Abstract
Telomerase expression is an important mechanism of tumor unlimited replicative potential. The aim of this study was to evaluate prognostic impact of telomerase activity in breast cancer patients and to correlate telomerase activity with established prognostic factors. We analyzed tissue of 102 malignant breast lesions and 20 healthy breast tissues. Telomerase activity was determined by telomeric repeat amplification protocol assay. Telomerase activity was present in 77 (75.49 %) of 102 breast cancers. Telomerase activity in breast cancers was statistically significantly higher in comparison with the activity in normal breast tissue. The levels of telomerase activity were significantly positively correlated with tumor size, axillary nodal status, histological grade, HER-2/neu protein expression in tumor tissue and expression of the nuclear antigen Ki-67. A statistically significant negative correlation was found between the presence of ER and telomerase activity. There was no correlation between telomerase activity and concentration of PR or the age of patients. Kaplan–Meier analysis showed that patients with higher telomerase activity had significantly shorter 10-year disease-free survival (p < 0.0001) and 10-year overall survival (p < 0.0001) than those with lower telomerase activity. These results were confirmed by logistic regression analysis. Our results support the prognostic role of telomerase activity and its relationship with the more aggressive phenotype of breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immortality is a striking difference between normal and cancer cells [1, 2]. Although this phenomenon has been known for more than 50 years, only after characterization of telomeres and telomerase by Nobel Prize laureates Elizabeth H. Blackburn, Carol W. Greider and Jack W. Szostak, its molecular basis become better understood [3]. Telomerase is a ribonucleoprotein enzyme composed of catalytic subunit (TERT, telomerase reverse transcriptase) and RNA component (TERC, telomerase RNA component) stabilized by dyskerin [3]. Telomerase activity is regulated at several levels including expression of telomerase subunits coding genes, post-translational level and protein phosphorylation [4, 5]. There are many positively acting transcription factors and co-regulators of telomerase expression such as Myc oncogene, HPV E6 oncoprotein, NF kappaB and beta-catenin [6]. Various tumor suppressor pathways such as Rb, WT1, TGF beta pathways negatively regulate telomerase and loss of these pathways leads to upregulation of telomerase expression [6].
Telomerase maintenance mechanisms are basis for unlimited replicative potential—a hallmark of cancer. Many tumors maintain telomere by expression of telomerase. A survey of telomerase activity in human cancers showed that a great majority of cancers (more than 85 %) have detectable levels of telomerase [7, 8]. Minority of tumors overcome Hayflick limit by alternative lengthening of telomeres [9]. A high percentage of adenocarcinomas express telomerase activity including breast cancer [10]. Besides telomere maintenance, telomerase has other functions in the cell. It plays a role in stress response, metabolism, mitochondrial function and chromatin regulation [3, 11]. Thus, telomerase role in malignant growth may include telomere-related and telomere non-related mechanisms. Additionally, telomerase activity is implicated in the mechanisms of drug resistance [10].
Telomerase activity has been studied as prognostic factor in breast cancer patients with divergent results [6]. Some studies suggested that telomerase activity may have significance in early diagnosis of breast cancer [12]. Telomerase is also considered to be a therapeutic target. Several therapeutic approaches have been proposed to inhibit telomerase in cancer: antisense oligonucleotides, small molecule inhibitors, nucleos(t)ide reverse-transcriptase inhibitors, expression modulators, immunotherapy, gene therapy, disruption of telomerase assembly, G-quadruplex stabilizers [13]. Some of these strategies are included into clinical trials. Experimental results suggest therapeutic efficacy of combination of telomerase inhibitors and standard chemotherapy [14]. However, anti-telomerase strategies are not without potential negative effects on stem cells and some normal cells. Additionally, cancer cells may develop escape mechanisms by induction of alternative lengthening of telomeres.
Breast is the most common site of cancer in women worldwide [15]. Despite advances in diagnosis and therapeutic procedures, breast cancer is the leading cause of cancer death in women [15]. In Croatia, in the year 2012 the number of new breast cancer cases was 2254 and breast cancer accounted for 24 % of all new cancer cases among Croatian women [16].
The aim of this study was to analyze prognostic significance of telomerase activity in breast cancer patients. We also studied relationship between telomerase activity and various clinical, pathological and biochemical parameters.
Materials and methods
Patients and samples
This study was carried out using tissue of 102 malignant breast lesions operated between April 1999 and April 2000 at the University Hospital Zagreb. The control group included 20 normal breast tissues. All samples and clinical information were obtained under institutional review board approval. The study was approved by the Ethics Committee of the Medical faculty at the Universities of Zagreb and the University Hospital Zagreb. Our patients did not receive chemotherapy or irradiation before surgery. The treatment of our patients after surgery was in accordance with national guidelines (based on St Gallen recommendations). Parameters tested for all patients and their tumors are given in Table 1. Postoperative follow-up of patients [overall survival (OS) and disease-free survival (DFS)] was 10 years (120 months). Steroid receptors [estrogen (ER) and progesterone (PR)] were determined by using the ligand-binding method according to Horwitz and McGuire [17]. A positive receptor status for ER and PR was defined as the presence of 10 fmol/mg of cytosol proteins and 20 fmol/mg of cytosol proteins, respectively. Tumor size, nodal status and histological grade (Scarff, Bloom and Richardson as grade I,II,III) were assayed by standard procedure at the Department of Pathology. HER-2/neu expression was determined immunohistochemically using antibodies against HER-2/neu (Herceptest, Dako,Glostrup, Denmark). An immunohistochemical score of 3+ according to Herceptest criteria or fluorescence in situ hybridization (FISH; Dako, Glostrup, Denmark) with amplification ratio ≥2 was accepted as HER-2/neu-positive result. Ki67 was determined immunohistochemically using monoclonal antibodies against Ki67 (MiB-1 clone, Dako, Glostrup, Denmark). The percentage of tumor cells (100 cells) with positive nuclear staining was evaluated and categorized as negative if nuclear staining was <10 % of cells and positive if there was nuclear staining ≥10 % of cells.
Telomeric repeat amplification protocol (TRAP) assay
The nitrogen-frozen breast tissue (cancer or healthy) about 0.1 mg was crushed and transferred to the lysis buffer homogenized and incubated for 30 min on ice. After centrifugation (16,000g, 20 min, 4 °C), the supernatant was transferred to a test tube, frozen in liquid nitrogen and stored at −70 °C until use. Total protein concentration was determined by the Lowry method [18]. The final supernatant was diluted to a concentration of 50 µg/mL protein with lysis buffer. Telomerase activity was detected with telomeric repeat amplification protocol (TRAP) assay as previously described [19, 20] using Telomerase PCR ELISA kit (Boehringer Mannheim, Germany). The cut-off value was defined as absorbance level 0.2 at 450 nm.
Statistical analysis
The results were analyzed using the statistical software MedCalc. Distribution of telomerase activity for the group of breast cancer patients and the control group is shown using Kruskal–Wallis test. The association of telomerase activity with clinicopathologic parameters was studied using Spearman’s correlation coefficient. Multivariate analysis was performed by logistic regression analysis. DFS and OS curves were calculated by Kaplan–Meier method and compared by log-rank tests. A p value <0.05 was considered significant.
Results
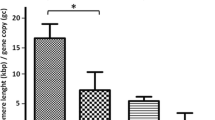
Clinical and pathological data of patients and their tumors are given in Table 1. Telomerase activity was present in 77 (75.49 %) of 102 breast cancers. The absorbance range of positive breast carcinomas was 0.202–3.459 with the median 1.032. The range of telomerase activity in the cytosol of normal breast tissue was 0.0170–0.089 with the median 0.053. Telomerase activity in breast cancers was statistically significantly higher in comparison with the activity in normal breast tissue (p = 0.0001) (Fig. 1). The levels of telomerase activity were significantly positively correlated with tumor size (p = 0.004), axillary nodal status (p < 0.001), histological grade (p < 0.001), HER-2 protein expression in tumor tissue (p = 0.003) and expression of the nuclear antigen Ki-67 (p = 0.0038) (Table 2). A statistically significant negative correlation was found between the presence of ER and telomerase activity (p < 0.001) (Table 2). There was no correlation between telomerase activity and concentration of PR or the age of patients (Table 2). After logistic regression analysis, telomerase activity was significantly associated with DFS and OS along with some clinicopathological factors (for DFS: tumor size, axillary lymph nodes status and ER; for OS: ER and HER-2 status) (Table 3).
Kaplan–Meier analysis showed that patients with higher telomerase activity had shorter 10-year DFS (p < 0.0001) and shorter 10-year OS (p < 0.0001) than those with lower telomerase activity (Figs. 2, 3, respectively).
Discussion
In our study, we analyzed relationship between telomerase activity in breast cancer patients and various clinical, pathological and biochemical factors. We also analyzed correlation of telomerase activity with disease-free survival and overall survival. Telomerase activity was determined by TRAP assay.
Several authors studied telomerase activity in breast cancer. In the study of Hiyama et al. [21], telomerase activity was present in 130 (93 %) out of 140 breast cancers, while Roos et al. [22] found telomerase activity in 85 % of 106 breast cancers. Comparatively small studies conducted by Mokbel et al. [23, 24] showed telomerase activity in 67 and 72 % of breast tumors respectively. In the literature analysis by Baykal et al. [25], telomerase activity was present in 13–100 % of breast cancers. These results confirm that telomerase maintenance by expression of telomerase is a frequent event in breast cancer.
We found correlation between telomerase activity and tumor size and axillary lymph nodes involvement. These results are in accordance with those of Hoos et al. [26]. Hiyama et al. [21] and Umbricht et al. [21, 27] found correlation between telomerase activity and tumor size, while some authors found no correlation [28–30]. Sugino et al. [29] did not find correlation between telomerase activity and lymph node metastasis.
In our study, telomerase activity was correlated with histological grade of tumors. Roos et al. [22] and Kalogeraki et al. [31] also found this correlation, while some other authors reported different results [28].
We found positive correlation between telomerase activity and some factors that influence telomerase expression. ER is important prognostic and predictive factor in breast cancer. We found a statistically significant negative correlation between telomerase activity and ER in our samples while there was no correlation with PR. In the study of Kalogeraki et al. [31], there was also a negative correlation between telomerase activity and ER, while Roos et al. [22] showed negative correlation between telomerase activity and both ER and PR. Estrogen plays a role in regulation of telomerase transcription catalytic subunit TERT [32]. However, in malignant cells many changes in gene expression occur and this regulation may be lost. We speculate that in breast cancer cells regulation of telomerase activity may be altered and that tumors with high levels of telomerase activity and negative ER belong to a group of tumors with more aggressive phenotype.
There are not many studies that related telomerase activity to HER-2/neu status. HER-2/neu is a negative prognostic factor although biological therapy dramatically improved outcome of HER-2/neu-positive patients. Kalogeraki et al. [31] found association between telomerase activity and HER-2/neu expression. This association was also shown in breast cancer cell lines [33]. Goueli and Janknecht [33] showed significant correlation between HER-2/neu expression and human telomerase reverse-transcriptase (hTERT) levels in human breast tumor specimens. In our analysis, there was a significant correlation between telomerase activity and HER-2/neu positivity. HER-2/neu upregulates catalytic telomerase subunit through ERK MAP kinase pathway [33]. Tumors with high telomerase activity and high HER-2 expression belong to more aggressive group and may be related to poor prognosis [33].
We also found correlation between telomerase activity and another negative prognostic factor- proliferation activity measured by Ki-67 index. Tumors with higher telomerase activity had higher values of Ki-67 index. These results were confirmed by other authors [22, 30]. We found no relationship between telomerase activity and the age of patients.
Studies that analyzed prognostic significance of telomerase activity yielded divergent results. Factors that may influence these results include: differences in methodology of telomerase analysis, amount of tissue analyzed and differences in study design. In a study with median follow-up of 74 months (range 26–98), Clark et al. [34] analyzed 400 breast cancer patients with lymph node metastasis. In the multivariate analysis, telomerase activity was strong predictor of OS but marginally predictive for DFS. Simickova et al. [35] found significant correlation between telomerase activity and DFS. In a study with follow-up of 5 years, Kimura et al. [36] demonstrated significant association between telomerase activity in breast cancer patients and recurrence of the disease. Poremba et al. [37] analyzed hTERT and the internal RNA component (hTR) expression from 611 breast carcinomas using tissue microarray. In this study with median follow-up of 63 months (range 1–151) an association was found between telomerase components and lower OS. Some studies did not find correlation between telomerase activity and patients’ outcome. Carey et al. [30] analyzed 203 breast cancer patients with no metastasis in lymph nodes and found no correlation between telomerase activity and relapse-free and OS. The median follow-up of this study was 5.5 years (range 0.1–10.4 years). In the study of Lu et al. [38] with median follow-up of 86 months (range 8–108), telomerase expression was not significantly associated with DFS and OS although an association may have been masked by the type of therapy.
In our study, we correlated telomerase activity with 10-year DFS and 10-year OS. Patients with higher levels of telomerase activity had shorter 10-year DFS and shorter 10-year OS than patients with lover levels of telomerase activity. These differences were statistically significant in the Kaplan–Meier analysis. Difference between low and high level of telomerase activity was significant in the logistic regression analysis as well.
In conclusion, our results suggest a relationship between telomerase activity and more aggressive phenotype. Our data also show association between telomerase activity and poor clinical outcome of breast cancer patients. According to our results, telomerase activity may be considered as a negative prognostic factor in breast cancer.
Taking into account the role of telomerase in tumor biology and permanent need for identification of additional prognostic factors, further studies on a larger number of patients are warranted in order to establish prognostic significance of telomerase activity.
References
Hayflick L. The illusion of cell immortality. Br J Cancer. 2000;83:841–6.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Zhu H, Belcher M, van der Harst P. Healthy aging and disease: role for telomere biology? Clin Sci (Lond). 2011;120:427–40.
Daniel M, Peek GW, Tollefsbol TO. Regulation of the human catalytic subunit of telomerase (hTERT). Gene. 2012;498:135–46.
Li AY, Lin HH, Kuo CY, Shih HM, Wang CC, Yen Y, et al. High-mobility group A2 protein modulates hTERT transcription to promote tumorigenesis. Mol Cell Biol. 2011;31:2605–17.
Reddel RR. Telomere maintenance mechanisms in cancer: clinical implications. Curr Pharm Des. 2014;20:6361–74.
Kim NW. Clinical implications of telomerase in cancer. Eur J Cancer. 1997;33:781–6.
Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–91.
Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995;14:4240–8.
Holysz H, Lipinska N, Paszel-Jaworska A, Rubis B. Telomerase as a useful target in cancer fighting-the breast cancer case. Tumour Biol. 2013;34:1371–80.
Saretzki G. Extra-telomeric functions of human telomerase: cancer, mitochondria and oxidative stress. Curr Pharm Des. 2014;20:6386–403.
Heaphy CM, Meeker AK. The potential utility of telomere-related markers for cancer diagnosis. J Cell Mol Med. 2011;15:1227–38.
Romaniuk A, Kopczynski P, Ksiazek K, Rubis B. Telomerase modulation in therapeutic approach. Curr Pharm Des. 2014;20:6438–51.
Tamakawa RA, Fleisig HB, Wong JM. Telomerase inhibition potentiates the effects of genotoxic agents in breast and colorectal cancer cells in a cell cycle-specific manner. Cancer Res. 2010;70:8684–94.
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Mathers C, Rebelo M, et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed 8 Oct 2015.
Croatian National Cancer Registry. Cancer incidence in Croatia 2012. Bulletin no. 37. http://www.hzjz.hr/wp-content/uploads/2013/11/Bilten-2012_final.pdf. Accessed 20 Dec 2015.
Horwitz KB, Mcguire WL. Progesterone and progesterone receptors in experimental breast cancer. Cancer Res. 1977;37:1733–8.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin Phenol reagent. J Biol Chem. 1951;193:265–75.
Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5.
Fajkus J. Detection of telomerase activity by the TRAP assay and its variants of alternatives. Clin Chim Acta. 2006;371:27–31.
Hiyama E, Gollahon L, Kataoka T, Kuroi K, Yokoyama T, Gazdar AF, et al. Telomerase activity in human breast tumors. J Natl Cancer Inst. 1996;88:116–22.
Roos G, Nilsson P, Cajander S, Nielsen NH, Arnerlöv C, Landberg G. Telomerase activity in relation to p53 status and clinico-pathological parameters in breast cancer. Int J Cancer. 1998;79:343–8.
Mokbell K, Parris CN, Ghilchik M, Newbold RF. Telomerase activity in the human breast. Breast. 1999;8:208–11.
Mokbel KM, Parris CN, Ghilchik M, Amerasinghe CN, Newbold RF. Telomerase activity and lymphovascular invasion in breast cancer. Eur J Surg Oncol. 2000;26:30–3.
Baykal A, Rosen D, Zhou C, Liu J, Sahin AA. Telomerase in breast cancer: a critical evaluation. Adv Anat Pathol. 2004;11:262–8.
Hoos A, Hepp HH, Kaul S, Ahlert T, Bastert G, Wallwiener D. Telomerase activity correlates with tumor aggressiveness and reflects therapy effect in breast cancer. Int J Cancer. 1998;79:8–12.
Umbricht CB, Sherman ME, Dome J, Carey LA, Marks J, Kim N, et al. Telomerase activity in ductal carcinoma in situ and invasive breast cancer. Oncogene. 1999;18:3407–14.
Loveday RL, Greenman J, Drew PJ, Monson JR, Kerin MJ. Genetic changes associated with telomerase activity in breast cancer. Int J Cancer. 1999;84:516–20.
Sugino T, Yoshida K, Bolodeoku J, Tahara H, Buley I, Manek S, et al. Telomerase activity in human breast cancer and benign breast lesions: diagnostic applications in clinical specimens, including fine needle aspirates. Int J Cancer. 1996;69:301–6.
Carey LA, Kim NW, Goodman S, Marks J, Henderson G, Umbricht CB, et al. Telomerase activity and prognosis in primary breast cancers. J Clin Oncol. 1999;17:3075–81.
Kalogeraki A, Kafousi M, Ieromonachou P, Giannikaki E, Vrekoussis T, Zoras O, et al. Telomerase activity as a marker of invasive ductal breast carcinomas on FNABs and relationship to other prognostic variables. Anticancer Res. 2005;25:1927–30.
Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, et al. Estrogen activates telomerase. Cancer Res. 1999;59:5917–21.
Goueli BS, Janknecht R. Upregulation of the catalytic telomerase subunit by the transcription factor ER81 and oncogenic HER2/Neu, Ras, or Raf. Mol Cell Biol. 2004;24:25–35.
Clark GM, Osborne CK, Levitt D, Wu F, Kim NW. Telomerase activity and survival of patients with node-positive breast cancer. J Natl Cancer Inst. 1997;89:1874–81.
Simícková M, Nekulová M, Pecen L, Cernoch M, Vagundová M, Pacovský Z. Quantitative determination of telomerase activity in breast cancer and benign breast diseases. Neoplasma. 2001;48:267–73.
Kimura M, Koida T, Yanagita Y. A study on telomerase activity and prognosis in breast cancer. Med Oncol. 2003;20:117–26.
Poremba C, Heine B, Diallo R, Heinecke A, Wai D, Schaefer KL, et al. Telomerase as a prognostic marker in breast cancer: high-throughput tissue microarray analysis of hTERT and hTR. J Pathol. 2002;198:181–9.
Lu L, Zhang C, Zhu G, Irwin M, Risch H, Menato G, et al. Telomerase expression and telomere length in breast cancer and their associations with adjuvant treatment and disease outcome. Breast Cancer Res. 2011;13:R56.
Acknowledgments
This work was supported by the Ministry of Science, Education and Sports, Republic of Croatia (Grant 108-1080058-0046).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study were in accordance with the ethical standards of the institution research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kulić, A., Plavetić, N.D., Gamulin, S. et al. Telomerase activity in breast cancer patients: association with poor prognosis and more aggressive phenotype. Med Oncol 33, 23 (2016). https://doi.org/10.1007/s12032-016-0736-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-016-0736-x