Abstract
Lenvatinib is a long-awaited alternative to sorafenib for the first-line targeted therapy of patients with advanced hepatocellular carcinoma (HCC). However, resistance to lenvatinib has also become a major obstacle to improving the prognosis of HCC patients. The underlying molecular mechanisms contributing to lenvatinib resistance in HCC are largely unknown. HGF/c-MET axis activation is related to tumor progression and several hallmarks of cancer and is considered as the key contributor to drug resistance. In the present study, we focused on the role of the HGF/c-MET axis in mediating lenvatinib resistance in HCC cells. We showed that HGF reduced the antiproliferative, proapoptotic, and anti-invasive effects of lenvatinib on HCC cells with high c-MET expression but did not significantly affect HCC cells with low c-MET expression. The c-MET inhibitor PHA-665752 rescued HCC cells from HGF-induced lenvatinib resistance. Furthermore, HGF/c-MET activated the downstream PI3K/AKT and MAPK/ERK pathways and promoted epithelial–mesenchymal transition (EMT) in HCC cells. Collectively, our results suggested that combining lenvatinib treatment with a c-MET inhibitor may improve its systemic therapeutic efficacy in HCC patients with high c-MET expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a common cause of cancer-related deaths [1]. Even with hepatectomy and liver transplantation, the 5-year recurrence rate of HCC is as high as 70%. Due to the lack of effective treatment, patients with advanced HCC have poor prognoses with a median overall survival time of less than 1 year. Sorafenib was the first systemic treatment drug to show an overall survival benefit in advanced HCC and was approved as a first-line treatment for patients with advanced HCC [2]. The successful clinical implementation of sorafenib increased the interest in developing new drugs for HCC. However, many drugs failed in first-line (linifanib [3], sunitinib [4], erlotinib [5], brivanib [6]) and second-line (everolimus [7]) HCC treatment settings in randomized controlled phase III trials.
Lenvatinib, a tyrosine kinase inhibitor, exhibited effects on overall survival not inferior to those of sorafenib in patients with untreated advanced HCC in the phase III REFLECT clinical trial and was recommended as another first-line drug for advanced HCC treatment last year [8]. Doctors currently faced the dilemma of advising patients about their choice between these two agents because of the lack of biomarkers that can predict a more favorable treatment response or survival benefit of one over the other [9]. Although lenvatinib has a significant benefit on the objective response rate in advanced HCC, this benefit is not sustained. We found that some patients with advanced HCC who received lenvatinib therapy in our center developed drug resistance after a period of time. This phenomenon prompted us to explore the potential mechanisms underlying lenvatinib resistance.
Abnormal expression of HGF and/or c-MET in tumor tissues or peripheral blood could offer prognostic biomarkers as well as predictive biomarkers for drug response [10]. Research over the past decades has shown that HGF/c-MET axis activity promotes tumor invasion and metastasis [11]. The HGF/c-MET axis can mediate multiple downstream pathways, including the PI3K/AKT, MAPK/ERK, STAT3, and SRC/FAK pathways. Activation of these pathways regulates several distinct biological processes in tumor cells, with effects including the promotion of proliferation, migration and invasion and the inhibition of apoptosis [12]. Moreover, c-MET is frequently overexpressed in tumor tissue, and the activation of the c-MET pathway is associated with drug resistance [13]. However, whether HGF/c-MET activation contributes to lenvatinib resistance has not yet been reported. Therefore, we conducted this study to investigate the underlying mechanism associated with lenvatinib resistance in HCC patients.
Materials and methods
Cell lines and reagents
MHCC97-L and SMMC-7721 cells were obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (shanghai, China). Lenvatinib, PHA-665752, and recombinant human HGF (R&D systems, USA) were purchased from MedChemExpress (Monmouth Junction, USA). The antibodies against c-MET, p-c-MET, AKT, p-AKT, ERK, p-ERK, E-cadherin, N-cadherin, Vimentin, and β-actin were purchased from Cell Signaling Technology (SCT, Danvers, MA, USA).
Cell culture
Cell lines were cultured in high-glucose DMEM (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS, Gibco), and an antibiotic mixture of 100 U/ml penicillin and 100 mg/ml streptomycin (Life Technologies, Inc., Rockville, MD). Cells were incubated in a humidified atmosphere containing 5% CO2 at 37 °C.
Cell proliferation assay
To measure cell proliferation, HCC cells were seeded in 96-well plates at 2 × 103 cells/well with or without HGF (50 ng/ml) for 24 h. The culture medium was then replaced with reduced serum (1% FBS) medium containing various concentrations of lenvatinib, HGF (50 ng/ml) and PHA-665752 (100 nM) for another 72 h. Cells were incubated for 1 h with CCK-8 reagent (100 μl/ml) (Dojindo Molecular Technologies, Kumamoto, Japan), and the absorbance was read at 450 nm in a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). The IC50 values were calculated in GraphPad Prism 7.0 (GraphPad software, La Jolla, CA).
Apoptosis analysis
HCC cells (5 × 105) were seeded in six-well plates with or without HGF (50 ng/ml) for 24 h and allowed to adhere. The culture medium was then replaced with reduced serum (1% FBS) medium containing lenvatinib (4 μM), HGF (50 ng/ml) and PHA-665752 (100 nM) for another 48 h. The cells were then harvested, stained with an Annexin V-FITC/PI kit (Dojindo Molecular Technologies) following the manufacturer’s instructions and analyzed in a FACSAria II flow cytometer (BD Biosciences, San Jose, USA).
Cell invasion assay
The cell invasion assay was performed using a 24-well Transwell chamber system (8 μm pores, Corning Incorporated, NY, USA) precoated with Matrigel (BD Biosciences). MHCC97-L and SMMC-7721 cells were resuspended in serum-free medium containing the indicated compounds and added into the upper chamber of each well (5 × 104 cells/well). Then, 600 μl of DMEM supplemented with 10% FBS was added to the lower chamber of each well. After incubation for 48 h, the cells were fixed with 4% formaldehyde and stained with 0.1% crystal violet for 30 min. The invaded cells were counted in five random fields under an inverted microscope (100 ×).
Western blot analysis
A total of 25–50 μg of prepared protein was separated by 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). After blocking in 4% bovine serum albumin (BSA) for 1 h, membranes were incubated first with primary antibodies against c-MET, p-c-MET, AKT, p-AKT, ERK, p-ERK, E-cadherin, N-cadherin, Vimentin, and β-actin at 4 °C overnight and then with secondary antibodies at room temperature for 1 h. Protein bands were visualized via enhanced chemiluminescence (ECL) in a chemiluminescence imaging system (Bio-Rad, Hercules, UA, USA).
Small interfering RNA (siRNA) transfection
MHCC97-L and SMMC-7721 cells were seeded in six-well plates at 5 × 105 cells/well overnight. Then, the cells were transfected with c-MET siRNA or control siRNA (50 nM) using Lipofectamine RNAiMAX reagent (Invitrogen, Carlsbad, CA, USA). Six hours later, the culture medium was refreshed and treated with lenvatinib (4 μM) and HGF (50 ng/ml) for another 24 h. The expression levels of epithelial–mesenchymal transition (EMT)-related proteins in the treated HCC cells were measured by Western blotting. The siRNA sequences were as follows: c-MET siRNA, 5′-GUGCCACUAACUACAUUUATT-3′ and control siRNA, 5′-UUCUCCGAACGUGUCACGUTT-3′ [14].
Statistical analysis
All data are presented as the mean ± standard deviations (SDs). Statistical comparisons between the two groups were conducted with an unpaired Student’s t test using GraphPad Prism 7.0. (*, **, and *** indicate P-values of < 0.05, 0.01, and 0.001, respectively).
Results
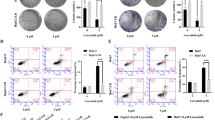
HGF reduced the antiproliferative effect of lenvatinib on HCC cells
Lenvatinib inhibited the proliferation of MHCC97-L and SMMC-7721 HCC cells in a concentration-dependent manner, with IC50 values of 1.81 μM and 1.83 μM, respectively. As shown in Fig. 1, HGF can significantly attenuate the proliferation inhibitory effect of lenvatinib in MHCC97-L cells. Combination treatment with PHA-665752 significantly restored the antiproliferative activity of lenvatinib that was reduced by HGF treatment in MHCC97-L cells but did not significantly affect SMMC-7721 cells.
HGF reduced the antiproliferative activity of lenvatinib in HCC cells. MHCC97-L and SMMC-7721 cells were treated with or without HGF (50 ng/ml) for 24 h and allowed to adhere. The cells were then treated with the indicated concentrations of lenvatinib or PHA-665752 (100 nM) for another 72 h. Cell proliferation was measured with a CCK-8 assay. All data are presented as the mean ± SDs for three independent experiments. *P < 0.05, ***P < 0.001
HGF reduced the proapoptotic effect of lenvatinib on HCC cells
The apoptosis rates of MHCC97-L and SMMC-7721 cells were measured by flow cytometry. As shown in Fig. 2, the apoptosis rates were 46.5% and 36.6% for lenvatinib (4 μM)-treated MHCC97-L and SMMC-7721 cells, respectively. Pretreatment with 50 ng/ml HGF significantly reduced the proapoptotic effect of lenvatinib on MHCC97-L cells, but did not significantly affect SMMC-7721 cells. However, PHA-665752 at a final concentration of 100 nM could restored the proapoptotic effect of lenatinib that reduced by HGF treatment in MHCC97-L cells but did not significantly effect SMMC-7721 cells.
HGF reduced the proapoptotic activity of lenvatinib in HCC cells. MHCC97-L and SMMC-7721 cells were treated with or without HGF (50 ng/ml) for 24 h allowed to adhere. Then, the cells were treated with lenvatinib (4 μM) or PHA-665752 (100 nM) for another 48 h. The apoptosis rate was measured by flow cytometry. The results are representative of three independent experiments. **P < 0.01, ***P < 0.001
HGF reduced the anti-invasive effect of lenvatinib on HCC cells
Transwell assays were used to measure the effect of lenvatinib on inhibiting the invasion of HCC cells. As shown in Fig. 3, lenvatinib (4 μM) markedly reduced the invasive ability of SMMC-7721 and MHCC97-L cells. However, HGF reduced the anti-invasive effect of lenvatinib on MHCC97-L cells, but did not significantly effect SMMC-7721 cells. PHA-665752 restored the anti-invasive effect of lenvatinib that was reduced by HGF treatment in MHCC97-L cells but did not significantly affect SMMC-7721 cells.
HGF reduced the anti-invasive activity of lenvatinib in HCC cells. MHCC97-L and SMMC-7721 cells were treated with or without HGF (50 ng/ml) for 24 h and allowed to adhere. Then, the cells were treated with lenvatinib (4 μM) or PHA-665752 (100 nM) for another 48 h. The invasion ability was measured with a Transwell assay. The results are representative of three independent experiments. ***P < 0.001
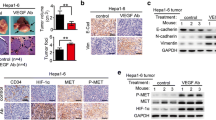
Difference expression of c-MET in HCC cell lines
The protein expression level of c-MET in MHCC97-L and SMMC-7721 cells was measured by Western blotting. As shown in Fig. 4a, c-MET was expressed at high levels in MHCC97-L cells but at low levels in SMMC-7721 cells. These results may partially explain why HGF reduced the antiproliferative, proapoptotic, and anti-invasive effects of lenvatinib in MHCC97-L cells but did not significantly affect SMMC-7721 cells.
Western blot analysis of the expression of c-MET and downstream pathway proteins in HCC cells. a Difference in the protein expression of c-MET in HCC cell lines. b HGF promoted c-MET autophosphorylation and downstream PI3K/AKT and MAPK/ ERK signaling pathways activation in HCC cells. c Knockdown of c-MET by siRNA abolished HGF-induced EMT in lenvatnib-treated HCC cells. The results are representative of two independent experiments
HGF/c-MET-mediated PI3K/AKT and MAPK/ERK pathway activation in HCC cells with high c-Met expression
The expression levels of proteins in pathways underlying HGF/c-MET-mediated drug resistance were measured by Western blotting. As shown in Fig. 4b, HGF treatment markedly increased the phosphorylation of c-MET and the downstream mediators AKT and ERK in MHCC97-L cells. However, PHA-665752 abolished HGF-dependent phosphorylation of c-MET and the downstream mediators AKT and ERK in MHCC97-L cells, which have high c-Met expression. However, neither HGF nor PHA-665752 significantly affected the expression level of c-MET and these downstream pathway proteins in SMMC-7721 cells. These results suggested that the HGF/c-MET axis mediated PI3K/AKT and MAPK/ERK pathway activation in HCC cells with high c-MET expression.
HGF promotes epithelial–mesenchymal transition (EMT) of HCC cells
HGF increased the protein expression level of N-cadherin and Vimentin, and reduced that of E-cadherin in lenvatinib-treated MHCC97-L cells but did not significantly affect lenvatinib-treated SMMC-7721 cells (Fig. 4c). Knockdown of c-MET expression by siRNA reversed EMT in HCC cells (Fig. 4c). These results indicated that the HGF/c-MET axis promoted lenvanitnib resistance via EMT in HCC cells with high c-MET expression.
Discussion
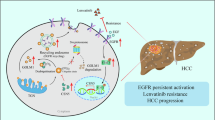
Lenvatinib was recently approved as a first-line treatment for advanced HCC patients in the USA, the European Union, Japan, and China and its clinical utility in advanced HCC has gradually increased [15]. However, some patients in our center who exhibited good initial responses to lenvatinib therapy quickly developed resistance. To extend the overall survival of HCC patients, exploration of the potential resistance mechanisms of lenvatinib in HCC is necessary. The HGF/c-MET axis has been proposed to be a key contributor among multiple resistance mechanisms [16]. In the present study, we focused on HGF-induced lenvatinib resistance and the related pathways contributing to lenvatinib resistance in HCC cells.
We demonstrated that lenvatinib inhibited the proliferation effect of HCC cells in a concentration-dependent manner. Pretreatment of HCC cells with HGF reduced the antiproliferative, proapoptotic, and anti-invasive effects of lenvatinib in MHCC97-L cells but did not significantly affect SMMC-7721 cells. Moreover, the c-MET inhibitor PHA-665752 rescued HGF-induced resistance to these antiproliferative, proapoptotic, and anti-invasive effects in MHCC97-L cells but did not significantly affect this resistance in SMMC-7721 cells. HCC is a highly heterogeneous disease [17]. A meta-analysis suggested that c-MET upregulation is an prognostic factor for recurrence and unfavorable survival in HCC patients who have undergone hepatectomy [18]. As c-MET is the receptor for HGF and the direct target of PHA-665752, we speculated that these inconsistent results derived from the differential expression of c-MET in HCC cell lines. Further Western blot assays confirmed that c-MET is expressed at high levels in MHCC97-L cell but at low levels in SMCC-7721 cells. These results indicated that high c-MET expression may play a critical role in promoting lenvatinib resistance in HCC cell lines.
In the canonical HGF/c-MET pathway, HGF binding results in autophosphorylation of c-MET and downstream activation of the PI3K/AKT and MAPK/ERK pathways. Activation of these pathways promotes cell survival by protecting cells from apoptosis. The PI3K/AKT pathway has been reported to mediate acquired resistance to sorafenib in HCC cells [19]. However, the role of the PI3K/AKT and MAPK/ERK pathways in lenvatinib resistance has not been reported. Our results showed that HGF led to c-MET autophosphorylation and activated the downstream of PI3K/AKT and MAPK/ERK pathways in lenvatinib-treated MHCC97-L cells but not SMMC-7721 cells. In addition, PHA-665752 abrogated HGF/c-MET-mediated downstream PI3K/AKT and MAPK/ERK activation in MHCC97-L cells. These results indicated that HGF-dependent c-MET activation induces lenvatinib resistance by reactivating the downstream PI3K/AKT and MAPK/ERK in HCC cells with high c-MET expression, suggesting that c-MET is a promising treatment target for the reversal of lenvatinib resistance in HCC patients.
EMT is a biological process in which epithelial cells loss polarity and gain of the characteristics of mesenchymal cells with the ability to invade and resist apoptosis [20]. Accumulating evidence has shown that EMT promotes drug resistance in tumor cells [21]. Long-term exposure to a systematic targeted drug could induce EMT in HCC cells, thus increasing invasion and the risk of rebound growth [22]. In this study, we found that HGF promoted EMT in lenvatinib-treated HCC cells with high c-MET expression, and that siRNA-medicated knockdown of c-MET reversed HGF-induced EMT. These results indicated that c-MET is the critical contributor to EMT and is associated with lenvatinib resistance in HCC cells with high c-MET expression.
In summary, our results showed that the HGF/c-MET axis induces lenvatinib resistance by activating the downstream PI3K/AKT and MAPK/ERK pathways and promoting EMT of HCC cells with high c-MET expression. Combination treatment with lenvatinib and a c-MET inhibitor reversed the drug resistance induced by HGF, resulting in improved antitumor effects in HCC cells with high c-MET expression. Our results suggest that c-MET is a predictive biomarker for lenvatinib resistance. Combining lenvatinib treatment with a c-MET inhibitor may improve treatment efficacy HCC patients with high c-MET expression. However, it should be noted that only one HCC cell line with high c-MET expression was tested in our study; future studies are needed to further verify and to promote our findings in clinical application.
References
Kanwal F, Singal AG. Surveillance for hepatocellular carcinoma: current best practice and future direction. Gastroenterology. 2019;157(1):54–64. https://doi.org/10.1053/j.gastro.2019.02.049.
Pinter M, Peck-Radosavljevic M. Review article: systemic treatment of hepatocellular carcinoma. Aliment Pharmacol Ther. 2018;48(6):598–609. https://doi.org/10.1111/apt.14913.
Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33(2):172–9. https://doi.org/10.1200/JCO.2013.54.3298.
Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–75. https://doi.org/10.1200/JCO.2012.45.8372.
Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33(6):559–66. https://doi.org/10.1200/JCO.2013.53.7746.
Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31(28):3517–24. https://doi.org/10.1200/JCO.2012.48.4410.
Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312(1):57–67. https://doi.org/10.1001/jama.2014.7189.
Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73. https://doi.org/10.1016/S0140-6736(18)30207-1.
Bangaru S, Marrero JA, Singal AG. Review article: new therapeutic interventions for advanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2020;51(1):78–89. https://doi.org/10.1111/apt.15573.
Moosavi F, Giovannetti E, Saso L, Firuzi O. HGF/MET pathway aberrations as diagnostic, prognostic, and predictive biomarkers in human cancers. Crit Rev Clin Lab Sci. 2019;56(8):533–66. https://doi.org/10.1080/10408363.2019.1653821.
You WK, McDonald DM. The hepatocyte growth factor/c-Met signaling pathway as a therapeutic target to inhibit angiogenesis. BMB Rep. 2008;41(12):833–9. https://doi.org/10.5483/bmbrep.2008.41.12.833.
Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. 2011;3(1 Suppl):S7–S19. https://doi.org/10.1177/1758834011422556.
Kim KH, Kim H. Progress of antibody-based inhibitors of the HGF-cMET axis in cancer therapy. Exp Mol Med. 2017;49(3):e307. https://doi.org/10.1038/emm.2017.17.
Huang X, Gan G, Wang X, Xu T, Xie W. The HGF-MET axis coordinates liver cancer metabolism and autophagy for chemotherapeutic resistance. Autophagy. 2019;15(7):1258–79. https://doi.org/10.1080/15548627.2019.1580105.
Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: a review in hepatocellular carcinoma. Drugs. 2019;79(6):665–74. https://doi.org/10.1007/s40265-019-01116-x.
Raghav KP, Gonzalez-Angulo AM, Blumenschein GR Jr. Role of HGF/MET axis in resistance of lung cancer to contemporary management. Transl Lung Cancer Res. 2012;1(3):179–93. https://doi.org/10.3978/j.issn.2218-6751.2012.09.04.
Jeng KS, Chang CF, Jeng WJ, Sheen IS, Jeng CJ. Heterogeneity of hepatocellular carcinoma contributes to cancer progression. Crit Rev Oncol Hematol. 2015;94(3):337–47. https://doi.org/10.1016/j.critrevonc.2015.01.009.
Kim JH, Kim HS, Kim BJ, Jang HJ, Lee J. Prognostic value of c-Met overexpression in hepatocellular carcinoma: a meta-analysis and review. Oncotarget. 2017;8(52):90351–7. https://doi.org/10.18632/oncotarget.20087.
Chen KF, Chen HL, Tai WT, Feng WC, Hsu CH, Chen PJ, et al. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther. 2011;337(1):155–61. https://doi.org/10.1124/jpet.110.175786.
Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol Cancer. 2016;15:18. https://doi.org/10.1186/s12943-016-0502-x.
Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–51. https://doi.org/10.1038/onc.2010.215.
van Malenstein H, Dekervel J, Verslype C, Van Cutsem E, Windmolders P, Nevens F, et al. Long-term exposure to sorafenib of liver cancer cells induces resistance with epithelial-to-mesenchymal transition, increased invasion and risk of rebound growth. Cancer Lett. 2013;329(1):74–83. https://doi.org/10.1016/j.canlet.2012.10.021.
Funding
This work was supported by the Medical Science and Technology Research Fund of Guangdong Province, China (Grant Number A2017387).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: HC and XZ; (II) Administrative support: HC and XZ; (III) Provision of study materials: HC and XZ; (IV) Collection and assembly of data: RF, SJ, and JL; (V) Data analysis and interpretation: RF, SJ, and XZ; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, R., Jiang, S., Li, J. et al. Activation of the HGF/c-MET axis promotes lenvatinib resistance in hepatocellular carcinoma cells with high c-MET expression. Med Oncol 37, 24 (2020). https://doi.org/10.1007/s12032-020-01350-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-020-01350-4