Abstract
ATP citrate lyase (ACLY) is responsible for the conversion of cytosolic citrate into acetyl-CoA and oxaloacetate, and the first rate-limiting enzyme involved in de novo lipogenesis. Recent studies have demonstrated that inhibition of elevated ACLY results in growth arrest and apoptosis in a subset of cancers; however, the expression pattern and underlying biological function of ACLY in pancreatic ductal adenocarcinoma (PDAC) remains unclear. In the current study, overexpressed ACLY was more commonly observed in PDAC compared to normal pancreatic tissues. Kaplan–Meier survival analysis showed that high expression level of ACLY resulted in a poor prognosis of PDAC patients. Silencing of endogenous ACLY expression by siRNA in PANC-1 cells led to reduced cell viability and increased cell apoptosis. Furthermore, significant decrease in glucose uptake and lactate production was observed after ACLY was knocked down, and this effect was blocked by 2-deoxy-d-glucose, indicating that ACLY functions in the Warburg effect affect PDAC cell growth. Collectively, this study reveals that suppression of ACLY plays an anti-tumor role through decreased Warburg effect, and ACLY-related inhibitors might be potential therapeutic approaches for PDAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite great advances in surgery, medical management, and screening, pancreatic cancer remains one of the most lethal human malignancies with a 5-year survival lower than 5 % [1]. The most common type of pancreatic cancer is pancreatic ductal adenocarcinoma (PDAC, accounting for 95 % of cases), which is characterized by a poor blood supply and a frequently hypoxic microenvironment [2]. In order to survive and grow under this stressful microenvironment, abnormal chain of metabolic alterations including enhanced glycolysis, diverted glutamine consumption, and anomalous pentose phosphate pathway (PPP) is often activated. This type of “metabolic addiction” provides nucleotides, proteins, and lipids from exogenous glucose and glutamine, which ultimately favors tumor progression [3].
It is also well known that proliferating cancer cells are highly dependent on de novo lipogenesis for fuelling membrane biogenesis. ATP citrate lyase (ACLY) is a key metabolic enzyme involved in the conversion of citrate produced by glycolysis into acetyl-CoA (AcCoA), an essential substrate for the synthesis of fatty acid and cholesterol [4]. Overexpressed ACLY has been observed in several types of cancers, including liver, breast, bladder, prostate, lung, and colorectal cancers, while its expression pattern in PDAC remains unknown [5–10]. Elevated ACLY promotes cancer cell survival and growth and enhances aggressive biological behaviors such as migration and invasion [11, 12]. In colorectal cancer, ACLY also mediates resistance of colorectal cancer HT29 cells to SN38 [9]. Inhibition of ACLY by siRNA or pharmacologic inhibitors results in growth arrest and induction of apoptosis in vitro and in vivo [6, 11], and sensitized chemonaive colorectal cancer cells to SN38 [9]. The active form of ACLY is closely correlated with tumor stage and prognosis [13]. However, the clinical significance and underlying molecular functions of ACLY in PDAC also remain unclear.
The Warburg effect, a hallmark of cancer cells, was characterized by increased glucose consumption and lactate generation even in the presence of oxygen (aerobic glycolysis) [14]. Given citrate is metabolite inhibitor of glycolysis, it is reasonable to hypothesize a possible influence of ACLY on glycolysis. Previous reports have demonstrated that glycolysis may have an implication on cancer cell differentiation by increasing cytosolic AcCoA and resultant lipogenesis [11]. Therefore, cancer cells with enhanced Warburg effect are much more sensitive to ACLY suppression. In PDAC, the oncogenic KRAS, which occurs in >90 % of cases, enables PDAC tumor maintenance through the increased uptake of glucose and subsequent shunting into glycolysis [15]. However, whether ACLY inhibition alters this metabolic phenotype of PDAC remain poorly defined.
In the present study, we firstly observed elevated ACLY expression during malignant transformation, and high ACLY expression indicates a poor prognosis. Suppression of ACLY resulted in decreased cell viability and increased caspase-3/7 activity and ultimately inhibited tumor growth. Mechanistically, ACLY inhibition accompanied with decreased Warburg effect as demonstrated by glucose consumption, lactate secretion, and glycolytic enzymes, and this effect was greatly reversed by 2-deoxy-d-glucose (2-DG) treatment.
Materials and methods
Cell culture and reagents
Human PANC-1 cells were all obtained from Cell Bank of the Chinese Academy of Sciences. Cells were cultured in DMEM media supplemented with 2 % (v/v) fetal bovine serum (FBS) and 1 % antibiotics at 37 °C in a humidified incubator with 5 % CO2. 2-DG (Sigma) was diluted to preferable concentrations in culture medium before use.
Immunohistochemistry
Two tissue microarrays (TMA) were analyzed in this study. TMA1 contained 40 cases of PDAC tissues, and normal pancreatic tissues from 10 cases were purchased from Alenabio Inc., Xi’an, China. TMA2 contained 81 cases of PDAC tissues and normal pancreatic tissues from 44 cases were purchased from Shanghai Outdo Biotech Inc. The tissue microarray of PDAC was deparaffinized and rehydrated using xylene and graded ethanol. After antigen retrieval and neutralization of endogenous peroxidase, slides were blocked with 5 % bovine serum albumin for 1 h. Slides were then incubated overnight at 4 °C with primary antibody (ACLY, Abcam; ki-67, Proteintech). After washing in phosphate-buffered saline (PBS) for three times, the section was labeled by HRP (rabbit) second antibody for 1 h and again washed three times with PBS. Visualization was performed by 3, 3′-diaminobenzidine tetrahydrochloride (DAB) and counterstained by hematoxylin. Scoring was conducted by the area of positive staining on a scale of 0–3: 0–10 % scored 0; 10–35 % scored 1; 36–70 % scored 2; more than 70 % scored 3. The scoring by the pathologists was done in a blinded manner.
Quantitative real-time PCR
All tissue samples were obtained with informed consent, and all procedures were performed in accordance with the Human Investigation Ethical Committee of the Nanjing Municipal Hospital of T.C.M. Total RNA from frozen tissue samples or PANC-1 cells was extracted with Trizol reagent (Invitrogen), and reverse transcription was performed using the PrimeScript RT-PCR kit (Takara, Japan). The mRNA levels of detected genes were quantified using an ABI Prism 7500 Sequence Detection System with SYBR Green Master Mix (Takara, Japan) and then normalized to β-actin. Primers used in this study were as follows: ACLY: forward: 5′-ATCGGTTCAAGTATGCTCGGG-3′, reverse: 5′-GACCAAGTTTTCCACGACGTT-3′; HK2: forward: 5′-TTGACCAGGAGATTGACATGGG-3′, reverse: 5′-CAACCGCATCAGGACCTCA-3′; PFKL: forward: 5′-GCTGGGCGGCACTATCATT-3′, reverse: 5′-TCAGGTGCGAGTAGGTCCG-3′; PGK2: forward: 5′-AAACTGGATGTTAGAGGGAAGCG-3′, reverse: 5′-GGCCGACCTAGATGACTCATAAG-3′; ENO1: forward: 5′-GCCGTGAACGAGAAGTCCTG-3′, reverse: 5′-ACGCCTGAAGAGACTCGGT-3′; PKM: forward: 5′-ATAACGCCTACATGGAAAAGTGT-3′, reverse: 5′-TAAGCCCATCATCCACGTAGA-3′; LDHA: forward: 5′-ATGGCAACTCTAAAGGATCAGC-3′, reverse: 5′-CCAACCCCAACAACTGTAATCT-3′; β-actin: forward: 5′-CATGTACGTTGCTATCCAGGC-3′, reverse: 5′-CTCCTTAATGTCACGCACGAT-3′.
siRNA transfection, cell viability, and caspase-3/7 activity assay
PANC-1 cells were transfected in a mixture of three siRNAs targeting ACLY as well as a negative control (GenePharma, Shanghai, China). Transfection was accomplished by seeding 2 × 105 cells into a 6-well plate, and after 24 h, the medium was aspirated and incubated with transfection complex according to the manufacturer’s protocol. The interference efficiency was detected by Western blotting. For cell viability assay, cells (3 × 103) were seeded into a 96-well plate per well supplemented in the presence of 2 % FBS (v/v) and cultured overnight. Cell viability was evaluated by Cell Counting Kit-8 (CCK-8, Dojindo, Japan) following the manufacturer’s protocols at 24, 48, 72, 96, and 120 h, and the absorbance was measured at 450 nm using a Multifunctional Microplate Reader (Tecan). The viable cells were distinguished with the fluorescent dyes Calcein-AM (Dojindo, Japan). The caspase-3/7 activity assay was performed at 72 h under serum deprivation according to the manufacturer’s instructions (Promega).
Cell invasion assay
Cell invasion assay was performed with 8.0-μm-pore inserts (Millipore, USA) in 24-well plate. Briefly, 2 × 104 cells were seeded into the matrigel-coated upper compartment of the transwell inserts. Cells were allowed to incubate for 48 h. The invaded cells were fixed and stained by 0.1 % (w/v) crystal violet. Each experiment was performed in triplicate.
Measurement of glucose and lactate
PANC-1 cells were cultured in fresh phenol red-free medium, and the culture medium was collected in the first 24 h after siRNA treatment. The lactate and glucose levels were measured using lactate assay kits (Biovision) or glucose assay kits (Life technologies) according to the manufacturer’s instructions. All experiments were repeated at least three times.
Statistical analysis
Data were presented as the mean ± SD of three independent experiments. Statistical analyses and graphical representations were performed with SPSS 16.0 (SPSS Inc.; Chicago, USA) and GraphPad Prism 5 (San Diego, CA) software. Overall survival rate was calculated according to the Kaplan–Meier method, and the difference in survival curves was evaluated by the log-rank test. The Student’s t test was used to determine the statistically significant differences among indicated experimental results. Values of p < 0.05 were considered statistically significant.
Results
Elevated expression of ACLY indicates a poor prognosis in PDAC
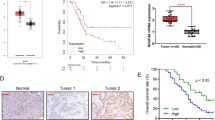
To observe the expression pattern of ACLY in PDAC, two different tissue microarrays (TMA) described in materials were analyzed using immunohistochemical staining. ACLY is expressed and active in normal pancreatic beta cells [16]. ACLY levels and activity are significantly reduced in pancreatic islets from patients with type 2 diabetes [17]. Consistent with this, in normal pancreas, ACLY immunoreactivity in the current study was exclusively distributed in islets, while immunoreactivity in acinar or ductal cells can merely be detected (Fig. 1a). However, ACLY expression was significantly up-regulated in PDAC tumor tissues (p < 0.001, Fig. 1b, c). Notably, ACLY was localized predominantly in the cytoplasm of PDAC cells (Fig. 1b). To further confirm this result, 20 paired PDAC and non-tumor tissues were collected to detect the expression of ACLY at the mRNA level using real-time quantitative PCR. Expectedly, ACLY expression was significantly up-regulated in PDAC tumor tissues compared with corresponding non-tumor tissues (p < 0.05, Fig. 1d). To determine the prognostic value of ACLY in PDAC, the relationship between ACLY expression and clinical follow-up information was analyzed by Kaplan–Meier analysis and log-rank test in TMA2. As shown in Fig. 1e, high ACLY expression was associated with decreased overall survival (p < 0.001). Taken together, these data above revealed that elevated expression of ACLY during malignant transformation indicated a poor prognosis in PDAC patients.
Elevated expression of ACLY indicates a poor prognosis in PDAC. a Representative images of the ACLY expression in normal pancreas, scale bar 200 μm. The arrows represent ACLY-positive staining pancreatic islets. b Representative images of the ACLY expression in PDAC, scale bar 100 μm. c Immunohistochemical analysis of ACLY expression in two tissue microarrays (***p < 0.001). 0–3 is the scale of ACLY expression levels. d Increased ACLY mRNA expression in 20 PDAC tissues compared with corresponding non-tumor tissues (*p < 0.05). e Kaplan–Meier curves for PDAC patients group based on ACLY expression in TMA2
Silencing of ACLY inhibits cell growth and promotes cell apoptosis
Given ACLY is a critical cytosolic enzyme involved in de novo lipogenesis, we hypothesized whether ACLY has an implication for cell growth. To test this hypothesis, cellular functions of PANC-1 cells were analyzed after transfected with a mixture of three siRNAs targeting ACLY. The protein expression of ACLY was pronounced decreased after siRNAs treatment (Fig. 2a). For CCK-8 assay, PANC-1 cells were cultured in 2 % FBS, a condition simulated the undernourished microenvironment due to intense stromal hyperplasia of PDAC. The result showed that silencing of ACLY exhibited markedly reduced cell viability in relation to the negative control cells (p < 0.05, Fig. 2b). The apoptosis assay was performed when PANC-1 cells were starved for 72 h. The data showed that the caspase-3/7 activity, an index of cell apoptosis, was significantly increased after ACLY was knocked down (p < 0.01, Fig. 2c). Viable cells as demonstrated by calcein-AM staining were also significantly decreased after ACLY was knocked down (Fig. 2d). Furthermore, cell invasion assay was performed after ACLY was silenced; however, no significant difference was found in the invaded cells between ACLY-silencing group and negative control group (Fig. 2e). To further confirm the effects of ACLY on tumor growth, the correlation between ACLY and ki-67, a marker of cell proliferation, was performed using immunohistochemical staining in TMA2 (Fig. 2f). Expectedly, ACLY expression levels in PDAC tissues were significantly associated with the levels of ki-67 (r = 0.382, p = 0.003). Collectively, above data indicated that elevated ACLY was closely correlated with tumor growth in PDAC.
Silencing of ACLY inhibits cell growth and promotes cell apoptosis. a The protein level of ACLY was detected by Western blotting in PANC-1 cells after ACLY-targeted siRNA treatment. b Cell viability of PANC-1 cells was decreased after ACLY was silenced (si-Ctrl vs. si-ACLY; *p < 0.05; **p < 0.01; ***p < 0.001). c Caspase-3/7 activity of PANC-1 cells was increased after ACLY was silenced (si-Ctrl vs. si-ACLY; **p < 0.01). d Calcein-AM staining demonstrated the survival cells upon ACLY knockdown, scale bar 100 μm. e Representative images of invaded cells in si-Ctrl and si-ACLY group, scale bar: 100 μm. f Statistical analysis of immunohistochemical results of ACLY and ki-67 expression in TMA2. p Values were calculated by the Spearman’s rank correlation test
ACLY knockdown correlates with decreased Warburg effect in PANC-1 cells
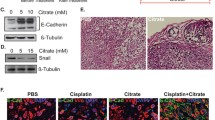
In PDAC, the mechanism of ACLY functions remains unclear. Occasionally, we found that acidification of the culture medium of ACLY-silenced PANC-1 cells was more slow than negative control cells (Fig. 3a). We therefore hypothesized whether ACLY functions through Warburg effect, a phenomenon characterized by increased lactate production and glucose consumption. Indeed, lactate secretion and glucose uptake were significantly decreased after ACLY was knocked down (p < 0.05, Fig. 3b, c). Meanwhile, several key glycolytic enzymes, such as phosphofructokinase (PFKL), Enolase 1 (ENO1), and lactate dehydrogenase A (LDHA), were also down-regulated upon ACLY-targeted siRNA treatment (p < 0.05, Fig. 3d). Given the Warburg effect was significantly reduced by ACLY knockdown, we thereby hypothesized whether ACLY functions through regulating the Warburg effect (Fig. 3e). To test this hypothesis, cell viability and caspase-3/7 activity were measured after treatment with 2-DG, a glycolysis inhibitor. Although decreased cell viability and increased caspase-3/7 activity were induced by 2-DG, the disadvantage conferred by ACLY knockdown was also completely abolished (Fig. 3f, g). Taken together, these findings suggested that decreased Warburg effect accounts for the growth arrest effect induced by ACLY inhibition.
ACLY knockdown correlates with decreased Warburg effect in PANC-1 cells. a Acidification of the culture medium was evaluated by visually inspecting the color of the medium. Lactate production in the culture medium (b) and glucose consumption (c) was measured and normalized based on protein concentration (si-Ctrl vs. si-ACLY; *p < 0.05; **p < 0.01). d Quantification of glycolytic enzymes by real-time quantitative PCR analysis (si-Ctrl vs. si-ACLY; *p < 0.05). e The relationship between ACLY and glycolysis. In the presence of 2-DG, cell viability (f) and caspase-3/7 activity (g) of PANC-1 cells were analyzed after ACLY was silenced
Discussion
It has been well established that ACLY is the first key enzyme for producing AcCoA, which is needed for de novo lipogenesis and substrate acetylation [18]. Overexpressed ACLY or activation has been found pervasively in cancers. In the present study, we first observed elevated ACLY in PDAC tissues and its clinical significance in prognosis by analysis of two different TMAs. By suppression of ACLY in PANC-1 cells, we showed that elevated ACLY was essential for tumor growth, and this effect was mediated by Warburg effect.
ACLY is widely distributed in many tissues, such as fat, liver, and pancreatic beta cells, and deletion of this gene results in embryonic lethality [19]. Consistent with this, we observed that immunoreactivity of ACLY was exclusively located in pancreatic islets. However, in addition to islets, intense immunoreactivity of ACLY was also observed in the cytoplasm of PDAC cancer cells. Importantly, high ACLY expression was significantly associated with poor prognosis in our cohort. Hence, we determined the cellular functions of ACLY in PDAC cells. Our data clearly suggested that ACLY knockdown has anti-tumor potential in PANC-1 cells as evidenced by decreased cell viability and increased caspase-3/7 activity. This effect was consistent with previous findings by other research groups [11, 20].
Abnormal ACLY signaling has been reported in many kinds of human cancers except PDAC. It was convinced that overexpressed ACLY is necessary for tumor growth through the production of AcCoA for lipogenesis so that inhibition of ACLY effectively abolished the growth of tumor. Previous reports have demonstrated that inhibition of ACLY/AKT exhibits an anti-tumor effect through increasing mitochondrial reactive oxygen species (ROS) generation [20]. In colorectal cancer, combined inhibition of AKT signaling and ACLY successfully resensitized SN38-resistant cells to SN38 [9]. Besides, ACLY knockdown activates AMP-activated protein kinase (AMPK), and activated AMPK may facilitate p53-induced senescence or apoptosis, ultimately leading to tumor progression [21]. Dysregulation of cellular metabolism is a hallmark of cancer cells [22]. Both elevated glycolysis and increased lipogenesis play crucial role in tumor growth. Because decreased citrate can regulate glycolysis through activating phosphofructokinase (PFK), increased oxaloacetate can promote gluconeogenesis and glycolysis, so that ACLY can also promote cell growth through coordination with cytosolic levels of citrate and oxaloacetate [23]. Because ACLY is a cross-link between glucose and lipid metabolism, one may expect a potential role of ACLY in glucose regulation. Our study suggested that ACLY knockdown showed decreased lactate secretion, glucose consumption, and reduced expression of glycolytic enzymes and may cause tumor growth inhibition. Similar phenomenon was also reported in glioblastomas, that is, an association of up-regulated ACLY and ENO1, indicating that ACLY acts as a positive regulator of glycolysis [24]. Meanwhile, supplying 2-DG into the culture medium completely blocked the growth disadvantage induced by ACLY suppression. This finding further confirmed that ACLY exhibits prosurvival role through Warburg effect.
In conclusion, our results from both clinical specimens and in vitro cell experiments demonstrated that ACLY expression was up-regulated in PDAC, and ACLY knockdown caused tumor growth arrest through reduced Warburg effect. We proposed that inhibition of ACLY-related pathway may represent a novel therapeutic strategy for controlling tumor growth in PDAC. Apart from PDAC, our researches in colorectal cancer and anal fistula also revealed an elevated expression of ACLY (data not shown), indicating the broad-spectrum roles of ACLY in human diseases. Notably, many ACLY inhibitors developed offering a new insight into potential clinical application.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi:10.3322/caac.21208.
The Lancet Oncology. Pancreatic cancer in the spotlight. Lancet Oncol. 2014;15(3):241. doi:10.1016/S1470-2045(14)70097-X.
Finley LW, Zhang J, Ye J, Ward PS, Thompson CB. SnapShot: cancer metabolism pathways. Cell Metab. 2013;17(3):466. doi:10.1016/j.cmet.2013.02.016.
Watson JA, Fang M, Lowenstein JM. Tricarballylate and hydroxycitrate: substrate and inhibitor of ATP: citrate oxaloacetate lyase. Arch Biochem Biophys. 1969;135(1):209–17.
Yancy HF, Mason JA, Peters S, Thompson CE 3rd, Littleton GK, Jett M, et al. Metastatic progression and gene expression between breast cancer cell lines from African American and Caucasian women. J Carcinog. 2007;6:8. doi:10.1186/1477-3163-6-8.
Migita T, Narita T, Nomura K, Miyagi E, Inazuka F, Matsuura M, et al. ATP citrate lyase: activation and therapeutic implications in non-small cell lung cancer. Cancer Res. 2008;68(20):8547–54. doi:10.1158/0008-5472.CAN-08-1235.
Varis A, Wolf M, Monni O, Vakkari ML, Kokkola A, Moskaluk C, et al. Targets of gene amplification and overexpression at 17q in gastric cancer. Cancer Res. 2002;62(9):2625–9.
Turyn J, Schlichtholz B, Dettlaff-Pokora A, Presler M, Goyke E, Matuszewski M, et al. Increased activity of glycerol 3-phosphate dehydrogenase and other lipogenic enzymes in human bladder cancer. Horm Metab Res. 2003;35(10):565–9. doi:10.1055/s-2003-43500.
Zhou Y, Bollu LR, Tozzi F, Ye X, Bhattacharya R, Gao G, et al. ATP citrate lyase mediates resistance of colorectal cancer cells to SN38. Mol Cancer Ther. 2013;12(12):2782–91. doi:10.1158/1535-7163.MCT-13-0098.
Gao Y, Islam MS, Tian J, Lui VW, Xiao D. Inactivation of ATP citrate lyase by Cucurbitacin B: a bioactive compound from cucumber, inhibits prostate cancer growth. Cancer Lett. 2014;349(1):15–25. doi:10.1016/j.canlet.2014.03.015.
Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8(4):311–21. doi:10.1016/j.ccr.2005.09.008.
Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009;100(9):1369–72. doi:10.1038/sj.bjc.6605007.
Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24(41):6314–22. doi:10.1038/sj.onc.1208773.
Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–70.
Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149(3):656–70. doi:10.1016/j.cell.2012.01.058.
MacDonald MJ, Smith AD 3rd, Hasan NM, Sabat G, Fahien LA. Feasibility of pathways for transfer of acyl groups from mitochondria to the cytosol to form short chain acyl-CoAs in the pancreatic beta cell. J Biol Chem. 2007;282(42):30596–606. doi:10.1074/jbc.M702732200.
MacDonald MJ, Longacre MJ, Langberg EC, Tibell A, Kendrick MA, Fukao T, et al. Decreased levels of metabolic enzymes in pancreatic islets of patients with type 2 diabetes. Diabetologia. 2009;52(6):1087–91. doi:10.1007/s00125-009-1319-6.
Zaidi N, Swinnen JV, Smans K. ATP-citrate lyase: a key player in cancer metabolism. Cancer Res. 2012;72(15):3709–14. doi:10.1158/0008-5472.CAN-11-4112.
Beigneux AP, Kosinski C, Gavino B, Horton JD, Skarnes WC, Young SG. ATP-citrate lyase deficiency in the mouse. J Biol Chem. 2004;279(10):9557–64. doi:10.1074/jbc.M310512200.
Migita T, Okabe S, Ikeda K, Igarashi S, Sugawara S, Tomida A, et al. Inhibition of ATP citrate lyase induces an anticancer effect via reactive oxygen species: AMPK as a predictive biomarker for therapeutic impact. Am J Pathol. 2013;182(5):1800–10. doi:10.1016/j.ajpath.2013.01.048.
Lee JH, Jang H, Lee SM, Lee JE, Choi J, Kim TW, et al. ATP-citrate lyase regulates cellular senescence via an AMPK- and p53-dependent pathway. FEBS J. 2015;282(2):361–71. doi:10.1111/febs.13139.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi:10.1016/j.cell.2011.02.013.
Potapova IA, El-Maghrabi MR, Doronin SV, Benjamin WB. Phosphorylation of recombinant human ATP:citrate lyase by cAMP-dependent protein kinase abolishes homotropic allosteric regulation of the enzyme by citrate and increases the enzyme activity. Allosteric activation of ATP:citrate lyase by phosphorylated sugars. Biochemistry. 2000;39(5):1169–79.
Beckner ME, Fellows-Mayle W, Zhang Z, Agostino NR, Kant JA, Day BW, et al. Identification of ATP citrate lyase as a positive regulator of glycolytic function in glioblastomas. Int J Cancer. 2010;126(10):2282–95. doi:10.1002/ijc.24918.
Acknowledgments
This research is supported by Health Bureau of Nanjing, China (Grant No. ZKX12040).
Conflict of interest
The authors declare that there is no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Haifeng Zong and Yang Zhang have contributed equally to this work.
"This article has been retracted by the authors as it was discovered that duplication of several key experiments described herein were not possible because: The difference between acidification of culture medium in si-Ctrl group and si-ACLY could not be observed and the results of lactate production and glucose consumption could not be repeated. The authors regret any confusion caused."
About this article
Cite this article
Zong, H., Zhang, Y., You, Y. et al. RETRACTED ARTICLE: Decreased Warburg effect induced by ATP citrate lyase suppression inhibits tumor growth in pancreatic cancer. Med Oncol 32, 85 (2015). https://doi.org/10.1007/s12032-015-0540-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0540-z