Abstract
To improve the poor prognosis for children with metastatic osteosarcoma (OS), interleukin-2 (IL-2) was added to the standard treatment due to its capacity to activate lymphocytes and differentiate lymphocyte subsets into lymphokine-activated killer (LAK) cells that are capable of recognizing and killing various tumor cells. This study concerns a cohort of unselected patients aged < 18 years with metastatic OS, who were treated with IL-2, high-dose methotrexate, doxorubicin, cisplatin, ifosfamide, LAK reinfusion, and surgery, between 1995 and 2010. Thirty-five patients aged 4–17 years were involved. Thirty-two of the 35 patients underwent surgery on their primary tumor, and 25 had surgery on lung metastases too. Twenty-seven patients received IL-2 plus LAK reinfusion. The median follow-up was 130 months (77–228), and the 3-year event-free and overall survival rates were 34.3 and 45.0%, respectively. Eleven patients remained alive, all of whom achieved a complete surgical removal of the primary tumor and lung metastases (1 patient did not receive lung resections due to complete lung metastases remission). Patients who had a complete surgical remission of the primary and metastatic sites and who responded well to chemotherapy had a better event-free survival. These results confirm the importance of complete surgical remission and point to a noteworthy (though still be ameliorate) survival rate in our series of patients, underling a potential role for immunotherapy with IL-2 and LAK/NK cell activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteosarcoma (OS) is the most common primary bone malignancy in children and young adults. Approximately 10–20% of patients have metastases at the time of their diagnosis, and the most common sites of metastatic disease are the lung (85–90% of all cases), followed by bones. The presence of metastatic disease at onset is a poor prognostic factor and is associated with 5-year survival rates ranging between 10 and 30% [1,2,3,4,5]. Intensifying the chemotherapeutic regimens has proven of limited use in improving the prognosis in cases of metastatic OS, which has reached an unsatisfactory steady level. This means that new therapeutic approaches are necessary, especially for patients with multiple sites of metastases [6, 7]. Among the unconventional treatments considered for OS, immunotherapy is based on an upregulation of the immune response in tumor-bearing host. Interleukin-2 (IL-2) has aroused particular interest because of its ability to activate lymphocytes and to differentiate lymphocyte subsets into lymphokine-activated killer (LAK) cells [8], and also because this phenomenon can be amplified by the in vitro pulsing of peripheral blood autologous mononuclear cells with IL-2 [9]. In vitro, LAK cells have the capacity to recognize and kill various tumor cells, irrespective of their histocompatibility expression status [10]. When LAK cells are injected intravenously, they have a peculiar tropism for the interstitium of the lung, which is the most common site of metastatic disease in cases of OS [11]. Some studies have shown that multidrug-resistant cancer cells are instead susceptible to adoptive cellular immunotherapy, and these data support the hypothesis that adoptive immunotherapy may be associated with chemotherapy to kill multidrug-resistant clones [12,13,14,15]. These observations provided the rationale for a prospective, single-institution trial that included conventional chemotherapy, surgery, and immunotherapy with IL-2 for pediatric OS. The aim of this study was to improve the prognosis for patients with metastatic OS at onset.
Materials and methods
All patients aged less than 18 years with OS revealing metastatic disease at onset diagnosed during the study period (1995–2010) and were eligible for the study. A histological diagnosis on biopsy of the primitive tumor was mandatory, while biopsy of metastatic site(s) was not essential for disease staging purposes. Normal renal function (judging from BUN level and creatinine clearance) and normal cardiac function (assessed on echocardiogram and ECG) were mandatory. The staging process included CT and/or NMR of the primary tumor, chest CT scan, TC-99 MDP bone scan, and CT of the metastatic skeletal site(s). The study was approved by the Institutional Review Boards (Istituto Nazionale per lo Studio e la Cura dei Tumori—Ethical Committee), and informed consent was obtained from parents or guardians.
Treatment
The study design is shown in Fig. 1. The treatment consisted of immunotherapy, chemotherapy, and surgery on both primary tumors and metastases. The goal of surgery was to obtain a complete resection of the primary tumor with wide margins, and the resection of all metastases, whenever feasible. The resection of lung metastases generally preceded the resection of the primary tumor. From 1995 to 2006, after the first cycle with IL-2, a leukapheresis was performed, and peripheral blood mononuclear cells were activated with IL-2 in vitro to obtain LAK cells, which were cryopreserved and then reinfused before proceeding with the subsequent courses of IL-2. Due to legal restrictions on cellular products, the protocol was amended in January 2007 and, from then on, immunotherapy consisted only of courses of IL-2, without any cryopreservation or infusion of LAK cells. The treatment started with a first cycle of recombinant IL-2 at a dose of 9 × 10e6 IU/sqm/day for 4 days as a continuous infusion. Leukapheresis was performed 36 h after the end of the first course of IL-2, aiming to collect at least 20 × 10e9 peripheral blood mononuclear cells/sqm. The peripheral blood mononuclear cells were then pulsed in vitro with IL-2 using a short-term method [11] and cryopreserved in liquid nitrogen. The harvest was thereafter divided into 3 doses, for thawing and reinfusion immediately before each of the 3 subsequent scheduled courses of IL-2.
Preoperative chemotherapy started 2 days after the IL-2 infusion at week 1 and consisted of 2 monthly courses of vincristine 1.4 mg/sqm (maximum 2 mg) + methotrexate 8 g/sqm in 6-h infusions on day 1 and day 8, and cisplatin 40 mg/sqm/day + ifosfamide 1.5 g/sqm/day × 3 on day 15 (cycle A; Fig. 1). The second course of IL-2 was scheduled at week 11. The surgical resection of lung metastases by sternotomy or bilateral thoracotomy was scheduled at week 13. A third cycle A and a third course of IL-2 were then given at week 16. Surgery on the primary tumor was scheduled at week 23 and was performed with conservative intent whenever feasible. The Huvos four-grade system was used for the histological assessment after resection, and a good histological response was defined as grade III–IV, with less than 10% of viable tumor cells. When the tumor was judged inoperable or demolitive surgery would be required, hyperfractionated accelerated radiotherapy (in two daily fractions of 3 Gy at 6- to 8-h intervals) was delivered for 5 days a week to the site of the primary tumor, up to a total dose of 48 Gy. After local treatment, patients received 3 courses of vincristine 1.4 mg/sqm (maximum 2 mg) + methotrexate 8 g/sqm on day 1, cisplatin 40 mg/sqm/day + ifosfamide 1.5 g/sqm/day × 3 on day 8, and cisplatin 50 mg/sqm/day × 2 + doxorubicin 90 mg/sqm on day 28 (cycle B). This was followed at week 44 by the fourth course of IL-2, which concluded the treatment.
During the IL-2 infusions, patients were continuously monitored for blood pressure, temperature, fluid intake, and diuresis. Paracetamol 10 mg/kg/q every 6 h and ranitidine 50 mg/sqm BID were mandatory supportive care. Folinic acid 15 mg iv. or orally was given 24 h after starting the methotrexate infusion, and every 6 h thereafter, for a total of 12 doses. Treatment-related side effects were graded according to the Common Terminology Criteria for Adverse Events (CTCAE).
Statistical analysis
Survival was defined as the time elapsing from the date of diagnosis to the date of follow-up or death due to any cause. Event-free survival (EFS) was defined as the time elapsing from the date of starting the systemic therapy up until the first event, or the last follow-up if no events had occurred. An event was defined as a progression of the existing disease for patients not in complete remission, a local recurrence, a systemic recurrence, or death from any cause. Overall survival (OS) and EFS distributions were estimated using the Kaplan and Meier model. The log-rank test was used to compare survival curves. All survivors had been seen or contacted within the 6 months preceding the analysis.
Different variables were considered in the univariate analysis for EFS.
Results
Between 1995 and 2010, 35 consecutive children with OS revealing metastases at diagnosis entered the study. The median age at diagnosis was 13 years (range 4–17). Table 1 shows the clinical characteristics of the sample. Four patients had an axial osteosarcoma. All children entered the pre-surgical phase. Twenty-four patients experienced a progression or relapse a median 10 months (range 4–50 months) and 23 died a median 18 months (range 8–150 months) after their diagnosis. One patient died in complete remission 30 months after being diagnosed as a result of severe viral infection. At the time of the present analysis, 11 patients (30%) were alive and in continuous complete remission (1 with skeletal metastases at diagnosis).
Eighteen patients had bilateral lung metastases at onset, 11 had monolateral lung involvement, 2 had both lung and skeletal involvement, 3 had skeletal metastases alone, and 1 had bone, visceral, and pleural involvement. High serum alkaline phosphatase (ALP) and lactic dehydrogenase (LDH) levels were recorded in 16 and 14 patients, respectively.
A total of 140 courses of IL-2 were administered, the side effects of which are detailed in Table 2. In 2 patients, the IL-2 treatment had to be stopped as a result of toxicity—at the fourth cycle due to transient anuria in both cases. All side effects were reversible after the IL-2 infusions came to an end. The 27 children enrolled between 1995 and 2006 underwent leukapheresis, and it proved possible to pulse and collect a median 32 × 10e9/sqm (range 20–315) of LAK cells by means of one or 2 aphereses (median 1.1). As expected, severe pancytopenia, mucositis, and infections were common after the chemotherapy courses and required a dose reduction or a delay of the subsequent treatment in 18% of cases. Reversible alterations in liver function were also common after high-dose methotrexate, while severely impaired renal function was rare (2%). No toxic deaths were recorded.
Local treatment consisted of surgery on the primary tumor in 32 patients (11 patients underwent demolitive surgery), while 3 received radiotherapy (1 patient with pelvis tumor not amenable to conservative surgery and 2 patients with rapidly progressing disease). Twenty-five patients underwent surgery on both primary tumors and lung metastases: 14 patients had a thoracotomy and 11 a sternotomy. Seven patients had surgery on primary tumors but not on lung metastases, due to a rapid progression of the pulmonary disease in 6 cases and to a complete radiological remission in 1. After a sternotomy, 1 patient did not undergo demolitive surgery on the primary tumor due to disease progression. Two patients underwent no surgery on either the primary tumor or multiple skeletal metastases.
Histological response to neoadjuvant chemotherapy on the primary tumor was evaluable in 31/35 patients. There were 14 patients who achieved necrosis of more than 90% of their tumor, while 17 were poor responders.
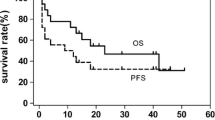
The 3- and 5-year EFS rates were 34.3 and 28.6% (SE = 8 and 7.6%), respectively, and the overall survival rates were 45.7 and 37.1% (SE = 8%), respectively (Figs. 2, 3).
Eleven patients were alive at 77–228 months (median 130 months) after their diagnosis: 10 were in first complete remission (CR), and 1 in second complete remission. One of these 11 patients developed a second tumor (squamous carcinoma) 118 months after being diagnosed with OS. All 11 survivors achieved complete surgical resection of their primary tumor, and all pulmonary metastatic lesions were removed in 10 of them, while surgery was avoided in 1 due to complete remission of all his metastatic lesions. Seven of these 11 surviving patients were good responders in terms of the percentage of necrosis on the primary tumor after neoadjuvant chemotherapy.
Complete surgical resection of the primary tumor positively affected the outcome, giving patients better 3- and 5-year EFS rates (37.5% [SE = 8.6%)] and 31.3% [SE = 8.2%], respectively) than patients who did not undergo surgery (EFS 0%; p = 0.0007). Patients requiring demolitive surgery (amputation or disarticulation) had a worse 3- and 5-year EFS (9% [SE = 9%]) than patients who had conservative surgery (EFS 52.4% [SE = 10.9%] and 42.9% [SE = 10.8%], respectively; p = 0.005). Patients whose lung metastases could be resected had better 3- and 5-year EFS rates (41.7% [SE = 10%] and 33.3% [SE = 9.6%], respectively) than patients whose metastases could not be treated surgically (3- and 5-year EFS 18.2% [SE = 11.6%]), but the difference was not statistically significant (p = 0.08; Table 3).
For patients with lung metastases, the 3- and 5-year EFS probabilities were 54.5% (SE = 15%) and 36.4% (SE = 14.5%), respectively, for patients with unilateral lung involvement, and 33.35% (SE = 11.1%) and 27.8% (SE = 10.6%), respectively, for patients with bilateral lung involvement; patients with skeletal metastases had instead a 3- and 5-year EFS of 16.7% (SE = 15.2%; p = 0.3944; Table 3). Five of the 6 patients with skeletal metastases died due to disease progression a median 18 months after being diagnosed, while the 1 surviving patient is disease-free 135 months after the diagnosis.
In the subgroup of 10 patients who did not achieve a macroscopically complete resection because of unresectable primary or metastatic disease, 8 died due to disease progression (with a 3- and 5-year EFS rate of 11% [SE = 10%] vs. 3- and 5-year EFS rates of 42.3 and 34.6% [SE = 9%], respectively, for patients who underwent complete resection).
Patients achieving a good necrosis after chemotherapy had better 3- and 5-year EFS rates (64.3% [SE = 12.8%] and 50% [SE = 13.4%], respectively) than patients responding less well to chemotherapy (11.8% [SE = 7.8%] and 5.9% [SE = 5.7%], respectively; p = 0.0045; Fig. 4).
Patients whose disease relapsed or progressed at least 24 months after they had been diagnosed had a better 3-year EFS than those whose disease relapsed or progressed earlier on (50% [SE = 25%] vs. 0%, p = 0.0001).
Log-rank tests revealed no differences in 3-year EFS rates by gender, histology, LDH level, ALP level, site of primary tumor, surgical approach to lung metastases, or treatment with LAK reinfusions.
Discussion
The presence of metastases at diagnosis is the most important prognostic factor in patients with OS and is an independent predictor of a poor outcome: patients with metastatic disease have long-term survival rates in the range of 10–30% [1,2,3,4,5]. Patients with primary metastatic OS form a heterogeneous group, however, and their prognosis depends on the pattern of metastatic disease at diagnosis. For instance, patients with lung metastases have a better prognosis than patients with multifocal bone dissemination, which seems to herald a catastrophic outcome. Among the patients with lung metastases alone, unilateral lung involvement carries a better prognosis than bilateral lung metastases [4, 5]. High serum alkaline phosphatase levels, visceral pleural involvement, and the chondroblastic subtype are reportedly associated with a poor prognosis in some series.
The survival of patients with metastatic OS has improved slightly with an optimal use of intensive chemotherapy, and especially thanks to more aggressive surgery, resecting both the primary tumor and metastases [4, 5, 16,17,18]. High-dose chemotherapy and autologous stem cell rescue did not show any survival advantage, but only a major hematological and non-hematological toxicity [6, 7].
Different strategies have been used in an effort to improve the current cure rate for metastatic OS patients, such as enhancing the local efficacy of chemotherapy for lung metastases by using liposomal, aerosolized drug formulations of cisplatin (sustained-release lipid inhalation targeting [SLITTM] cisplatin) or aerosolized granulocyte–monocyte colony-stimulating factor (GM-CSF), natural killer (NK) cell infusion and aerosol IL-2, or adding immunostimulating as muramyl tripeptide phosphatidylethanolamine (MTP-PE, i.e., mifamurtide) and alpha interferon [19,20,21,22,23].
Some encouraging responses to IL-2 have been reported in clinical trials on adult patients with various tumors, but it only proved effective for renal cell carcinoma and melanoma [24]. Data on the use of IL-2 for solid tumors in children are limited, however, and neuroblastoma is the only pediatric tumor for which IL-2 has been included in phase III protocols to date [13].
The present study reports the clinical results obtained in a series of 35 consecutive children with metastatic OS treated according to a program that included IL-2 in addition to chemotherapy and surgery. In the first part of the study period, patients also received repeated reinfusions of autologous LAK cells. The decision to administer LAK cells together with the IL-2 infusions stemmed from the fact that LAK cells are distributed preferentially to the lungs—the most common site of metastatic spread from OS. No statistically significant differences emerged in the 3-year EFS rate between the subsets of patients given pulsed LAK + IL2 as opposed to IL2 alone, suggesting a limited role for in vitro pulsing of PBMC cells. Though it is still not satisfying, the survival rate in our series was higher than reported elsewhere. The survival of more than 40% of patients at 3 years is encouraging—especially if we consider that a considerable percentage of patients in our series (17%) had extrapulmonary metastases, which is associated with a catastrophic prognosis. Our results point to a potential role for immunotherapy with IL-2 and LAK/NK cell activation in controlling lung metastases.
Histological response to neoadjuvant chemotherapy is a well-recognized prognostic factor in patients with non-metastatic OS, and this applies to metastatic patients too. Our findings confirm as much, but the small number of patients in our series may represent a bias.
The results of our study confirm other reports in the literature: complete surgical resection of all metastatic sites carries a better prognosis [4, 5]; patients cannot be cured without the concomitant surgical resection of all clinically detectable lesions, and all resectable metastases should be removed regardless of their number and site. Patients with clinically detectable tumor have a fivefold higher risk of dying than patients achieving a complete surgical resection of all detectable tumors [25].
Further prospective studies on larger numbers of patients could establish the efficacy of adding IL2 to chemotherapy for OS.
The present study has 3 principal biases: the small number of cases, the lack of a control arm, and the long-term of the enrollment. Moreover, we miss the date of tumor infiltrating lymphocytes at the time point of surgery. The rarity of the presentation justifies the first two biases, and further the study population is composed by an unselected cohort of consecutive patients. The long period of the enrollment was due to our institutional choice to prolong the study conceived in the early 1990s.
The prognosis for patients with metastatic OS remains dismal, especially in cases with multiple unresectable metastases. In this setting, it is crucial to identify and validate new agents that might be administered, alone or as adjuvants to conventional chemotherapy, in an effort to better control local and metastatic disease, and thus improve not only patients’ chances of survival and cure, but also their quality of life. The combination of genomic complexity, low incidence, and inter- and intratumoral heterogeneity make OS biology difficult to investigate thoroughly, however.
References
Bacci G, Briccoli A, Ferrari S, Saeter G, Donati D, Longhi A, Manfrini M, Bertoni F, Rimondini S, Monti C, Forni C. Neoadjuvant chemotherapy for osteosarcoma of the extremities with synchronous lung metastases: treatment with cisplatin, adriamycin and high-dose methotrexate and ifosfamide. Oncol Rep. 2000;7:339–46.
Bielack SS, Kempf-Bielack B, Dilling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1702 patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2002;20:776–90.
Harris MB, Gieser P, Goorin AM, Ayala A, Shochat SJ, Ferguson WS, Holbrook T, Link MP. Treatment of metastatic osteosarcoma at diagnosis: a Pediatric Oncology Group Study. J Clin Oncol. 1998;16:3641–8.
Kager L, Zoubek A, Potschger U, Kastner U, Flege S, Kempf-Bielack B, Branscheid D, Kotz R, Salzer-Kuntschik M, Winkelmann W, Jundt G, Kabisch H, Reichardt P, Jürgens H, Gadner H, Bielack SS. Primary metastatic osteosarcoma: presentation and outcome of patients treated on Neoadjuvant Cooperative Osteosarcoma Study Group Protocols. J Clin Oncol. 2003;21:2011–8.
Mialou V, Philip T, Kalifa C, Perol D, Gentet JC, Marec-Berard P, Pacquement H, Chastagner P, Defaschelles AS, Hartmann O. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome. The French Pediatric experience. Cancer. 2005;104:1100–9.
Janinis J, McTiernan A, Driver D, Mitchell C, Cassoni AM, Pringle J, Kilby A, Whelan JS. A pilot study of short-course intensive multiagent chemotherapy in metastatic and axial skeletal osteosarcoma. Ann Oncol. 2002;13:1935–44.
Boye K, Brach Del Prever A, Eriksson M, Saeter G, Tienghi A, Lindholm P, Fagioli F, Skjeldal S, Ferrari S, Hall KS. High-dose chemotherapy with stem cell rescue in the primary treatment of metastatic and pelvic osteosarcoma: final results of the ISG/SSG II Study. Pediatr Blood Cancer. 2014;61:840–5.
Stern JB, Smith KA. Interleukin-2 induction of T-cell G1 progression and c-myb expression. Science. 1986;233:203–6.
Yang SC, Grimm EA, Parkinson DR, Carinhas J, Fry KD, Mendiguren-Rodriguez A, Licciardello J, Owen-Schaub LB, Hong WK, Roth JA. Clinical and immunomodulatory effects of combination immunotherapy with low-dose interleukin 2 and tumor necrosis alpha in patients with advanced non small cell lung cancer: a phase I trial. Cancer Res. 1991;5:3669–76.
Oppenheim MH, Lotze MT. Interleukin-2: solid tumor therapy. Oncology. 1994;51:154–69.
Kjaergaard J, Hokland ME, Agger R, Skovbo A, Nannmark U, Basse PH. Biodistribution and tumor localization of lymphokine-activated killer T cells following different routes of administration into tumor-bearing animals. Cancer Immunol Immunother. 2000;48:550–60.
Ladenstein R, Pötschger U, Siabalis D, Garaventa A, Bergeron C, Lewis IJ, Stein J, Kohler J, Shaw PJ, Holter W, Pistoia V, Michon J. Dose finding study for the use of subcutaneous recombinant interleukin-2 to augment natural killer cell numbers in an outpatient setting for stage 4 neuroblastoma after megatherapy and autologous stem-cell reinfusion. J Clin Oncol. 2011;1(29):441–8.
Dilloo D, Laws HJ, Hanenberg H, Körholz D, Nürnberger W, Burdach SE. Induction of two distinct natural killer-cell populations, activated T cells and antineoplastic cytokines, by interleukin-2 therapy in children with solid tumors. Exp Hematol. 1994;22:1081–8.
Luksch R, Perotti D, Cefalo G, Gambacorti Passerini C, Massimino M, Spreafico F, Casanova M, Ferrari A, Terenziani M, Polastri D, Gambirasio F, Podda M, Bozzi F, Ravagnani F, Parmiani G, Fossati Bellani F. Immunomodulation in a treatment program including pre- and post-operative interleukin-2 and chemotherapy for childhood osteosarcoma. Tumori. 2003;89:263–8.
Horton SA, Oldham RK, Yannelli JR. Generation of human lymphokine-activated killer cells following brief exposure to high-dose interleukin-2. Cancer Res. 1990;50:1686–92.
Bacci G, Briccoli A, Rocca M, Ferrari S, Donati D, Longhi A, Bertoni F, Bacchini P, Giacomini S, Forni C, Manfrini M, Galletti S. Neoadjuvant chemotherapy for osteosarcoma of the extremities with metastases at presentation: recent experience at the Rizzoli Institute in 57 patients treated with cisplatin, doxorubicin, and a high dose of methotrexate and ifosfamide. Ann Oncol. 2003;14:1126–34.
Janeway KA, Barkauskas DA, Krailo MD, Meyers PA, Schwartz CL, Ebb DH, Seibel NL, Grier HE, Gorlick R, Marina N. Outcome for adolescent and young adult patients with osteosarcoma: a report from the Children’s Oncology Group. Cancer. 2012;118:4597–605.
Goorin AM, Harris MB, Bernstein M, Ferguson W, Devidas M, Siegal GP, Gebhardt MC, Schwartz CL, Link M, Grier HE. Phase II/III trial of etoposide and high-dose ifosfamide in newly diagnosed metastatic osteosarcoma: a Pediatric Oncology Group trial. J Clin Oncol. 2002;20:426–33.
Anderson PM, Markovic SN, Sloan JA, Clawson ML, Wylam M, Arndt CA, Smithson WA, Burch P, Gornet M, Rahman E. Aerosol granulocyte macrophage-colony stimulating factor: a low toxicity, lung-specific biological therapy in patients with lung metastases. Clin Cancer Res. 1999;5:2316–23.
Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM, Harris M, Kleinerman E, Link MP, Nadel H, Nieder M, Siegal GP, Weiner MA, Wells RJ, Womer RB, Grier HE. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival. A report from the Children’s Oncology Group. J Clin Oncol. 2008;26:633–8.
Chou AJ, Kleinerman ES, Krailo MD, Chen Z, Betcher DL, Healey JH, Conrad EU 3rd, Nieder ML, Weiner MA, Wells RJ, Womer RB, Meyers PA. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma. A report from the Children’s Oncology Group. Cancer. 2009;115:5339–48.
Bielack SS, Smeland S, Whelan JS, Marina N, Jovic G, Hook JM, Krailo MD, Gebhardt M, Pápai Z, Meyer J, Nadel H, Randall RL, Deffenbaugh C, Nagarajan R, Brennan B, Letson GD, Teot LA, Goorin A, Baumhoer D, Kager L, Werner M, Lau CC, Sundby Hall K, Gelderblom H, Meyers P, Gorlick R, Windhager R, Helmke K, Eriksson M, Hoogerbrugge PM, Schomberg P, Tunn PU, Kühne T, Jürgens H, van den Berg H, Böhling T, Picton S, Renard M, Reichardt P, Gerss J, Butterfass-Bahloul T, Morris C, Hogendoorn PC, Seddon B, Calaminus G, Michelagnoli M, Dhooge C, Sydes MR, Bernstein M. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon alpha-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol. 2015;33:2279–87.
Guma SR, Lee DA, Yu L, Gordon N, Hughes D, Stewart J, Kleinerman ES. Natural killer (NK) cell therapy and aerosol interleukin-2 for treatment of osteosarcoma lung metastasis. Pediatr Blood Cancer. 2014;61(4):618–26.
Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–8.
Briccoli A, Rocca M, Salone M, Guzzardella GA, Balladelli A, Bacci G. High-grade osteosarcoma of the extremities metastatic to the lung: long-term results in 323 patients treated combining surgery and chemotherapy, 1985–2005. Surg Oncol. 2010;19:193–9.
Acknowledgements
The authors wish to thank the “Associazione Bianca Garavaglia, per l’aiuto e il sostegno nel campo dei tumori infantili” for the support provided to our Pediatric Oncology Unit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by Ethical Committee and parents’ or guardians’ patients gave their consensus to participate in the study.
Rights and permissions
About this article
Cite this article
Meazza, C., Cefalo, G., Massimino, M. et al. Primary metastatic osteosarcoma: results of a prospective study in children given chemotherapy and interleukin-2. Med Oncol 34, 191 (2017). https://doi.org/10.1007/s12032-017-1052-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-017-1052-9