Abstract
The purpose of this study was to assess the impact of pentoxifylline and vitamin E on the incidence and severity of radiotherapy-induced oral mucositis and dysphagia in head and neck cancer patients. This is a prospective, randomized, controlled study. Head and neck cancer patients receiving 30–35 radiotherapy fractions with or without concurrent chemotherapy excluding those intolerant to xanthines, with any bleeding tendency were included. Sixty patients were enrolled; 30 patients received radiotherapy (control group) and 30 patients received radiotherapy with pentoxifylline and vitamin E (intervention group). The incidence, severity, onset and duration of oral mucositis and/or dysphagia were assessed. Locoregional control, quality of life, need for hospitalization, radiotherapy breaks, and adverse events were recorded and compared between groups. Pentoxifylline and vitamin E combination did not affect the incidence or the onset of oral mucositis or dysphagia. After adjusting for age, the combination reduced the incidence of severe oral mucositis (p = 0.01) and dysphagia (p = 0.012). The combination decreased the duration of oral mucositis and dysphagia by 5 weeks (p = 0.002) and 4 weeks (p = 0.003), respectively. The study drugs reduced the need for hospitalization (p = 0.002) and for radiotherapy breaks (p = 0.002) with improvement of FOIS (p = 0.014), EQ-5D index (p = 0.009) and VAS score (p = 0.012). Pentoxifylline and vitamin E decreased the occurrence of dysgeusia (p = 0.026) and fatigue (p = 0.026) without compromising locoregional control. Pentoxifylline/vitamin E combination reduced the severity and duration of acute radiotherapy-induced oral mucositis and dysphagia in head and neck cancer patients.
Trial registry ClinicalTrials.gov registration number: NCT02397486.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck cancer is defined as any malignancy arising in the skin, sinuses, nasal or oral cavities, salivary glands, pharynx, and larynx [1]. In 2015, there were 932,000 new cases of head and neck cancer worldwide [2]. Radiotherapy alone or after surgery or combined with chemotherapy is the cornerstone for the management of head and neck cancers [3]. Head and neck cancer radiotherapy is accompanied by a broad spectrum of acute and chronic toxicities [4]. Acute radiotherapy-induced toxicities are a main source of pain and distress to patients, may cause treatment regimen interruptions, develop into late complications and even complicate the course of other comorbidities leading to worse prognosis [5]. Till now, proper oral hygiene and usage of modern conformal radiotherapy techniques are the only available approaches for prevention of radiotherapy-induced oral toxicity. However, the incidence of oral toxicities remains high despite proper oral hygiene and application of conformal techniques [6].
Inhibition of transforming growth factor-beta (TGF-β1) as an inflammatory mediator has become an attractive target for radiation protection [7]. Pentoxifylline is a non-specific phosphodiesterase inhibitor that blocks the transcription of TGF-β1 while vitamin E is an antioxidant with inhibitory effect on the production of TGF-β1 [8]. Pentoxifylline studied in combination with vitamin E showed promising reversing effect on osteoradionecrosis [9]. When administered to lung cancer patients treated with concurrent chemo-radiotherapy, the combination demonstrated considerable reduction in both acute and subacute radiotherapy-induced lung toxicity [8]. The aim of this study was to assess the impact of pentoxifylline and vitamin E combination on the incidence and severity of acute radiotherapy-induced toxicity in head and neck cancer patients.
Materials and methods
The study was a prospective, randomized, open label, controlled study conducted at the Clinical Oncology Department, Ain Shams University Hospitals. The study was approved by the organization ethical committee and registered at ClinicalTrials.gov registry. Eligible patients were adults; aged 18 years or more; diagnosed with squamous cell carcinoma of the head and neck eligible for treatment with radiotherapy. Patients had to sign a written informed consent in accordance with the Declaration of Helsinki. Additional inclusion criteria included; a platelet count more than 100 × 109/L, an absolute neutrophil count greater than 1.5 × 109/L, aspartate aminotransferase level up to 2.5 times the upper limit normal, serum bilirubin level not more than 1.5 times the institutional upper limit normal and serum creatinine levels up to 1.5 mg% and 1.4 mg% for males and females, respectively. Patients were excluded if they were treated with anticoagulants, vitamin E and/or pentoxifylline or patients with recent cerebral and/or retinal hemorrhage or those who have previously exhibited intolerance to pentoxifylline or methylxanthines.
All sixty patients recruited in the study were first stratified according to receipt of cisplatin-based concurrent chemotherapy followed by permuted block randomization using randomly fixed size blocks to either intervention group or control group. Randomization was performed by an independent researcher using computer-generated allocation sequence. Intervention group included 30 patients who received 30–35 fractions of 3D conformal radiotherapy to a total of 60–70 Gy over a period of 6–7 weeks. They also received pentoxifylline 400 mg oral tablets twice daily and vitamin E 1000 mg oral soft gelatin capsules once daily throughout the 6–7 weeks. Twenty of those patients also received concurrent cisplatin 100 mg/m2 every 21 days. Control group included 30 patients who received 30–35 fractions of 3D conformal radiotherapy to a total of 60–70 Gy over a period of 6–7 weeks. Nineteen of those patients received concurrent cisplatin 100 mg/m2 every 21 days. No patients received cetuximab since it was not reimbursed by the hospital.
At baseline, we collected disease history, patients’ demographic data, and computed tomography scans of the head and neck, pelvi-abdominal and chest regions. Patients were followed up on weekly basis up to 90 days since radiotherapy initiation. Patients were interviewed to assess the occurrence and severity of oral mucositis and/or dysphagia (primary outcome). Severity of oral symptoms was graded using the national cancer institute common terminology criteria for adverse events version 4.03 (NCI CTCAE v4.03). Time to onset of oral mucositis and/or dysphagia with the starting date being the day the patient received the first radiotherapy fraction and the date of event is the first day the patient started experiencing oral symptoms and duration till complete resolution of oral symptoms or end of the 90 days of the study were recorded for each patient. Disease locoregional control was assessed clinically and radiologically 6–8 weeks after administration of the last radiotherapy fraction in accordance with the routine standard of care at the study setting. Functional oral intake scale (FOIS) score and the validated Arabic version of the EuroQOL-5D 3 level (EQ-5D-3L) version 5.0 were assessed at baseline and after the 90-day follow-up period [10, 11]. The outcome health states of the EQ-5D-3L questionnaire were converted into EQ-5D Index scores that were used together with patients’ VAS score to describe the patients’ QoL [12]. Adverse events encountered by patients in both groups, need for hospitalization due to oral symptoms, and unplanned radiotherapy break due to oral toxicity were recorded on weekly basis (secondary outcomes).
Data management and analysis were performed using Statistical Package for Social Sciences (SPSS) version 20. The anticipated incidence of acute radiotherapy-induced oral mucositis in head and neck cancer patients was 68% [13]. To detect a reduction in the incidence of oral mucositis to 30% in the intervention group, 24 patients were to be assigned to each group in order to achieve study power of more than 80% and a two-sided type I error less than 0.05.
Parametric numerical data were summarized as means and standard deviations. Non- parametric numerical data were summarized as medians and inter-quartile ranges (IQR). Categorical data were represented as percentages. Two-sided tests were used throughout data analysis with p-values less than 0.05 considered to be statistically significant. Independent t-test was used to compare groups with respect to parametric numerical data. Mann–Whitney U test was used to compare groups with respect to non-parametric numerical data. The χ2 test and Fisher’s exact test were used to compare groups with respect to categorical data. Quade’s test (Rank analysis of co-variance) was used to compare non-parametric data between groups while adjusting for confounders. Binary logistic regression models were used to compare binary outcomes between groups while adjusting for confounders. Wald χ2 test was used to assess the significance of effect of different variables on outcomes within the model while Hosmer and Lemeshow test was used to assess the goodness of fit of the model. Cox regression model was used to compare time to onset of oral symptoms while adjusting for confounders.
Results
From May 2015 to March 2018, a total of ninety head and neck cancer patients were assessed for study eligibility. Only sixty patients were enrolled in the study. At baseline, both groups were comparable in demographic and clinical characteristics except for age upon diagnosis. The means of patients’ ages were 56.57 and 63.53 years in intervention and control groups, respectively (p-value < 0.05). Patients’ demographics and clinical characteristics at baseline are summarized in Table 1.
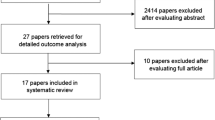
Out of the 60 patients, 4 patients were inevaluable by the end of the 90 day follow-up period due to early death (two patients developed severe methicillin resistant Staphylococcus aureus infections while two patients died of thromboembolic events that were not related to their cancer), one patient dropped out due to noncompliance to follow-up and eight patients did not comply with radiotherapy (Fig. 1). Although addition of pentoxifylline and vitamin E did not significantly affect the incidence and the time to develop any grade dysphagia and/or mucositis, the combination significantly decreased the severity and the duration till complete resolution of oral side effects as shown in Tables 2 and 3. Pentoxifylline and vitamin E reduced the incidence of severe (grade 3 or 4) dysphagia and/or oral mucositis from 36.7% in the control group to 3.3% in the intervention group. The median onsets of dysphagia in the intervention and control group were 3 weeks and 4 weeks, respectively, while those of oral mucositis were 5.5 weeks and 6 weeks, respectively.
In addition to the 13 patients who dropped out of the study during the 90-day follow-up period, two patients were not evaluable for locoregional control due to their death before completion of response assessment. Addition of pentoxifylline and vitamin E did not significantly affect patients’ response to radiotherapy as shown in Table 3. By the end of the 90-day follow-up period, there was a statistically significant difference between the study groups after adjusting for age as a confounder in FOIS score, EQ-5D Index score, and the VAS score of patients in favor of the intervention group (Table 3). The studied drugs significantly decreased the need for hospitalization due to oral side effects from 30% in the intervention group to 6.7% in the intervention group after adjusting for age (Table 2). Pentoxifylline and vitamin E significantly diminished the need for unplanned breaks in radiotherapy (Table 3).
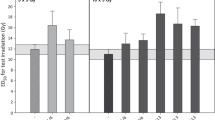
One patient in the intervention group reported grade 3 hoarseness. In the control group, there were 4 reports of grade 3 productive cough, 2 reports of grade 3 hypotension and radiation dermatitis, and 1 report of grade 3 hoarseness, hypokalemia and hypoglycemia. There was a statistically significant difference between the study groups in the incidence of dysgeusia (3.3% in the intervention group vs. 26.7% in the control group, p-value = 0.026) and fatigue (0% in the intervention group vs. 20% in the control group, p-value = 0.024) (Fig. 2).
Discussion
The current study is the first to assess the efficacy of pentoxifylline and vitamin E combination to prevent acute radiotherapy-induced toxicity in head and neck cancer patients. Although the addition of pentoxifylline and vitamin E decreased the incidence of any grade dysphagia and oral mucositis by 10% and 16.7%, respectively, these effects were not statistically significant. This could be attributed to the small sample size of the current study. Similarly, there was no statistically significant difference between the intervention and control groups in onset of dysphagia (3 weeks vs. 4 weeks, respectively) and oral mucositis (5.5 weeks vs. 6 weeks, respectively). However, the studied drugs significantly decreased the incidence of severe (grade 3 or 4) dysphagia and oral mucositis by 33.4%. Pentoxifylline and vitamin E significantly shortened the median duration of dysphagia and oral mucositis till complete resolution of symptoms by 4 weeks and 5 weeks, respectively. The duration of oral mucositis in the control group in this study (5 weeks) was similar to that reported in the literature (37 days) [14].
Locoregional control was evaluated and compared between both study groups to ensure that addition of pentoxifylline and vitamin E did not compromise patients’ response to radiotherapy. There was no statistically significant difference between both groups in locoregional disease control achievement. Despite prior assumption that pentoxifylline can act as a radio-sensitizer via increasing oxygenation of malignant tissue [15], this assumption was not proven in clinical studies [16]. There was a statistically significant difference in FOIS scores in favor of the intervention group. This could be attributed to the lower incidence of severe (grade 3 or 4) dysphagia in the intervention group. FOIS is very sensitive to changes in oral intake ability since it discriminates clearly between feeding tube dependency and the ability to take food by mouth [17].
By the end of the 90-day follow-up period, the median EQ-5D Index score and VAS score were significantly higher in the intervention group than the control group. Previous studies have shown a strong correlation between acute radiotherapy-induced oral symptoms including dysphagia, oral mucositis and dysgeusia and impaired patients’ health related quality of life [18, 19]. The higher incidence and longer duration of severe (grade 3 or 4) oral mucositis and/or severe (grade 3 or 4) dysphagia in addition to the higher incidence of dysgeusia in the control group could have contributed to the worse quality of life measures in this group compared to the intervention group.
The current study showed that pentoxifylline and vitamin E significantly decreased the odds of being hospitalized due to oral symptoms. This could be attributed to the decrease in the severity of oral mucositis and/or dysphagia encountered by patients in the intervention group which decreased the need for patient admission to treat severe oral symptoms and their consequences. Radiotherapy delay or interruption increases the risk of treatment failure [20]. Unplanned radiotherapy breaks were found to be 3.8 times more common among patients suffering from severe oral mucositis [21]. Similarly in the current study, no unplanned radiotherapy breaks occurred in the patients receiving pentoxifylline and vitamin E while 20% of the patients in the control group who suffered from higher incidence of severe oral mucositis needed unplanned radiotherapy breaks.
Pentoxifylline and vitamin E were well tolerated without significant increase in the incidence of adverse effects. Elevated levels of tumor necrosis factor- alpha have been implicated in the pathogenesis of taste alterations occurring in head and neck cancer patients [22]. Being an anti- tumor necrosis factor, pentoxifylline could have decreased the frequency of patient reported dysgeusia in the intervention group. The significant increase in incidence of fatigue in the control group could be a downstream effect of the higher severity of oral symptoms that increase the risk of malnutrition presenting as fatigue. Symptoms attributed to oral mucositis such as copious viscid secretions, and oral pain can affect sleeping pattern leading to development of fatigue.
The results of this study are limited by the heterogeneity of the patients recruited. The lack of a placebo control group contributed to inability to design a double-blinded trial, but the authors were reluctant to use pills filled with edible vegetable oils that might mimic the antioxidant role of vitamin E. The high drop-out rate with the small sample size could have affected the study results.
Conclusion
Pentoxifylline and vitamin E reduced the severity and duration of radiotherapy-induced oral mucositis and dysphagia with significant improvement of oral intake and quality of life. The combination was well tolerated and decreased the need for hospitalization and the need for radiotherapy interruption. The antioxidant properties of vitamin E did not affect tumor response to radiotherapy. Based on the favorable results of this study, we recommend the conduction of randomized, multi-centered trials that assess the effectiveness of pentoxifylline and vitamin E in preventing acute radiotherapy-induced toxicity and their effect on quality adjusted life years and the economic burden of hospitalization. Future studies are needed to assess the impact of pentoxifylline and vitamin E on the incidence and severity of late radiotherapy-induced toxicities.
References
Walden MJ, Aygun N. Head and neck cancer. Semin Roentgenol. 2013;48(1):75–86. https://doi.org/10.1053/j.ro.2012.09.002.
Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–48. https://doi.org/10.1001/jamaoncol.2016.5688.
Mendenhall WM, Dagan R, Bryant CM, Fernandes R. Radiation oncology for head and neck cancer: current standards and future changes. Oral Maxillofac Surg Clin N Am. 2018. https://doi.org/10.1016/j.coms.2018.08.003.
Wuthrick EJ, Zhang Q, Machtay M, Rosenthal DI, Nguyen-Tan PF, Fortin A, et al. Institutional clinical trial accrual volume and survival of patients with head and neck cancer. J Clin Oncol. 2015;33(2):156–64. https://doi.org/10.1200/jco.2014.56.5218.
Andreassen CN, Eriksen JG, Jensen K, Hansen CR, Sørensen BS, Lassen P, et al. IMRT—biomarkers for dose escalation, dose de-escalation and personalized medicine in radiotherapy for head and neck cancer. Oral Oncol. 2018;86:91–9. https://doi.org/10.1016/j.oraloncology.2018.09.001.
Pareek P, Samdariya S, Sharma A, Gupta N, Shekhar S, Kirubakaran R. Pentoxifylline and vitamin E alone or in combination for preventing and treating side effects of radiation therapy and concomitant chemoradiotherapy. Cochrane Database Syst Rev. 2016. https://doi.org/10.1002/14651858.cd012117.
Patyar RR, Patyar S. Role of drugs in the prevention and amelioration of radiation induced toxic effects. Eur J Pharmacol. 2018;819:207–16. https://doi.org/10.1016/j.ejphar.2017.12.011.
Misirlioglu CH, Demirkasimoglu T, Kucukplakci B, Sanri E, Altundag K. Pentoxifylline and alpha-tocopherol in prevention of radiation-induced lung toxicity in patients with lung cancer. Med Oncol. 2007;24(3):308–11. https://doi.org/10.1007/s12032-007-0006-z.
Lyons AJ, Brennan PA. Pentoxifylline—a review of its use in osteoradionecrosis. Br J Oral Maxillofac Surg. 2017;55(3):230–4. https://doi.org/10.1016/j.bjoms.2016.12.006.
Crary MA, Mann GDC, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. 2005;86(8):1516–20. https://doi.org/10.1016/j.apmr.2004.11.049.
EuroQolGroup. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy (Amsterdam, Netherlands). 1990;16(3):199–208.
Jelsma J, Hansen K, de Weerdt W, de Cock P, Kind P. How do Zimbabweans value health states? Popul Health Metrics. 2003;1(1):11. https://doi.org/10.1186/1478-7954-1-11.
Nishimura N, Nakano K, Ueda K, Kodaira M, Yamada S, Mishima Y, et al. Prospective evaluation of incidence and severity of oral mucositis induced by conventional chemotherapy in solid tumors and malignant lymphomas. Support Care Cancer. 2012;20(9):2053–9. https://doi.org/10.1007/s00520-011-1314-6.
Le QT, Kim HE, Schneider CJ, Murakozy G, Skladowski K, Reinisch S, et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo-controlled study. J Clin Oncol. 2011;29(20):2808–14. https://doi.org/10.1200/jco.2010.32.4095.
Moss RW. Do antioxidants interfere with radiation therapy for cancer? Integr Cancer Ther. 2007;6(3):281–92. https://doi.org/10.1177/1534735407305655.
Nieder C, Zimmermann FB, Adam M, Molls M. The role of pentoxifylline as a modifier of radiation therapy. Cancer Treat Rev. 2005;31(6):448–55. https://doi.org/10.1016/j.ctrv.2005.07.007.
Bhatt AD, Goodwin N, Cash E, Bhatt G, Silverman CL, Spanos WJ, et al. Impact of transcutaneous neuromuscular electrical stimulation on dysphagia in patients with head and neck cancer treated with definitive chemoradiation. Head Neck. 2014;37(7):1051–6. https://doi.org/10.1002/hed.23708.
Sroussi HY, Jessri M, Epstein J. Oral assessment and management of the patient with head and neck cancer. Oral Maxillofacl Surg Clin North Am. 2018;30(4):445–58. https://doi.org/10.1016/j.coms.2018.06.006.
Carmignani I, Locatello LG, Desideri I, Bonomo P, Olmetto E, Livi L, et al. Analysis of dysphagia in advanced-stage head-and-neck cancer patients: impact on quality of life and development of a preventive swallowing treatment. Eur Arch Oto-Rhino-Laryngol. 2018;275(8):2159–67. https://doi.org/10.1007/s00405-018-5054-9.
Duncan W, MacDougall RH, Kerr GR, Downing D. Adverse effect of treatment gaps in the outcome of radiotherapy for laryngeal cancer. Radiother Oncol. 1996;41(3):203–7. https://doi.org/10.1016/s0167-8140(96)01838-5.
Russo G, Haddad R, Posner M, Machtay M. Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist. 2008;13(8):886–98. https://doi.org/10.1634/theoncologist.2008-0024.
Murtaza B, Hichami A, Khan AS, Ghiringhelli F, Khan NA. Alteration in taste perception in cancer: causes and strategies of treatment. Front Physiol. 2017. https://doi.org/10.3389/fphys.2017.00134.
Funding
No pharmaceutical or industrial support. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data acquisition and analysis were performed by RS. The first draft of the manuscript was written by RS and LEWl. All authors revised and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (Research Ethics Committee-Ain Shams University) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sayed, R., El Wakeel, L., Saad, A.S. et al. Pentoxifylline and vitamin E reduce the severity of radiotherapy-induced oral mucositis and dysphagia in head and neck cancer patients: a randomized, controlled study. Med Oncol 37, 8 (2020). https://doi.org/10.1007/s12032-019-1334-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-019-1334-5