotene (Sigma-Aldrich) was prepared and used by diluting the experiment day freshly and filtering with 0.21 μm filters. For 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich M5655) analysis, C6 cells were cultured in 96-well plates (at a density of 15 × 103 cells per well) and the adhered cells were treated with 12.5, 25, 50, 100, 200, 400 and 800 µM bexarotene for 24, 48 and 72 h. Following the bexarotene incubation, cells were incubated with 100 µL of 0.5 mg/mL freshly prepared MTT solution for 3 h at 37 °C in the dark. After the incubation, the supernatant was discarded and the formazan crystals formed were dissolved in 100 µL 100% DMSO. Finally, the absorbance values of each well were measured at 570 nm using a microplate reader (800TS, BioTek Instruments, Winooski, Vermont, USA). To calculate cell viability, cells not treated with bexarotene were considered as the control group and these cells were assumed to be 100% viable. The following formula was used to calculate the percent viability of C6 cells untreated and treated with bexarotene:
The 25% (IC25), 50% (IC50), and 75% (IC75) concentrations of bexarotene were determined by the respective plot according to the MTT results. The 25, 50, and 75 µM bexarotene concentrations were used for biochemical analysis.
Cell lysate preparation and biochemical analysis
For biochemical analyses, C6 cells (5 × 103) were grown in a 96-well plate and the cohesive cells were treated with 25, 50, and 75 µM bexarotene concentrations for 24 h. Following treatment, the cells were washed with phosphate buffer (PBS; pH 7.4), and adhered cells were separated from the plate with the aid of trypsin and then the pellets were collected in an Eppendorf tube by centrifugation at 1000xg for 5 min at 4 °C. The pellets were washed 2 times in PBS, and then the pellets resuspended in 500 mL of radioimmunoprecipitation assay (RIPA, Santa Cruz Biotechnology, USA) in lysis buffer were incubated with RIPA for 20 min at 4 °C in an orbital shaker (Isolab). Consequently, the pellets were centrifuged to obtain cell debris at 10000xg for 20 min at 4 °C.
PPARγ, NF-κB, 8-OHdG, and TAS levels in the cell lysates treated with IC25, IC50, and IC75 concentrations of bexarotene were measured according to the manufacturer's instructions using commercially available ELISA kits (SEA886Ra, SEB824Ra, CSB-E10526r, and Rel Assay Diagnostics TAS, respectively).
Analysis of ROS production
A commercially available kit (MCH100111, Merck Millipore) based on the reaction of dihydroethidium (DHE) with superoxide anions was used to detect ROS formation in C6 cells incubated with IC25, IC50, and IC75 bexarotene concentrations for 24 h. Briefly, first of all, all chemicals were brought to room temperature. Following, 10 μL of the treated and untreated cell suspension (2 × 106) was taken and 190 μL of Muse® Oxidative Stress Reagent working solution was added and mixed thoroughly by pipetting. The mixture was incubated at 37 °C for 30 min and then measured in a Muse® Cell Analyzer.
Quantitative real-time reverse transcription-polymerase chain reaction for PPARγ
The PPARγ/β-actin mRNA ratio was determined by quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis of PPARγ mRNA expressions in C6 cells (2 × 106) untreated and treated with bexarotene for 24 h. To briefly explain, the RT-PCR procedure, total RNA was extracted using an RNA isolation kit (BS88133/BS88136, Bio Basic, Canada) according to the manufacturer's instructions. After the purified RNA was washed in RNase-free water, measurements were made by NanoDrop™ 2000/2000c (Thermo Fisher Scientific, USA) using the OD260/OD280 ratio to determine its quality and concentration. Synthesis of cDNA from total RNA was performed according to the manufacturer’s instructions using the SuperScript™ IV One-Step RT-PCR System (12,594,100, Thermo Fisher Scientific).
The PPARγ and β-actin primers were as follows: PPARγ forward primer 5′-CCC TTT ACC ACG GTT GAT TTC TC-3′, and the reverse primer 5′-GCA GGC TCT ACT TTG ATC GCA CT-3′; β-actin forward primer 5′-GTG GGG CGC CCC AGG CAC CA-3′ and the reverse primer 5′-CTT CCT TAA TGT CAC GCA CGA TTT C-3'.
SYBR Green Master Mix (Applied Biosystems, USA) was used to amplify cDNA according to the manufacturer's instructions in the StepOnePlus™ Real-Time PCR System (ThermoFisher Scientific) and its software program. The thermal cycling profile was as follows: a pre-incubation step for 10 min at 95 °C, followed by 45 cycles of denaturation step at 95 °C for 10 s, annealing step at 55 °C for 1 min, and extension step at 72 °C for 10 s. PPARγ and β-actin transcription levels were determined according to the post-amplification Ct values. β-actin mRNA levels were used as an internal standard, and the relative expression was determined using the formula 2¯ΔΔCT.
Annexin V-FITC/PI staining and caspase 3/7 for apoptosis profile
Annexin V-fluorescein isothiocyanate (FITC) staining/PI (propidium iodide) cross staining kit (Merck Millipore, Germany) was used to evaluate bexarotene-induced apoptosis in C6 cells. Shortly, C6 cells (2 × 106) were grown in six-well plates and adherent cells were incubated with 25, 50, and 75 µM bexarotene concentrations for 24 h. Subsequently, the cells were detached with trypsin and washed with PBS. Following, the cells were incubated with 5 μL Annexin V-FITC and 1 μL propidium iodide at 25 °C for 15 min in the dark. After incubation, cells were analyzed for apoptotic cell death by flow cytometry (Muse® Cell Analyzer, Merck Millipore, Germany).
The apoptotic status in the bexarotene-treated groups and control group cells was determined by measuring the activation of caspase-3/7. Quantitative measurement of viable, early and late apoptotic and dead cells was performed using the Muse® Caspase-3/7 kit (MCH100108, Merck Millipore). To summarize, early apoptotic, late apoptotic and dead cells were determined with the aid of a dead cell marker (7-AAD) based on cellular plasma membrane permeability. The cells (2 × 106) were incubated with 25, 50, and 75 µM bexarotene concentrations for 24 h. Following, the trypsinized cells were washed with PBS and 5 μL of Muse® Caspase-3/7 working solution was added to 50 μL of cells. After 30 min of incubation at 37 °C, 150 μL of 7-AAD was added to the cell medium, and then mixed thoroughly and measured on Muse® Cell Analyzer.
Statistical analysis
All experiments were performed as a triple repetition of three independent experiments and results are expressed as mean ± standard deviation (SD). The results were analyzed statistically using GraphPad Prism software 7 (GraphPad Software, San Diego, CA, USA). One-Way Analysis of Variance (ANOVA) was used to identify differences between experimental groups followed by Tukey Post hoc analysis. P < 0.05 was considered to indicate statistical significance.
Results
Inhibitory effects of bexarotene on C6 cell viability
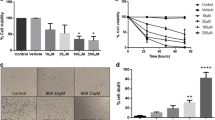
The effects of bexarotene on cell viability are shown in Fig. 1. Bexarotene between 0 and 800 µM concentrations was applied to C6 cells for 24, 48, and 72 h. The cells showed a concentration- and time-dependent decrease following bexarotene treatment. Notably, we could not detect any cell at the bexarotene concentrations of 100 µM and above. Notwithstanding, no significant decrease in cell viability was found in the treatment of 12.5 µM bexarotene concentration for 24 h and 48 h (a decrease by 3.7% and 5.2%, respectively; P > 0.05 vs control) as well as in the treatment of 25 µM bexarotene concentration for 24 h (a decrease by 4.1%; P > 0.05 vs control).
The cells incubated with 12.5 µM bexarotene for 72 h resulted in a 28.6% reduction compared to the control group (P < 0.05). Besides, 25 µM bexarotene treatment for 48 and 72 h showed a 35.2% and 41.8% reduction in C6 cells compared to the control group, respectively (P < 0.01). The C6 cells treated with 50 µM bexarotene for 24 and 48 h caused a decrease of 48.3% and 97.1%, respectively, compared to the control group, while the 72 h treatment completely suppressed the cell viability. According to MTT analysis results, IC25, IC50, and IC75 concentrations of bexarotene were found to be 25, 50, and 75 µM, respectively. In further analysis, C6 cells were incubated with IC25, IC50, and IC75 concentrations of bexarotene for 24 h.
Bexarotene regulated PPARγ, NF-κB, 8-OHdG, and TAS levels in C6 cells
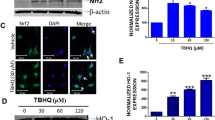
PPARγ, NF-κB, 8-OHdG, and TAS levels in C6 cells incubated with IC25, IC50, and IC75 concentrations of bexarotene are presented in Fig. 2. Generally, PPARγ, NF-κB, 8-OHdG, and TAS levels exhibited a concentration-dependent increase in the bexarotene-treated cells. The cells incubated with 25, 50, and 75 µM bexarotene concentrations for 24 h demonstrated a statistically significant increase of 47.2%, 94.8%, and 141.6% in PPARγ levels compared to the control group, respectively (Fig. 2a).
C6 cells treated with 25 µM bexarotene concentration showed a moderate decrease in NF-κB levels (Fig. 2b, P > 0.05). However, the treatment of 50 and 75 µM bexarotene concentrations caused a decrease in NF-κB levels of 23.6% and 27.1%, respectively (P < 0.01). Thus, NF-κB production in C6 cells was suppressed due to the increase in bexarotene concentration.
Bexarotene treatment upregulated 8-OHdG levels in C6 cells (Fig. 2C). The cells treated with bexarotene concentrations (25, 50, and 75 μM) for 24 h increased 8-OHdG levels (P < 0.01 vs control). The maximum increase in 8-OHdG levels was observed in cells treated with 75 µM bexarotene (an increase by 91.5%, P < 0.01 vs control).
The decrease in TAS levels is closely related to the increase in oxidative burden in the bexarotene-treated cells. C6 cells treated with bexarotene concentrations (25, 50, and 75 µM) for 24 h decreased TAS levels (Fig. 2d). The maximum decrease in TAS levels was observed in cells treated with 75 µM bexarotene; this value is 40.6% lower than that detected in the control group (P < 0.01). Furthermore, 12.5% (P < 0.05 vs control) and 37.2% (P < 0.01 vs control) decreases in TAS levels were observed in C6 glioma cells treated with 25 and 50 µM bexarotene concentrations for 24 h, respectively.
Oxidative stress stimulated by bexarotene
Excessive ROS accumulation in cancer cells can trigger oxidative mechanisms that induce cell damage and death pathways. ROS generation levels were measured to evaluate oxidative stress in C6 cells treated with bexarotene. ROS levels in the groups treated with bexarotene for 24 h and control cells are shown in Fig. 3. Figure 3 shows the image providing data on living cells (M1) and ROS-producing cells (M2). It is noteworthy that ROS accumulation in the bexarotene-treated cells showed a concentration-dependent increase. C6 cells after 24 h treatment with IC75 bexarotene concentration were determined to have higher ROS levels (a rate of 72.59%) than the other groups (Fig. 3d, P < 0.01).
Effect of bexarotene on PPARγ mRNA levels of C6 cells
PPARγ mRNA levels in the C6 cells treated and untreated were measured. PPARγ mRNA levels in bexarotene-treated groups were significantly different from the controls (Fig. 4). Besides, the PPARγ mRNA expression depends on the bexarotene concentration. The PPAR-γ mRNA levels increased significantly in a concentration-dependent manner compared to the control. The PPAR-mRNA levels of cells treated with IC75 bexarotene concentration increased approximately two-fold compared to the control group (P < 0.01).
Bexarotene triggered apoptotic cell death in C6 cells
Phosphatidylserine is used to evaluate apoptotic cell death by translocating to the outer surface of the cell membrane during apoptosis. Annexin V-FITC binds to phosphatidylserine on the outer surface of apoptotic cells and provides data on the apoptotic status of the cells by flow cytometric analysis. A concentration-dependent increase in apoptotic cell death was detected after 24 h bexarotene treatment (Fig. 5). The treatments with IC25, IC 50 and IC75 bexarotene concentrations reduced viable cell ratio by 25.8%, 43.6% and 64.3%, respectively (P < 0.01). Remarkably, the early apoptotic cells ratio (31.85%) in the cells treated with 50 µM bexarotene was higher than the other treatment groups (Fig. 5c). As shown in Fig. 5d, the highest late apoptotic cell rate and death rate were found in cells treated with 75 µM bexarotene for 24 h (29.6% and 6.4%, respectively).
Caspase 3/7 activation is an important factor in initiating and promoting the apoptotic cell death. C6 glioma cells treated with 25, 50, and 75 µM bexarotene for 24 h were found to increase caspase-3/7 activation (Fig. 6). Exposure to 25 and 50 µM bexarotene did not show a significant decrease in apoptosis when compared to control (Fig. 6b and Fig. 6c; 3.2% and 3.18%, respectively; P > 0.05), although the viability of C6 cells was notably reduced. Importantly, the caspase-3/7 activation was distinctly enhanced by a ratio of 55.04% in C6 cells treated with 75 µM bexarotene (Fig. 6d; P < 0.01 vs control).
Discussion
The study aimed to explore whether bexarotene exposure at different durations (24, 48 and 72 h) and concentrations (0–800 µM) could cause anti-tumoral effects in C6 glioma cells by triggering various biochemical mechanisms. We determined three cytotoxic doses (IC25, IC50, and IC75) for C6 cells and analyzed oxidative, apoptotic and DNA damage mechanisms through the PPARγ/NF-κB signaling pathway. Interestingly, we found that bexarotene treatment upregulated PPARγ levels and lowered NF-κB in C6 cells, suggesting that PPARγ and NF-κB regulation induce DNA damage by inducing oxidative and apoptotic mechanisms.
Bexarotene, an RXR agonist with high blood–brain barrier permeability, is the third-generation retinoid used for the treatment of CTCL [17]. This synthetic retinoid shows its biological effect by forming heterodimers with many nuclear receptors, particularly PPARs, through RXR activation [18]. PPARs, which are nuclear receptor proteins, regulate cellular differentiation, proliferation, and metabolism as well as the expression of genes associated with the tumoral activity as transcription factors. Wang et al. [19] showed that bexarotene exposure hampered cell proliferation by reducing adhesion in CTCL lineages (MJ, HuT78, HuT102, and SeAx). In another report, bexarotene treatment in malignant breast cells was shown to inhibit cell proliferation and tumor formation by preventing the cell cycle from G1 to S phase through G1 blockade [20]. Consistent with the aforementioned studies, we found a concentration- and time-dependent decrease in the C6 glioma cells treated with bexarotene. However, we detect an insignificant reduction in cell proliferation at the bexarotene concentrations 12.5 µM for 48 h as well as 25 µM for 24 h. A previous study reported the effect of bexarotene (0.1 µM and 10 µM) on MJ, Hut78, and HH cell lines for 24, 48, 72, and 96 h [21]. In this study, the cell viability in MJ, Hut78 and HH cell lines treated with 0.1, 1, and 10 µM bexarotene for 96 h was reduced by approximately 26.3%, 35.4%, and 40.8%, respectively, whereas the cell viability in C6 treated with 50 µM bexarotene for 24 decreased by 48.3%. In contrast, bexarotene has been shown to significantly preserve the viability of neurons against glutamate excitotoxicity in mouse primary cortical neuron cultures containing 90% neurons and 10% glial cells [22]. Recently, it was reported that bexarotene inhibited the viability of cancer cells and prevented tumor formation [23], especially by restricting the proliferation of glioblastoma cells [24]. These data suggest that cancer cells, especially C6 glioma cells, are more sensitive to bexarotene than normal neurons.
ROS causes oxidation of cellular macro-biomolecules (DNA, proteins, and lipids) [25]. High proliferation of cancer cells results in increased ROS production and abnormal redox homeostasis. [26] Cancer cells prevent oxidation of ROS-induced biomolecules and induction of apoptosis by upregulating antioxidant defense mechanisms to optimize oxidative burden [27]. This antioxidant capacity in tumor cells obstructs excessive ROS production, allowing resistance to apoptosis [28]. However, excessive ROS accumulation causing anti-tumorigenic effects in cancers induces increased oxidative stress and DNA damage along with genomic instability [29]. As a result, when cellular levels of antioxidants fail to combat oxidants, biological molecules and cells are damaged and thus excessive induction of ROS production causes death in cancers. In this study, we found that ROS production and DNA damage were increased in a dose-dependent manner in C6 cells treated with bexarotene, while cell proliferation was suppressed. Additionally, bexarotene-induced ROS production in C6 cells was proportional to the decreased TAS levels. Similarly, PPAR agonist treatment in C6 glioma cells failed to alter the expression and enzymatic activity of catalase, an important antioxidant enzyme [30]. 8-OHdG, which is formed as a result of the interaction of nucleobases in the DNA helix with ROS, is the most abundant DNA lesion and a potential biomarker [31]. Moreover, we showed that the increase in cellular ROS levels causes DNA damage, increasing 8-OHdG levels. Consistently, Bhuyan et al. [32] also reported that bexarotene treatment increases ROS production by inducing oxidative stress in erythrocytes.
Caspases are a family of cysteine-dependent aspartate-specific proteases that are critical in initiating programmed cell death through extrinsic and intrinsic pro-apoptotic signal mechanisms [33]. Some of these are called effector caspases (caspase-3, -6, and -7), which are activated by the initiator caspases (caspase-8, -9, and -10) to promote apoptotic processes and involved in the main demolition of the cell by proteolysis of structural proteins. The activation of caspase-3/7, which coordinates the destruction phase of apoptosis, is a characteristic feature of apoptosis [34]. PPARγ activation with PPARγ agonists can induce apoptosis in glioma cells through several signaling pathways, such as increasing the expression of pro-apoptotic proteins in cancer cell lines or causing the release of cytochrome c to the cytosol by altering the mitochondrial membrane potential [35]. A previous study reported that PPARγ activation decreased the expression of the anti-apoptotic protein and increased the expression of the apoptotic protein in the A375 cell line [36]. In another study, Ai et al. [23] pointed out that the expression of anti-apoptotic proteins and tumor suppressor genes were decreased, while caspase-3/-7 activity was increased in bexarotene-treated A549 cells. Recently, bexarotene and its derivatives have been shown to induce apoptosis in C6 and U-87 MG glioma cells via the caspase-3 and caspase 9 activation pathways [23]. Similarly, we found as a result of caspase-3/-7 activity and Annexin V assays that bexarotene treatment in C6 glioma cells promoted apoptosis by enhancing the expression and activation of PPARγ. These results might be promising for anti-cancer drug resistance in the treatment of gliomas.
One of the remarkable results of this study was that bexarotene as a PPARγ agonist upregulated PPARγ expression and subsequently inhibited proliferation of C6 cells, which correlated with previous studies. In particular, bexarotene mediates the potential of PPARγ to inhibit inflammation, angiogenesis and metastasis [37]. NF-κB, the main regulator of adaptive immune reactions, induces migration and invasion by inhibiting apoptosis and promoting cell proliferation. Especially, NF‑κB, structurally active in cancer cells, upregulates oxidative stress and DNA damage as well as necrotic cell death [12]. Physical interaction of PPARγ with NF-κB was reported to inhibit the NF-κB signaling pathway by reducing the NF-κB binding affinity on inflammatory promoters [38]. In the study of Hwang et al. [39], docosahexaenoic acid treatment in MCF‑7 breast cancer cells was demonstrated to cause NF-κB inhibition through the increase in PPARγ expression. They found that PPARγ-dependent NF-κB suppression inhibits the proliferation and invasion of cells. Also, previous studies reported that inactivation or inhibition of NF-κB in C6 glioma cells triggered apoptosis by downregulating anti-apoptotic proteins as well as upregulating apoptotic protein proteins, thereby activating the caspase cascade [40, 41]. In the current study, consistently, PPARγ expression and activation levels increased, while NF-κB levels decreased in C6 cells treated with bexarotene. Moreover, the decrease in NF-κB levels was correlated with increased caspase-3/-7 activation. Therefore, in our study, inhibition of NF-κB by PPARγ may have suppressed the proliferation of C6 glioma cells and triggered apoptosis.
Conclusion
To summarize, bexarotene as a PPARγ agonist showed a concentration- and time-dependent anti-tumoral effect in C6 glioma cells. The increase in cellular PPARγ levels induced DNA damage and apoptosis by causing NF-κB inhibition. Besides, an increase in oxidant levels and a decrease in antioxidant levels were observed in bexarotene-treated cells. This study demonstrates the cytotoxic effects of bexarotene through the PPARγ/NF-κB signaling pathway in glioma cells. The important drawback of this study may be that our study design was not supported by an in vivo model. Bexarotene has a potency to be a promising therapeutic agent for the treatment of neuroblastomas. We think that our study will provide important data for future studies on enlightening the other action mechanisms of bexarotene.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–50. https://doi.org/10.1001/jama.2013.280319.
Wirsching HG, Galanis E, Weller M. Glioblastoma. Handb Clin Neurol. 2016;134:381–97. https://doi.org/10.1016/B978-0-12-802997-8.00023-2.
Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392(10145):432–46. https://doi.org/10.1016/S0140-6736(18)30990-5.
Grobben B, De Deyn PP, Slegers H. Rat C6 glioma as experimental model system for the study of glioblastoma growth and invasion. Cell Tissue Res. 2002;310(3):257–70. https://doi.org/10.1007/s00441-002-0651-7.
Farol LT, Hymes KB. Bexarotene: a clinical review. Expert Rev Anticancer Ther. 2004;4(2):180–8. https://doi.org/10.1586/14737140.4.2.180.
Qu L, Tang X. Bexarotene: a promising anticancer agent. Cancer Chemother Pharmacol. 2010;65(2):201–5. https://doi.org/10.1007/s00280-009-1140-4.
Hong F, Pan S, Guo Y, Xu P, Zhai Y. PPARs as Nuclear Receptors for Nutrient and Energy Metabolism. Molecules. 2019;24(14):2545. https://doi.org/10.3390/molecules24142545.
Brunmeir R, Xu F. Functional Regulation of PPARs through Post-Translational Modifications. Int J Mol Sci. 2018;19(6):1738. https://doi.org/10.3390/ijms19061738.
Li J, Liu YP. The roles of PPARs in human diseases. Nucleosides Nucleotides Nucleic Acids. 2018;37(7):361–82. https://doi.org/10.1080/15257770.2018.1475673.
Wang P, Li B, Cai G, Huang M, Jiang L, Pu J, Li L, Wu Q, Zuo L, Wang Q, Zhou P. Activation of PPAR-γ by pioglitazone attenuates oxidative stress in aging rat cerebral arteries through upregulating UCP2. J Cardiovasc Pharmacol. 2014;64(6):497–506. https://doi.org/10.1097/FJC.0000000000000143.
Li CC, Yang HT, Hou YC, Chiu YS, Chiu WC. Dietary fish oil reduces systemic inflammation and ameliorates sepsis-induced liver injury by up-regulating the peroxisome proliferator-activated receptor gamma-mediated pathway in septic mice. J Nutr Biochem. 2014;25(1):19–25. https://doi.org/10.1016/j.jnutbio.2013.08.010.
Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309–24. https://doi.org/10.1038/nri.2017.142.
Chen GG, Lee JF, Wang SH, Chan UP, Ip PC, Lau WY. Apoptosis induced by activation of peroxisome-proliferator activated receptor-gamma is associated with Bcl-2 and NF-kappaB in human colon cancer. Life Sci. 2002;70(22):2631–46. https://doi.org/10.1016/s0024-3205(02)01510-2.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. https://doi.org/10.1016/j.cell.2010.01.025.
Joyce D, Albanese C, Steer J, Fu M, Bouzahzah B, Pestell RG. NF-kappaB and cell-cycle regulation: the cyclin connection. Cytokine Growth Factor Rev. 2001;12(1):73–90. https://doi.org/10.1016/s1359-6101(00)00018-6.
Janssens S, Tschopp J. Signals from within: the DNA-damage-induced NF-kappaB response. Cell Death Differ. 2006;13(5):773–84. https://doi.org/10.1038/sj.cdd.4401843.
Shen D, Yu X, Wu Y, Chen Y, Li G, Cheng F, Xia L. Emerging roles of bexarotene in the prevention, treatment and anti-drug resistance of cancers. Expert Rev Anticancer Ther. 2018;18(5):487–99. https://doi.org/10.1080/14737140.2018.
Evans RM, Mangelsdorf DJ. Nuclear Receptors, RXR, and the Big Bang. Cell. 2014;157(1):255–66. https://doi.org/10.1016/j.cell.2014.03.012.
Wang L, DeMarco SS, Chen J, Phillips CM, Bridges LC. Retinoids Bias Integrin Expression and Function in Cutaneous T-Cell Lymphoma. J Invest Dermatol. 2015;135(8):2102–8. https://doi.org/10.1038/jid.2015.122.
Wu K, DuPre E, Kim H, Tin-U CK, Bissonnette RP, Lamph WW, Brown PH. Receptor-selective retinoids inhibit the growth of normal and malignant breast cells by inducing G1 cell cycle blockade. Breast Cancer Res Treat. 2006;96(2):147–57. https://doi.org/10.1007/s10549-005-9071-1.
Zhang C, Hazarika P, Ni X, Weidner DA, Duvic M. Induction of apoptosis by bexarotene in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. Clin Cancer. 2002;8(5):1234–40.
Huuskonen MT, Loppi S, Dhungana H, Keksa-Goldsteine V, Lemarchant S, Korhonen P, Wojciechowski S, Pollari E, Valonen P, Koponen J, Takashima A, Landreth G, Goldsteins G, Malm T, Koistinaho J, Kanninen KM. Bexarotene targets autophagy and is protective against thromboembolic stroke in aged mice with tauopathy. Sci Rep. 2016;6:33176. https://doi.org/10.1038/srep33176.
Ai X, Mao F, Shen S, Shentu Y, Wang J, Lu S. Bexarotene inhibits the viability of non-small cell lung cancer cells via slc10a2/PPARγ/PTEN/mTOR signaling pathway. BMC Cancer. 2018;18(1):407. https://doi.org/10.1186/s12885-018-4224-x.
Heo JC, Jung TH, Lee S, Kim HY, Choi G, Jung M, Jung D, Lee HK, Lee JO, Park JH, Hwang D, Seol HJ, Cho H. Effect of bexarotene on differentiation of glioblastoma multiforme compared with ATRA. Clin Exp Metastasis. 2016;33(5):417–29. https://doi.org/10.1007/s10585-016-9786-x.
Hanafi R, Anestopoulos I, Voulgaridou GP, Franco R, Georgakilas AG, Ziech D, Malamou-Mitsi V, Pappa A, Panayiotidis MI. Oxidative stress based-biomarkers in oral carcinogenesis: how far have we gone? Curr Mol Med. 2012;12(6):698–703. https://doi.org/10.2174/156652412800792598.
Klaunig JE. Oxidative Stress and Cancer. Curr Pharm Des. 2018;24(40):4771–8. https://doi.org/10.2174/1381612825666190215121712.
Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50–64. https://doi.org/10.1016/j.semcdb.2017.05.023.
Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–96. https://doi.org/10.2741/1667.
Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12(12):931–47. https://doi.org/10.1038/nrd4002.
Khoo NK, Hebbar S, Zhao W, Moore SA, Domann FE, Robbins ME. Differential activation of catalase expression and activity by PPAR agonists: implications for astrocyte protection in anti-glioma therapy. Redox Biol. 2013;1(1):70–9. https://doi.org/10.1016/j.redox.2012.12.006.
Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2’ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(2):120–39. https://doi.org/10.1080/10590500902885684.
Al Mamun Bhuyan A, Bissinger R, Cao H, Lang F. Triggering of Suicidal Erythrocyte Death by Bexarotene. Cell Physiol Biochem. 2016;40(5):1239–51. https://doi.org/10.1159/000453178.
Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–6. https://doi.org/10.1038/35037710.
Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–19. https://doi.org/10.1016/s0092-8674(04)00046-7.
Zander T, Kraus JA, Grommes C, Schlegel U, Feinstein D, Klockgether T, Landreth G, Koenigsknecht J, Heneka MT. Induction of apoptosis in human and rat glioma by agonists of the nuclear receptor PPARgamma. J Neurochem. 2002;81(5):1052–60. https://doi.org/10.1046/j.1471-4159.2002.00899.x.
Liu Y, Meng Y, Li H, Li J, Fu J, Liu Y, Chen XG. Growth inhibition and differentiation induced by peroxisome proliferator activated receptor gamma ligand rosiglitazone in human melanoma cell line A375. Med Oncol. 2006;23(3):393–402. https://doi.org/10.1385/mo:23:3:393.
Gretskaya NM, Gamisonia AM, Dudina PV, Zakharov SS, Sherstyanykh G, Akasov R, Burov S, Serkov IV, Akimov MG, Bezuglov VV, Markvicheva E. Novel bexarotene derivatives: Synthesis and cytotoxicity evaluation for glioma cells in 2D and 3D in vitro models. Eur J Pharmacol. 2020;883:173346. https://doi.org/10.1016/j.ejphar.2020.173346.
Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–35. https://doi.org/10.1146/annurev.med.53.082901.104018.
Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol. 2001;169(3):453–9. https://doi.org/10.1677/joe.0.1690453.
Hwang JK, Yu HN, Noh EM, Kim JM, Hong OY, Youn HJ, Jung SH, Kwon KB, Kim JS, Lee YR. DHA blocks TPA-induced cell invasion by inhibiting MMP-9 expression via suppression of the PPAR-γ/NF-κB pathway in MCF-7 cells. Oncol Lett. 2017;13(1):243–9. https://doi.org/10.3892/ol.2016.5382.
Kiekow CJ, Figueiro F, Dietrich F, Vechia LD, Pires EN, Jandrey EH, Gnoatto SC, Salbego CG, Battastini AM, Gosmann G. Quercetin derivative induces cell death in glioma cells by modulating NF-κB nuclear translocation and caspase-3 activation. Eur J Pharm Sci. 2016;84:116–22. https://doi.org/10.1016/j.ejps.2016.01.019.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by CH, FK and SK. The first draft of the manuscript was written by CH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hacioglu, C., Kar, F., Kacar, S. et al. Bexarotene inhibits cell proliferation by inducing oxidative stress, DNA damage and apoptosis via PPARγ/ NF-κB signaling pathway in C6 glioma cells. Med Oncol 38, 31 (2021). https://doi.org/10.1007/s12032-021-01476-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01476-z