Abstract
Early growth response-1 (EGR-1), also termed NEFI-A and Krox-24, as a multi-domain protein is implicated in several vital physiological processes, including development, metabolism, cell growth and proliferation. Previous studies have implied that EGR-1 was producing in response to the tissue injury, immune response and fibrosis. Meanwhile, emerging studies stressed the pronounced correlation of EGR-1 and human cancers. Nevertheless, the intricate mechanisms of cancer-reduce EGR-1 alteration still poorly characterized. In the review, we evaluated the effects of EGR-1 in tumor cell proliferation, apoptosis, migration, invasion and tumor microenvironment, and then, we dwell on the intricate signaling pathways that EGR-1 involved in. The aberrantly expressed of EGR-1 in cancers are expected to provide a new cancer therapy strategy or a new marker for assessing treatment efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early growth response-1 (EGR-1) is a member of early growth response proteins family that has been studied in a variety of physiological progress and identified as the downstream molecules of growth factors, hormones, neurotransmitters and metabolite [1]. EGR-1 as a transcription factor is susceptible to hypoxia, fluid shear stress and vascular injury rapidly [2]. Early growth response proteins family is categorized by an identical protein organization that encompasses four family numbers: EGR-1, EGR-2, EGR-3 and EGR-4 [3, 4]. The identical protein organization is characterized by three zinc fingers component conserved regions in the C-terminus achieving interaction with the target genes that harbor specific GC-rich consensus sequences [1]. Transcriptional activation domain, orienting in the N-terminus, holds the binding sites for other proteins that augment the transcriptional control of EGR-1. Linking the activation domain and the DNA-binding domain is the inhibitory domain that provides binding sites for transcriptional co-factors, NAB1 and NAB2, both are inhibitors of EGR-1 biological activation. Besides, CArG elements, a kind of serum response elements (SREs), located in the EGR-1 promoter are essential for radiation and chemotherapy by target the consensus sequence CC(A/T)6GG. Mechanism research indicated that CArG elements also regulated multiple immediate-early genes after stimulated by mitogenic (Fig. 1).
Analogous to EGR-1, transcription factor Sp1 inclines to bind the GC-rich sequence as well. It is tempting to speculate that Sp1 and EGR-1 compete for the overlapping sites of target genes [5]. Initially, EGR-1 was known for the “facilitated inhibition” of Sp1 trans-activation activity for inhibiting Sp1 binding to the GC-rich region [6, 7]. A string of studies have testified that Sp1 transcriptionally activate numerous oncogenes in human cancers cells. Comparatively, EGR-1 is a cancer suppressor for blunting the activation of Sp1 via “facilitated inhibition” effect [8, 9]. Preliminary indication of the effect of EGR-1 in cancers has been made, whereas defined EGR-1 as an anti-oncogene still far less certain. In further studies, EGR-1 has been identified as an early response gene to ionizing radiation. Mechanism analysis manifested that ionizing radiation could increase the transcription of EGR-1 via trigger the transcriptional activation domain, and then, upregulated EGR-1 synergistically intensified tumor cell apoptosis in various parallel signaling pathways [10]. Noteworthy was that EGR-1 boosted PTEN-induced apoptosis after ionizing radiation, following a directly binding site detected in the 5′ non-coding regions of PTEN [11]. CArG elements in the promoter of EGR-1 are the structural basis for synergistic kill effect of EGR-1 after ionizing radiation in cancer cells. For that, a suicide gene therapy vector was built in the method to put EGR-1 promoter in the upstream of GADD45α cDNA. Vitro models indicated that vector suppressed lung cell proliferation combined with resveratrol [12]. In a sense, gene vectors provide new strategies for the treatment of malignant tumors.
A number of heterogeneous natural compounds involved in modulating the biological property of EGR-1 in cancer development (Table 1). Notably, curumian inhibited EGR-1 expression, and kept a significant antitumor effect, while resveratrol promoted cancer cells apoptosis by the way of increasing the expression of EGR-1 [4, 13]. Similarly, a mount of cell-signaling studies provided that EGR-1 was the downstream of MAPK/ERK signal pathway that kept a role in promoting the survival of tumor cells. On the other hand, EGR-1 also induced tumor cells apoptosis via regulating the expression of NAG-1 and PTEN [14,15,16]. Several discrepancy could illustrate the controversial results of EGR-1 in cancer, like the tumor types, some known or unknown signaling pathways as well as specific functions of EGR-1. In our review, we have evaluated the effects of EGR-1 in tumor cell proliferation, apoptosis, migration, invasion and tumor microenvironment, and then summarized the possible signaling events that mediated by EGR-1 in cancers.
EGR-1 in DNA repair mechanisms
p53 gene as an anti-oncogene has been widely discovered by researchers and kept a high correlation with human tumors. Protein p53, a tetrameric transcription factor, was initially described as “the guardian of the genome”. The ability of p53 protein is DNA-damage monitoring and conserving stability. In recent years, anticancer activity of p53-lacked cells provided a new view to tumor treatment. EGR-1 has been detected for the function of inducing p53-lacked prostate cells apoptosis via increasing the expression of tumor necrosis factor-α (TNF-α) [10]. Acting in concert, EGR-1 and p53 combination could potentiate the anticancer efficiency of cisplatin in NSCLC xenografts mice [17]. Meanwhile, EGR-1 and p53 are also essential in the quercetin-mediated colon carcinoma cells apoptosis [18]. p21 represents a major target of p53 activity and a focal point of DNA damage as well as cell cycle arrest. Studies have indicated that p21 was the primary mediator of cell cycle regulation after p53 activation. Choi and coworkers reported that EGR-1 augmented the p21 gene expression, and then induced cancer cell apoptosis independent of p53 in DNA repair processes [19].
p53 gene mutation could be detected in a myriad of human cancers [20]. A study showed that p53 protein 156, 246, 247 and 273 point mutations hold high affinity with EGR-1 activation [21]. Further explorations on prostate cancer have indicated that mutant p53 initiated ERK1/2-mediated upregulation of EGR-1, in turn, a feedback loop of EGR-1/EGFR/ERK also detected [22]. Taken together, these findings showed that EGR-1 made a central role in DNA repair mechanisms under the physiological conditions, however, EGR-1 exacerbated the tumor progression after p53 mutation.
EGR-1 augments cell proliferation
Cell proliferation is a dynamic process that relies on various growth factors. High expression of growth factor and its receptor is a characteristic change of cancer cells. The earlier data demonstrated that upregulation of EGR-1 has been recognized as a potent prostate cancer event. Moreover, the protein level of EGR-1 in prostate cancer tissues kept positive correlation with Gleason scores and remarkable increase could be detected in 8–10 scores patients than those at lower Gleason scores [23]. Similar conclusion also appropriated in gastric cancer, for that EGR-1 at a high level when the patients diagnosed at malignant histological grade [24]. MAPK/ERK pathway is a classical proliferation signaling pathway which is triggered by growth factors. Treated with the MAPK/ERK pathway inhibitor, PD98059, EGR-1 expression level significantly reduced in vitro test, which implied that EGR-1 was the downstream gene of the MAPK/ERK pathway [25]. Furthermore, evidence has revealed that attenuated the nuclear fraction of EGR-1 pronounced suppressed breast cancer cells survival by means of inhibiting MAPK phosphorylation [26]. It was worth mentioning, transient overexpression of EGR-1 not only reinforced tumor growth, but also activated the p38 MAPK-signaling pathway [27].
Cyclin D1, a cell cycle regulatory molecule, induces cell proliferation via promoting G1 phase into S phase. Mitogen might increase cyclin D1 expression, utilizing the ERK signaling pathway activation [28]. Besides, bombesin-induced cell proliferation has been identified related to the activation of the MAPK pathway. Activated MAPK pathway enhanced the interaction of EGR-1 and cyclin D1, and then increased the cyclin D1 protein level in prostate cancer cells [29, 30]. Similarly, JNKs, another member of the MAPK family, also increased the expression of EGR-1 [31].
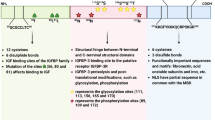
In principle, the mechanism of EGR-1 in cancer cell proliferation is a circular process. As the first step, EGR-1 as well as numerous growth factor activates the MAPK/ERK signaling pathway, then the activated MAPK/ERK signaling pathway further strengthen the expression of EGR-1. Up-regulated EGR-1 promotes cell proliferation via regulation cell cycle protein expression, and continue to enhance the activation of MAPK/ERK-signaling pathway by a positive feedback loop. Need to supplement, other growth factors, like insulin-like growth factor-1 (IGF-1) and its receptor, also hold the mobilizing function of the MAPK/ERK signaling pathway (Fig. 2).
EGR-1 promotes cell apoptosis
Apoptosis is a procession of programmed cell death, which occurs in physiological condition. Following the awareness of the anticancer functions of nonsteroidal anti-inflammatory drugs (NSAIDs), the underlying mechanisms have been extensively explored. Without rival, the NSAID-activated gene-1 (NAG-1) is a crucial regulator in NSAIDs-mediated multiform tumor cell growth arrest, whether rely on COX-2 or not, which belongs to the transforming growth factor-β (TGF-β) superfamily. Studies have detected an EGR-1-binding site in the promoter of NAG-1. Meanwhile, EGR-1 expression obviously facilitated NAG-1-mediated colon carcinoma cells, lung cancer cells and hepatocellular carcinoma cells apoptosis [32, 33]. Importantly, NSAIDs could directly up-regulate EGR-1-mediated NAG-1 expression, and then promoted cell apoptosis in a COX-2-independent manner. By either route, NSAIDs induced apoptosis on a COX-2-dependent manner by driving the activation of the PPARγ/EGR-1/NAG-1 signal pathway [34]. It is worth mentioned, amounts of natural compounds, drugs and molecules keep ability of anticancer via the EGR-1/NAG-1-mediated cell apoptosis (Table 2).
Phosphatase and tensin homolog (PTEN) is an important tumor suppressor gene. Virolle T and partners have found an Egr-1-binding site in the PTEN 5′-untranslated region. This suggests that EGR-1 could regulate PTEN expression and trigger cancer cells apoptotic via target the promoter of it. In breast cancer, studies indicated insulin-like growth factor-II (IGF-II) increased EGR-1-mediated PTEN expression [35]. Analogously, unconjugated bilirubin (UCB) activated APE1/Ref-1 pathway, and then promoted EGR-1 transcriptional regulated PTEN expression [36]. Vitamin D induced apoptosis of cancer cells via increasing PTEN expression in a vitamin D receptor, Egr-1 and p300 synergistic manner [37].
EGR-1 regulates cell metastasis
In malignant carcinomas, distant metastasis is always concurrent with poor prognosis. Originally, epithelial–mesenchymal transition (EMT) was discerned in embryogenesis. Through the multiple strategies and tools displayed by cancer cells to gain metastasis advantages, EMT is one of the most concealed. In the tumor progress, EMT refers to epithelial cells not curbing their movements in the epithelial cell layer and orienting to the mesenchymal cells layer, following significant morphology changes that from epithelial to mesenchymal morphology. Given that premise, EMT has been extensively delved into the regulation of cell invasion, intravasation and systemic dissemination. EMT-TFs, a series of transcription factors, orchestrate the EMT programs [38]. E-cadherin is an EMT-TF with extensive research as the key regulator to maintain cell–cell adhesion and its repression promotes cells break through the basement membrane. Similarly, Snail is indicated to enhance the expressions of mesenchyme genes, such as matrix metalloproteinase-9 (MMP9) and zincfinger ebox binding homeobox 1 (ZEB1), or reduce the protein level of epithelium marker [39]. CXCL5/ENA78, a CXC-type chemokine, is capable of promoting the expression of Snail via increasing Raf/MEK/ERK pathway-mediated EGR-1 transcription in the hormone-independent prostate cancer [40]. In ovarian cancer cell, epidermal growth factor (EGF) not only increases the invasive capability by the way of p38 MAPK-triggered Snail upregulation, but also upregulates Slug via ERK1/2 and PI3 K/Akt-mediated EGR-1 overexpression (Fig. 3) [41].
Besides EMT, cancer stem cells (CSCs) are another modulator of tumors distant metastasis. CSCs also termed as the tumor-initiating cells, have been widely detected in cells aggression and self-renewal, which kept high correlation with the progression of dormancy, colonization and secondary tumor formation in cancer metastasis. In the hypoxia condition, EGR-1 could inhibit the growth of breast cancer stem spheroids [42].
Additionally, Shao G investigated the regulation ability of EGR-1 in prostate cancer and its relationship to metastasis. It showed inactivating EGR-1 may attenuate IL-6-related prostate cancer metastases through weakening PI3 K/PTEN/Akt signaling pathway [43]. As a pleiotropic transcription factor, EGR-1 also promoted ionizing radiation-induced EMT in non-small cell lung cancer cells via the mut-p53/Egr-1/cathepsin L axis [44]. This suggests that the metastatic cascade is an especially complex, multipotent and high-synergistic biological process, rather than assembling one molecule after another.
EGR-1 in tumor microenvironment
Stephen Paget described the interplay between tumor and its microenvironment using the “seed and soil” theory in 1889. That is to say, the tumor cells was the seed that rooted in the tumor microenvironment.
Angiogenesis is an important physiology and pathology process in wound healing and tissue repair. Besides, angiogenesis also accelerated tumors getting the malignant characteristic and angiogenesis inhibitors have been extensively used in the therapeutic of cancers [45]. 5-Fluorouracil (5-FU), a potent anticancer drug, is commonly used in solid tumors chemotherapy. Thrombospondin-1 (TSP-1) has emerged as an angiogenesis suppressor for that inhibiting vascular endothelial growth factor (VEGF) and matrix metalloproteinase-9 (MMP9) expression, promoting endothelial cell apoptosis, and attenuating circulating endothelial cell progenitors. Increasing evidence verified that 5-FU augmented the p38 MAPK pathway mediated EGR-1 up-regulation, and then EGR-1 enhanced TSP-1 gene transcription by the way of identifying the transcriptional regulatory element in its promoter in human colon cells [46, 47]. Moreover, EGR-1 could increase the angiogenic growth factors basic fibroblast growth factor (bFGF) and VEGF transcription directly. Nevertheless, sustained expression of EGR-1 led a feedback inhibit of bFGF and VEGF expression that regulated by the corepressor NAB2 [48]. Besides, fibroblast growth factor 2 (FGF-2) is a key downstream target of EGR-1 as well that has been widely studied in anti-angiogenic therapies [49, 50].
Hypoxia is a powerful stimulator of angiogenesis that accompany with significant heterogeneity in endothelial cells. Hypoxia-inducible factors (HIFs) primarily activated when cells sensed the low oxygen tension, and then up-regulated HIFs mediated the transcriptional responses to hypoxia [51]. To improve intratumoral hypoxia, HIFs enhanced the expression of VEGF family members that promoted neovascularization through formation new capillaries from preexisting vessels, maintaining tumor cells survival in a hostile microenvironment [52,53,54]. Further exploration indicated that EGR-1 induced by ERK1/2 pathway augmented HIF-1α-mediated VEGF-A expression in the hypoxic microenvironment. Besides, EGR-1 directly activated VEGF-A expression by binding to the proximal region of the VEGF-A promoter in lung cancer cells [55]. Lymphatic endothelial cells (LECs) proliferation and migration is another vital functional response to hypoxia. EGR-1 has been reported involved in hypoxia-induced lymphangiogenesis via VEGF signaling cascades. However, the underlying molecular mechanism of EGR-1 in lymphangiogenesis still needs further studies. [56]. TNFα is an inflammatory mediator that secreted by macrophagocyte in hypoxic, and then increases EGR-1-transactivated GROα and MMP-9 expression within the tumor microenvironment [57, 58] (Fig. 4).
EGR-1 potentiates tumor treatment
Serum response elements (SRFs), a member of MADS box family, regulates various genes associated with cell growth and differentiation [59]. Deletion analysis provided insights that murine EGR-1 promoter holds two regions that kept two SREs. CArG element is the core of SREs [60]. Studies exhibited that up-regulation of EGR-1 by X-rays was conferred by CArG elements. Moreover, ROIs facilitated EGR-1 expression via activating CArG elements [61]. Seung LP and his companions ligated the CArG elements from EGR-1 promoter to the transcriptional start site of the human TNF cDNA and then this construct was transfected into tumor cell lines via cationic liposomes. Results showed, together with ionizing radiation or anticancer drugs, this genetic radiotherapy or chemo-inducible gene therapy could overcome tumor resistance to cytotoxic agents [62, 63]. Analogously, combination of hypoxia responsive elements (HREs) from the erythropoietin (EPO) and CArG elements from EGR-1 formed a novel chimeric gene promoter. Using such chimeric promoter may effectively address the problem of hypoxia in radiotherapy in cancer cells [64]. Wang WD constructed a special adenoviral vector including CArG elements and the upstream of the human wt-p53 gene. An enhanced antitumor response has been detected in the human NSCLC cells xenografts mice when treatment with AdEgr-p53 and cisplatin synchronously [65].
In conclusion, studies confirmed the potential antineoplastic effects of gene therapy vectors. Synergistically with radiation therapy and chemotherapy, the vector could achieve better therapeutic effects and lower normal tissues damage. These findings not only lead to a better understanding of the mechanism, but also shed light on the potential new strategy for developing treatment of cancer patients.
Conclusion
A worldwide epidemic of cancer-associated disaster is expected to come. The detection of molecular interaction in tumors may make a new point to the discovery of new therapeutic targets. Activation of EGR-1 is essential in normal cell growth, but the exact role kept on the tumorigenesis is unclear. EGR-1 is the key molecule in many signal pathways, and it is conceivable that some of these hold the tumor-promoting effects, whereas others are used to reduce tumor cells survival. Aberrant expression of EGR-1 seems commonly in a mass of human cancers. Undoubtedly, elucidation of the underlying molecular mechanism of EGR-1 signaling crosstalk and specific regulators will be necessary for effective anticancer strategies.
Our review sets out the case for EGR-1 as “friend” in various visual angles. Primarily, EGR-1 is “the guardian of the genome” in p53-lacked prostate cells via inducing TNFα activation, or cooperates with p53 gene promoting the anticancer efficiency of cisplatin as well as quercentin under the physiologic motion. Besides, EGR-1 potentiates the tumor cells apoptosis via upregulating tumor suppressors, NAG-1 and PTEN directly. Next, EGR-1 could inhibit the growth of tumor stem spheroids under low oxygen tension. Furthermore, EGR-1 augments the anticancer effects of 5FU by the way of TSP-1-mediated anti-angiogenesis in solid tumors. Most notably, the CArG elements located in EGR-1 promoter are essential for the construction of suicide gene vector and increase the radiation and chemotherapy sensitivity in vitro. We also discuss the “foes” manners of EGR-1 that are potent correlated with hostile microenvironment and pro-survival function. As the downstream molecule of MAPK-signaling pathway, EGR-1 could be upregulated by a vast of growth factors in several cancer cells, and then promote the transcriptional activation of cyclinD1 to maintain tumor cells mitosis. Meanwhile, EGR-1 also augments tumor metastasis via EGR-1-induced Slug and Snail expression. In a consistent manner, EGR-1 facilitates HIF-1α-mediated VEGF-A expression or directly activated VEGF-A transcription to enhance the angiogenesis and lymphangiogenesis in intratumoral hypoxia microenvironment. To conclude, EGR-1 is anti-oncogenes that monitor DNA-damage conserving, promotes tumor cells apoptosis, and adjuvant increases anticancer efficiency of radiotherapy and chemotherapy. However, at the hostile environment, the EGR-1 expression level increased to maintain tumor cell survival, proliferation, metastasis and angiogenesis as an oncogene. Identifying and utilizing the “foes” role of EGR-1 in cancer could lead an opening of horizons for the gene treatment in patients with malignant tumors.
References
Bhattacharyya S, Fang F, Tourtellotte W, Varga J. Egr-1: new conductor for the tissue repair orchestra directs harmony (regeneration) or cacophony (fibrosis). J Pathol. 2013;229(2):286–97. https://doi.org/10.1002/path.4131.
Pagel JI, Deindl E. Disease progression mediated by egr-1 associated signaling in response to oxidative stress. Int J Mol Sci. 2012;13(10):13104–17. https://doi.org/10.3390/ijms131013104.
Braddock M. The transcription factor Egr-1: a potential drug in wound healing and tissue repair. Ann Med. 2001;33(5):313–8.
Thiel G, Rossler OG. Resveratrol regulates gene transcription via activation of stimulus-responsive transcription factors. Pharmacol Res. 2017;117:166–76. https://doi.org/10.1016/j.phrs.2016.12.029.
Nag JK, Bar-Shavit R. Transcriptional landscape of PARs in epithelial malignancies. Int J Mol Sci. 2018;19(11):E3451. https://doi.org/10.3390/ijms19113451.
Rettino A, Rafanelli F, Genovese G, Goracci M, Cifarelli RA, Cittadini A, Sgambato A. Identification of Sp1 and GC-boxes as transcriptional regulators of mouse Dag1 gene promoter. Am J Physiol Cell Physiol. 2009;297(5):C1113–23. https://doi.org/10.1152/ajpcell.00189.2009.
Li H, Chen D, Zhang J. Analysis of intron sequence features associated with transcriptional regulation in human genes. PLoS ONE. 2012;7(10):e46784. https://doi.org/10.1371/journal.pone.0046784.
Courey AJ, Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988;55(5):887–98.
Huang RP, Fan Y, Ni Z, Mercola D, Adamson ED. Reciprocal modulation between Sp1 and Egr-1. J Cell Biochem. 1997;66(4):489–99.
Ahmed MM, Sells SF, Venkatasubbarao K, et al. Ionizing radiation-inducible apoptosis in the absence of p53 linked to transcription factor EGR-1. J Biol Chem. 1997;272:33056–61.
Virolle T, Adamson ED, Baron V, et al. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat Cell Biol. 2001;3:1124–8. https://doi.org/10.1038/ncb1201-1124.
Shi Q. Resveratrol-responsive CArG elements from the Egr-1 promoter for the induction of GADD45α to arrest the G2/M transition. Methods Mol Biol. 2019;1895:111–22. https://doi.org/10.1007/978-1-4939-8922-5_9.
Wong KE, Ngai SC, Chan KG, et al. Curcumin nanoformulations for colorectal cancer: a review. Front Pharmacol. 2019;10:152. https://doi.org/10.3389/fphar.2019.00152.
Auyeung KK, Cho CH. A novel anticancer effect of Astragalus saponins: transcriptional activation of NSAID-activated gene. Int J Cancer. 2009;125:1082–91. https://doi.org/10.1002/ijc.24397.
Secchiero P, Rimondi E, di Iasio MG, et al. C-Reactive protein downregulates TRAIL expression in human peripheral monocytes via an Egr-1-dependent pathway. Clin Cancer Res. 2013;19:1949–59. https://doi.org/10.1158/1078-0432.ccr-12-3027.
Thiel J, Alter C, Luppus S, et al. MicroRNA-183 and microRNA-96 are associated with autoimmune responses by regulating T cell activation. J Autoimmun. 2019;96:94–103. https://doi.org/10.1016/j.jaut.2018.08.010.
Wang WD, Li R, Chen ZT, et al. Cisplatin-controlled p53 gene therapy for human non-small cell lung cancer xenografts in athymic nude mice via the CArG elements. Cancer Sci. 2005;96:706–12. https://doi.org/10.1111/j.1349-7006.2005.00105.x.
Lim JH, Park JW, Min DS, et al. NAG-1 up-regulation mediated by EGR-1 and p53 is critical for quercetin-induced apoptosis in HCT116 colon carcinoma cells. Apoptosis. 2007;12:411–21. https://doi.org/10.1007/s10495-006-0576-9.
Choi BH, Kim CG, Bae YS, Lim Y, Lee YH, Shin SY. p21 Waf1/Cip1 expression by curcumin in U-87MG human glioma cells: role of early growth response-1 expression. Cancer Res. 2008;68(5):1369–77. https://doi.org/10.1158/0008-5472.can-07-5222.
Purohit V, Rapaka R, Kwon OS, Song BJ. Roles of alcohol and tobacco exposure in the development of hepatocellular carcinoma. Life Sci. 2013;92(1):3–9. https://doi.org/10.1016/j.lfs.2012.10.009.
Liu J, Grogan L, Nau MM, Allegra CJ, Chu E, Wright JJ. Physical interaction between p53 and primary response gene Egr-1. Int J Oncol. 2001;18(4):863–70. https://doi.org/10.3892/ijo.18.4.863.
Sauer L, Gitenay D, Vo C, Baron VT. Mutant p53 initiates a feedback loop that involves Egr-1/EGF receptor/ERK in prostate cancer cells. Oncogene. 2010;29(18):2628–37. https://doi.org/10.1038/onc.2010.24.
Eid MA, Kumar MV, Iczkowski KA, Bostwick DG, Tindall DJ. Expression of early growth response genes in human prostate cancer. Cancer Res. 1998;58(11):2461–8.
Park SY, Kim JY, Lee SM, et al. Expression of early growth response gene-1 in precancerous lesions of gastric cancer. Oncol Lett. 2016;12:2710–5. https://doi.org/10.3892/ol.2016.4962.
Schwachtgen JL, Houston P, Campbell C, et al. Fluid shear stress activation of egr-1 transcription in cultured human endothelial and epithelial cells is mediated via the extracellular signal-related kinase 1/2 mitogen-activated protein kinase pathway. J Clin Invest. 1998;101:2540–9. https://doi.org/10.1172/jci1404.
Burnatowska-Hledin MA, Kossoris JB, Van Dort CJ, et al. T47D breast cancer cell growth is inhibited by expression of VACM-1, a cul-5 gene. Biochem Biophys Res Commun. 2004;319:817–25. https://doi.org/10.1016/j.bbrc.2004.05.057.
Park SY, Kim JY, Lee SM, Chung JO, Lee KH, Jun CH, Lee YH. Expression of early growth response gene-1 in precancerous lesions of gastric cancer. Oncol Lett. 2016;12(4):2710–5. https://doi.org/10.3892/ol.2016.4962.
Lei B, Sun S, Zhang X, Feng C, Xu J, Wen Y, Yu Y. Bisphenol AF exerts estrogenic activity in MCF-7cells through activation of Erk and PI3 K/Akt signals via GPER signaling pathway. Chemosphere. 2019;220:362–70. https://doi.org/10.1016/j.chemosphere.2018.12.122.
Stangelberger A, Schally AV, Varga JL, Zarandi M, Cai RZ, Baker B, Kanashiro CA. Inhibition of human androgen-independent PC-3 and DU-145 prostate cancers by antagonists of bombesin and growth hormone releasing hormone is linked to PKC, MAPK and c-jun intracellular signalling. Eur J Cancer. 2005;41(17):2735–44. https://doi.org/10.1016/j.ejca.2005.08.022.
Xiao D, Chinnappan D, Pestell R, Albanese C, Weber HC. Bombesin regulates cyclin D1 expression through the early growth response protein Egr-1 in prostate cancer cells. Cancer Res. 2005;65(21):9934–42. https://doi.org/10.1158/0008-5472.can-05-1830.
Jablonski SA, Robinson-Drummer PA, Schreiber WB, Asok A. Impairment of the context preexposure facilitation effect in juvenile rats by neonatal alcohol exposure is associated with decreased Egr-1 mRNA expression in the prefrontal cortex. Behav Neurosci. 2018;132(6):497–511. https://doi.org/10.1037/bne0000272.
Chen YL, Lin PC, Chen SP, Lin CC, Tsai NM, Cheng YL, Harn HJ. Activation of nonsteroidal anti-inflammatory drug-activated gene-1 via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase revealed a isochaihulactone-triggered apoptotic pathway in human lung cancer A549 cells. J Pharmacol Exp Ther. 2007;323(2):746–56. https://doi.org/10.1124/jpet.107.126193.
Shin DY, Kim GY, Kim ND, Jung JH, Kim SK, Kang HS, Choi YH. Induction of apoptosis by pectenotoxin-2 is mediated with the induction of DR4/DR5, Egr-1 and NAG-1, activation of caspases and modulation of the Bcl-2 family in p53-deficient Hep3B hepatocellular carcinoma cells. Oncol Rep. 2008;19(2):517–26.
Chintharlapalli S, Papineni S, Baek SJ, et al. 1,1-Bis(3’-indolyl)-1-(p-substitutedphenyl)methanes are peroxisome proliferator-activated receptor gamma agonists but decrease HCT-116 colon cancer cell survival through receptor-independent activation of early growth response-1 and nonsteroidal anti-inflammatory drug-activated gene-1. Mol Pharmacol. 2005;68:1782–92. https://doi.org/10.1124/mol.105.017046.
Moorehead RA, Hojilla CV, De Belle I, et al. Insulin-like growth factor-II regulates PTEN expression in the mammary gland. J Biol Chem. 2003;278:50422–7. https://doi.org/10.1074/jbc.m306894200.
Cesaratto L, Calligaris SD, Vascotto C, et al. Bilirubin-induced cell toxicity involves PTEN activation through an APE1/Ref-1-dependent pathway. J Mol Med (Berl). 2007;85:1099–112. https://doi.org/10.1007/s00109-007-0204-3.
Pan L, Matloob AF, Du J, et al. Vitamin D stimulates apoptosis in gastric cancer cells in synergy with trichostatin A/sodium butyrate-induced and 5-aza-2’-deoxycytidine-induced PTEN upregulation. FEBS J. 2010;277:989–99. https://doi.org/10.1111/j.1742-4658.2009.07542.x.
Scheel C. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012;22:396–403. https://doi.org/10.1016/j.semcancer.2012.04.001.
Du J. High PKD2 predicts poor prognosis in lung adenocarcinoma via promoting Epithelial-mesenchymal Transition. Sci Rep. 2019;9(1):1324. https://doi.org/10.1038/s41598-018-37285-0.
Kuo PL, Chen YH, Chen TC, Shen KH, Hsu YL. CXCL5/ENA78 increased cell migration and epithelial-to-mesenchymal transition of hormone-independent prostate cancer by early growth response-1/snail signaling pathway. J Cell Physiol. 2011;226(5):1224–31. https://doi.org/10.1002/jcp.22445.
Cheng JC, Chang HM, Leung PC. Egr-1 mediates epidermal growth factor-induced downregulation of E-cadherin expression via Slug in human ovarian cancer cells. Oncogene. 2013;32(8):1041–9. https://doi.org/10.1038/onc.2012.127.
Forte E, Chimenti I, Rosa P, et al. EMT/MET at the crossroad of stemness, regeneration and oncogenesis: the Ying-Yang Equilibrium recapitulated in cell spheroids. Cancers (Basel). 2017. https://doi.org/10.3390/cancers9080098.
Shao G, Liu Y, Ma T, et al. GCN5 inhibition prevents IL-6-induced prostate cancer metastases through PI3 K/PTEN/Akt signaling by inactivating Egr-1. Biosci Rep. 2018. https://doi.org/10.1042/bsr20180816.
Wang W, Xiong Y, Ding X, et al. Cathepsin L activated by mutant p53 and Egr-1 promotes ionizing radiation-induced EMT in human NSCLC. J Exp Clin Cancer Res. 2019;38:61. https://doi.org/10.1186/s13046-019-1054-x.
Wang LF, Liu YS, Yang B, Li P, Cheng XS, Xiao CX, Guleng B. The extracellular matrix protein mindin attenuates colon cancer progression by blocking angiogenesis via Egr-1-mediated regulation. Oncogene. 2018;37(5):601–15. https://doi.org/10.1038/onc.2017.359.
Ren B, Yee KO, Lawler J. Regulation of tumor angiogenesis by thrombospondin-1. Biochim Biophys Acta. 2006;1765:178–88. https://doi.org/10.1016/j.bbcan.2005.11.002.
Zhao HY, Ooyama A, Yamamoto M, et al. Molecular basis for the induction of an angiogenesis inhibitor, thrombospondin-1, by 5-fluorouracil. Cancer Res. 2008;68:7035–41. https://doi.org/10.1158/0008-5472.can-07-6496.
Lucerna M, Pomyje J, Mechtcheriakova D, et al. Sustained expression of early growth response protein-1 blocks angiogenesis and tumor growth. Cancer Res. 2006;66:6708–13. https://doi.org/10.1158/0008-5472.can-05-2732.
Ganesan P, Matsubara K, Sugawara T. Marine algal carotenoids inhibit angiogenesis by down-regulating FGF-2-mediated intracellular signals in vascular endothelial cells. Mol Cell Biochem. 2013;380:1–9. https://doi.org/10.1007/s11010-013-1651-5.
Brown KC, Lau JK, Dom AM, et al. MG624, an α7-nAChR antagonist, inhibits angiogenesis via the Egr-1/FGF2 pathway. Angiogenesis. 2012;15:99–114. https://doi.org/10.1007/s10456-011-9246-9.
Eyries M, Siegfried G, Ciumas M, et al. Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ Res. 2008;103:432–40. https://doi.org/10.1161/circresaha.108.179333.
Shweiki D, Itin A, Soffer D. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–5. https://doi.org/10.1038/359843a0.
Michiels C, Arnould T. Endothelial cell responses to hypoxia: initiation of a cascade of cellular interactions. Biochim Biophys Acta. 2000;1497:1–10.
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:3721–32.
Ji RC. Hypoxia and lymphangiogenesis in tumor microenvironment and metastasis. Cancer Lett. 2014;346(1):6–16. https://doi.org/10.1016/j.canlet.2013.12.001.
Shimoyamada H, Yazawa T, Sato H, et al. Early growth response-1 induces and enhances vascular endothelial growth factor-A expression in lung cancer cells. Am J Pathol. 2010;177:70–83. https://doi.org/10.2353/ajpath.2010.091164.
Shin SY, Lee JM, Lim Y, Lee YH. Transcriptional regulation of the growth-regulated oncogene alpha gene by early growth response protein-1 in response to tumor necrosis factor alpha stimulation. Biochim Biophys Acta. 2013;1829(10):1066–74. https://doi.org/10.1016/j.bbagrm.2013.07.005.
Shin SY, Kim JH, Baker A, Lim Y, Lee YH. Transcription factor Egr-1 is essential for maximal matrix metalloproteinase-9 transcription by tumor necrosis factor alpha. Mol Cancer Res. 2010;8(4):507–19. https://doi.org/10.1158/1541-7786.mcr-09-0454.
Spencer JA, Major ML, Misra RP. Basic fibroblast growth factor activates serum response factor gene expression by multiple distinct signaling mechanisms. Mol Cell Biol. 1999;19(6):3977–88. https://doi.org/10.1128/mcb.19.6.3977.
Duan WR, Ito M, Park Y, Maizels ET, Hunzicker-Dunn M, Jameson JL. GnRH regulates early growth response protein 1 transcription through multiple promoter elements. Mol Endocrinol. 2002;16(2):221–33. https://doi.org/10.1210/mend.16.2.0779.
Datta R, Taneja N, Sukhatme VP, Qureshi SA, Weichselbaum R, Kufe DW. Reactive oxygen intermediates target CC(A/T)6GG sequences to mediate activation of the early growth response 1 transcription factor gene by ionizing radiation. Proc Natl Acad Sci USA. 1993;90(6):2419–22. https://doi.org/10.1073/pnas.90.6.2419.
Seung LP, Mauceri HJ, Beckett MA, Hallahan DE, Hellman S, Weichselbaum RR. Genetic radiotherapy overcomes tumor resistance to cytotoxic agents. Cancer Res. 1995;55(23):5561–5.
Bickenbach KA, Veerapong J, Shao MY, Mauceri HJ, Posner MC, Kron SJ, Weichselbaum RR. Resveratrol is an effective inducer of CArG-driven TNF-alpha gene therapy. Cancer Gene Ther. 2008;15(3):133–9. https://doi.org/10.1038/sj.cgt.7701103.
Greco O, Marples B, Dachs GU, Williams KJ, Patterson AV, Scott SD. Novel chimeric gene promoters responsive to hypoxia and ionizing radiation. Gene Ther. 2002;9(20):1403–11. https://doi.org/10.1038/sj.gt.3301823.
Wang WD, Li R, Chen ZT, Li DZ, Duan YZ, Cao ZH. Cisplatin-controlled p53 gene therapy for human non-small cell lung cancer xenografts in athymic nude mice via the CArG elements. Cancer Sci. 2005;96(10):706–12. https://doi.org/10.1111/j.1349-7006.2005.00105.x.
Chen A, Xu J. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25:278–87. https://doi.org/10.1038/sj.onc.1209019.
Chen QY, Jiao DM, Wang LF, et al. Curcumin inhibits proliferation-migration of NSCLC by steering crosstalk between a Wnt signaling pathway and an adherens junction via EGR-1. Mol BioSyst. 2015;11:859–68. https://doi.org/10.1039/c4mb00336e.
Yang MH, Kim J, Khan IA, et al. Nonsteroidal anti-inflammatory drug activated gene-1 (NAG-1) modulators from natural products as anti-cancer agents. Life Sci. 2014;100:75–84. https://doi.org/10.1016/j.lfs.2014.01.075.
Jeung YJ, Kim HG, Ahn J, et al. Shikonin induces apoptosis of lung cancer cells via activation of FOXO3a/EGR1/SIRT1 signaling antagonized by p300. Biochim Biophys Acta. 2016;1863:2584–93. https://doi.org/10.1016/j.bbamcr.2016.07.005.
Xia Y, Lian S, Khoi PN, et al. Chrysin inhibits cell invasion by inhibition of Recepteur d’origine Nantais via suppressing early growth response-1 and NF-κB transcription factor activities in gastric cancer cells. Int J Oncol. 2015;46:1835–43. https://doi.org/10.3892/ijo.2015.2847.
Han MH, Kim GY, Yoo YH. Sanguinarine induces apoptosis in human colorectal cancer HCT-116 cells through ROS-mediated Egr-1 activation and mitochondrial dysfunction. Toxicol Lett. 2013;220:157–66. https://doi.org/10.1016/j.toxlet.2013.04.020.
Zcharia E, Atzmon R, Nagler A, et al. Inhibition of matrix metalloproteinase-2 by halofuginone is mediated by the Egr1 transcription factor. Anticancer Drugs. 2012;23:1022–31. https://doi.org/10.1097/cad.0b013e328357d186.
Baek SJ, Kim JS, Nixon JB, et al. Expression of NAG-1, a transforming growth factor-beta superfamily member, by troglitazone requires the early growth response gene EGR-1. J Biol Chem. 2004;279:6883–92. https://doi.org/10.1074/jbc.m305295200.
Baek SJ, Kim JS, Nixon JB, et al. Expression of NAG-1, a transforming growth factor-beta superfamily member, by troglitazone requires the early growth response gene EGR-1. J Biol Chem. 2004;279(8):6883–92. https://doi.org/10.1074/jbc.m305295200.
Chen YL, Lin PC, Chen SP, et al. Activation of nonsteroidal anti-inflammatory drug-activated gene-1 via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase revealed a isochaihulactone-triggered apoptotic pathway in human lung cancer A549 cells. J Pharmacol Exp Ther. 2007;323:746–56. https://doi.org/10.1124/jpet.107.126193.
Chiu SC, Wang MJ, Yang HH, et al. Activation of NAG-1 via JNK signaling revealed an isochaihulactone-triggered cell death in human LNCaP prostate cancer cells. BMC Cancer. 2011;11:146. https://doi.org/10.1186/1471-2407-11-146.
Shin DY, Kim GY, Li W, et al. Implication of intracellular ROS formation, caspase-3 activation and Egr-1 induction in platycodon D-induced apoptosis of U937 human leukemia cells. Biomed Pharmacother. 2009;63:86–94. https://doi.org/10.1016/j.biopha.2008.08.001.
Auyeung KK. Coptis chinensis inhibits hepatocellular carcinoma cell growth through nonsteroidal anti-inflammatory drug-activated gene activation. Int J Mol Med. 2009;24:571–7.
Shin SY, Kim JH, Lee JH, et al. 2’-Hydroxyflavanone induces apoptosis through Egr-1 involving expression of Bax, p21, and NAG-1 in colon cancer cells. Mol Nutr Food Res. 2012;56:761–74. https://doi.org/10.1002/mnfr.201100651.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 81572349, 81872080), Jiangsu Provincial Medical Talent (ZDRCA2016055), the Science and Technology Department of Jiangsu Province (BK20181148) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Tt., Liu, Mr. & Pei, Ds. Friend or foe, the role of EGR-1 in cancer. Med Oncol 37, 7 (2020). https://doi.org/10.1007/s12032-019-1333-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-019-1333-6