Abstract
Sacral chordoma is an aggressive, locally invasive neoplasm, and has a poor prognosis. However, the molecular basis for the clinical behavior remains unknown. The purpose of this study was to investigate the expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and mammalian target of rapamycin (mTOR) in sacral chordoma, and explore their roles in the prognosis. PTEN and mTOR were detected immunohistochemically in 40 sacral chordoma tissues and 10 adjacent normal tissues. Correlations between PTEN and mTOR expression and clinicopathological factors were analyzed. Kaplan–Meier survival curves and log-rank test were used to analyze the continuous disease-free survival time (CDFS). The expression of PTEN in sacral chordoma was significantly lower than that in adjacent normal tissues, while the levels of mTOR expression in sacral chordoma were significantly higher than that in adjacent normal tissues (P = 0.000, P = 0.030). The positive expression of mTOR appears to correlate with the negative expression of PTEN in sacral chordoma (P = 0.021). PTEN-negative expression and mTOR-positive expression were associated with tumor invasion into the surrounding muscles (P = 0.038, P = 0.014). Log-rank test showed that PTEN-negative and mTOR-positive expressions had an important impact on the patients’ CDFS (P = 0.011, P = 0.015). Our results suggest that PTEN and mTOR might play an important role in the local invasiveness of sacral chordoma. PTEN and mTOR might be recognized as important prognostic predictors of recurrence and could be used as potential therapeutic targets for the treatment for sacral chordoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chordomas are relatively rare, slow-growing, and locally aggressive malignant bone tumors with an incidence of about 0.1/100000/year, and approximately 50–60 % of chordomas occur at the sacrum [1]. Surgery is the principal treatment option as the tumors are largely resistant to chemotherapy and radiotherapy. However, despite aggressive surgical resection, local recurrence rates of sacral chordoma were over 40 %, which leading to a poor quality of life and outcome [2]. The overall median survival time for chordoma has been estimated to be approximately 6 years, with a survival rate of 70 % at 5 years, falling to 40 % at 10 years [3]. To date, there is inadequate understanding of the biology of sacral chordoma.
Phosphatase and tensin homolog deleted on chromosome ten (PTEN) is a tumor suppressor gene that locates on chromosome 10q23 and encodes proteins regulating various signal transduction pathways and modulating cell growth processes, cell migration, and apoptosis [4]. PTEN is a negative regulator of the phosphoinositide 3-kinase (PI3 K) signal transduction pathway. Sitting downstream of PI3 K is the mammalian target of rapamycin (mTOR), a 289-kDa serine/threonine kinase that phosphorylates a series of downstream effectors involved in protein biosynthesis, ribosome biogenesis, and transcription of genes crucial to cell growth [5]. The PI3 K-AKT-mTOR pathway is an important cellular pathway involved in cell growth, tumorigenesis, cell invasion, and drug response. Negative expression of PTEN may result in increased mTOR activity [6].
To our knowledge, few studies have examined the clinical significance of the expression of PTEN and mTOR in sacral chordoma. Therefore, the aim of this present study was to evaluate the immunohistochemical expression levels of PTEN and mTOR in sacral chordoma and explore their correlations with patient’s pathological parameters and prognosis.
Materials and methods
Patients and tumor samples
Forty patients diagnosed with sacral chordoma and treated by primary tumor resection at the First Affiliated Hospital of Soochow University (Suzhou, China) between 1996 and 2011 were identified. In addition, ten adjacent normal tissues, which obtained at least 3 cm apart from the surgical margins, were included as a control group. Of the 40 patients, seventeen were females and twenty-three were males. The mean age at the time of surgery was 52.4 years (range 18–77 years). The medical records were reviewed to obtain clinical information for each case, including age, gender, tumor location, tumor size, and surrounding muscle invasion. Surrounding muscle invasion means the tumor invasion into the surrounding muscles, which was analyzed by preoperative magnetic resonance (MR) images. Histologic sections were stained routinely with hematoxylin–eosin for diagnostic purpose. The histology of all patients was confirmed. The following immunohistochemical studies were performed using sections from 10 % formalin-fixed, paraffin-embedded tissues, highlighting the representative areas of the tumors. All patients gave informed consent. This study was approved by the local Institutional Ethics Committee.
Immunohistochemical staining
Immunohistochemical staining was performed with EnVision two-step staining method on 4-μm-thick histologic sections of paraffin-embedded tissues. The samples were dewaxed with xylene/ethanol before antigen retrieval. The primary antibodies used were mouse monoclonal anti-PTEN and rabbit monoclonal anti-mTOR (Ser473) antibody (Cell Signaling Technologies, Beverly, MA, USA; diluted at 1:50). Reagents for immunohistochemistry were purchased from Bio Genex (SanRamon, CA, USA). The standard streptavidin–biotin–peroxidase complex method was employed to bind to the primary antibody along with Multilink concentrated biotinylated anti-IgG as a secondary antibody. The antibody binding was visualized with DAB before brief counterstaining with hematoxylin. As a negative control, sections were treated with phosphate-buffered saline with omission of the primary antibody.
Immunohistochemical staining was evaluated by two independent experienced pathologists who were blinded to the clinicopathological parameters and clinical outcomes of the patients. A semiquantitative assessment of protein expression was utilized to score PTEN and mTOR in chordoma [7]. The intensity of staining was scored as 0 (negative), 1 (weak), 2 (medium), or 3 (strong). The extent of staining was scored as 0(0 %), 1 (<20 %), 2 (20–50 %), and 3 (>50 %), according to the percentage of cells stained positive for each protein. The sum of the intensity and extent scores was utilized as the final score (0–6). Tissue specimens having a final score less than 2 were considered negative. Scores 3–6 were graded as positive.
Follow-up
Follow-up including plain radiographs, computed tomography scans, and MR imaging evaluation was performed at 3-month intervals in the first 2 years postoperatively and at 6-month intervals after 3 years. Continuous disease-free survival time (CDFS) was defined as the time interval from preliminary diagnosis to disease relapse.
Statistical analysis
Statistical analyses were completed using SPSS 16.0 statistical software (SPSS Inc., Chicago, IL). Comparisons of quantitative data between two groups were performed using a Student’s t test. Fisher’s exact test was utilized to compare the clinicopathological characteristics of the patients. Correlation between two proteases in scores was described by using the Spearman’s rank correlation test. CDFS was estimated by using Kaplan–Meier survival curves, and log-rank test was used to evaluate the influences of PTEN and mTOR expression on CDFS. P < 0.05 was considered statistically significant.
Results
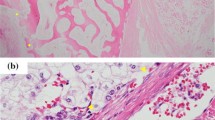
Expression of PTEN showed a nuclear and cytoplasmic staining pattern with diverse intensity. The positive expression of PTEN in the sacral chordoma was 25 % (10/40), significantly lower than that in the normal tissues (P = 0.000). Expression of mTOR showed mainly in the cytoplasm of tumor cells. The positive expression of mTOR in the sacral chordoma was 62.5 % (25/40), significantly higher than that in the normal tissues (P = 0.030) (Fig. 1; Table 1). The overall mean expression scores for PTEN and mTOR staining in sacral chordoma were 1.23 and 3.62, respectively. Positive expression of mTOR appears to correlate with negative expression of PTEN in sacral chordoma tissues (P = 0.021). Patients who had surrounding muscle invasion had a higher PTEN-negative expression rate (93.3 %) and mTOR-positive expression rate (86.7 %) than those without surrounding muscle invasion (64, 48 %). PTEN-negative expression and mTOR-positive expression were associated with tumor invasion into the surrounding muscles (P = 0.038, P = 0.014). No significant correlations were observed between the mTOR or PTEN expression and the patients’ age, gender, tumor size, and tumor location (Table 2).
Follow-up information was obtained in all cases. The median CDFS time was 46.5 months (range 6–160 months). There were eighteen (45 %) patients who developed local recurrences. The average recurrence time was 30.7 months (range 6–62 months). In ten patients with PTEN positive, only 10 % (1/10) had recurrence, while in the mTOR-positive group, 83.3 % (15/18) had recurrence. Log-rank test showed that patients with negative PTEN expression had a poorer prognosis than those with positive PTEN expression (P = 0.011). Meanwhile, the difference in CDFS rates between positive mTOR group and negative mTOR group was also statistically significant (P = 0.015) (Fig. 2). The results showed that mTOR and PTEN had an important influence on the prognosis of sacral chordoma.
Discussion
Sacral chordoma is an aggressive, locally invasive neoplasm, and has a poor prognosis. Our previous studies reported that several factors might predict the survival of sacral chordoma patients and one of the most important factors was the local invasion. [8]. However, the molecular basis for the clinical behavior remains unknown. Consequently, great efforts have been taken recently to identify novel prognostic and predictive biomarkers for patients with sacral chordoma [9, 10].
Current studies have suggested that the PI3 K-AKT-mTOR pathway is involved in cellular survival by inhibiting apoptotic processes and stimulating cell growth and proliferation [11, 12]. The PI3 K-AKT-mTOR pathway is commonly activated in human cancers and has been recognized as a potential therapeutic target for chordoma [13, 14]. In addition, it was demonstrated that PTEN could downregulate the PI3 K/AKT/mTOR pathway via its lipid phosphatase activity, which places PTEN into a mechanistically critical position [15]. However, the clinical significance of the activation of PTEN and mTOR in sacral chordoma remains uncertain. Therefore, in this present study, we aimed to detect the protein levels and correlations of PTEN and mTOR in sacral chordoma tissues and investigate the correlations of the expression levels of PTEN and mTOR with the clinicopathological variables and CDFS of sacral chordoma.
PTEN mutations are frequently found in glioblastoma, hepatocellular carcinoma, lung carcinoma, melanoma, endometrial carcinoma, and prostate cancer [6, 16, 17]. Given the predominance of 1p heterozygosity in chordoma, activating mutations in the mTOR gene that is located on 1p36.2 may be present in chordoma [15]. By immunohistochemical staining, we showed that the positive rates of PTEN and mTOR expressions in sacral chordoma were 25 % (10/40) and 62.5 % (25/40), respectively, which is significantly different in adjacent normal tissues (P < 0.05). Presneau et al. [13] reported that the positive rate of mTOR expression in 50 chordoma cases was 75 %. Our results indicated that the negative expression of the tumor suppressor PTEN and positive expression of the mTOR might be an important step in sacral chordoma development.
In addition, a significant correlation was found between negative expression of PTEN and positive expression of mTOR in sacral chordomas, indicating that mTOR and PTEN might co-regulate the progression of the tumor and participate in proliferation, invasion, and metastasis of sacral chordoma. Moreover, Bakarakos et al. [18] discovered that the loss of PTEN and activation of mTOR were closely correlated with breast cancer. In vitro, studies suggested that PTEN is capable of inhibiting cell proliferation and promoting apoptosis via inhibition of the activity of the PI3 K-Akt-mTOR pathway [19]. Han et al. [20] demonstrated that the loss of PTEN, which result in constitutive activation of Akt/mTORC1 signaling, may contribute to the advance of sporadic chordomas. Our results suggest that the inactivation of PTEN may be responsible for mTOR activation and consequent development of sacral chordoma.
Patients with sacral chordoma develop fatal local recurrences frequently. Previous study from our institution demonstrated that the presence of surrounding muscle invasion was one of the most important factors of prognosis [8]. However, the molecular basis remains unknown. Wang et al. [21] reported that PTEN and mTOR were crucial determinants of the prognosis in patients with non-small cell lung cancer. McBride et al. [6] reported that the PTEN methylated patients tend to have statistically significantly shorter survival time. Current study revealed that PTEN-negative expression and mTOR-positive expression were associated with local invasion (P < 0.05). More importantly, the log-rank test revealed that the CDFS was significantly shorter in patients with negative expression of PTEN and positive expression of mTOR. The results suggested that positive expression of mTOR and negative expression of PTEN linked to the recurrence of sacral chordoma. Based on the published data and our current data, mTOR and PTEN may be recognized as important prognostic indicators for sacral chordoma patients.
In conclusion, we found that negative expression of PTEN and positive expression of mTOR are strongly associated with sacral chordoma. Our results suggest that PTEN and mTOR were predictive factors for the prognosis of sacral chordoma. Expression of PTEN and mTOR in sacral chordoma samples may prove to be a valuable diagnostic and prognostic biomarker in sacral chordoma patients.
References
Casali PG, Stacchiotti S, Sangalli C, Olmi P, Gronchi A. Chordoma. Curr Opin Oncol. 2007;19:367–70.
Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005;87:2211–6.
Chugh R, Tawbi H, Lucas DR, Biermann JS, Schuetze SM, Baker LH. Chordoma: the nonsarcoma primary bone tumor. Oncologist. 2007;12:1344–50.
Pal SK, Figlin RA, Reckamp KL. The role of targeting mammalian target of rapamycin in lung cancer. Clin Lung Cancer. 2008;9:340–5.
Contreras AG, Dormond O, Edelbauer M, Calzadilla K, Hoerning A, Pal S, Briscoe DM. mTOR-understanding the clinical effects. Transplant Proc. 2008;40:S9–12.
McBride SM, Perez DA, Polley MY, et al. Activation of PI3 K/mTOR pathway occurs in most adult low-grade gliomas and predicts patient survival. J Neurooncol. 2010;97:33–40.
Han W, Ming M, He TC, He YY. Immunosuppressive cyclospor in Aactivates AKT in keratinocytes through PTEN suppression: implications in skin carcinogenesis. J Biol Chem. 2010;285:11369–77.
Chen KW, Yang HL, Lu J, Liu JY, Chen XQ. Prognostic factors of sacral chordoma after surgical therapy: a study of 36 patients. Spinal Cord. 2010;48:166–71.
Chen KW, Yang HL, Lu J, et al. Expression of vascular endothelial growth factor and matrix metalloproteinase-9 in sacral chordoma. J Neurooncol. 2011;101:357–63.
Chen C, Yang HL, Chen KW, et al. High expression of survivin in sacral chordoma. Med Oncol. 2013;30:529.
Rosner M, Hanneder M, Siegel N, Valli A, Fuchs C, Hengstschlager M. The mTOR pathway and its role in human genetic diseases. Mutat Res. 2008;659:284–92.
Scheper MA, Chaisuparat R, Nikitakis NG, Sauk JJ. Expression and alterations of the PTEN/AKT/mTOR pathway in ameloblastomas. Oral Dis. 2008;14:561–8.
Presneau N, Shalaby A, Idowu B, et al. Potential therapeutic targets for chordoma: PI3 K/AKT/TSC1/TSC2/mTOR pathway. Br J Cancer. 2009;100:1406–14.
Schwab J, Antonescu C, Boland P, et al. Combination of PI3 K/mTOR inhibition demonstrates efficacy in human chordoma. Anticancer Res. 2009;29:1867–71.
Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45.
Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7.
Risinger JI, Hayes AK, Berchuck A, Barrett JC. PTEN/MMAC1 mutations in endometrial cancers. Cancer Res. 1997;57:4736–8.
Bakarakos P, Theohari I, Nomikos A, Mylona E, et al. Immunohistochemical study of PTEN and phosphorylated mTOR proteins in familial and sporadic invasive breastcarcinomas. Histopathology. 2010;56:876–82.
Cheng ZY, Guo XL, Yang XY, et al. PTEN and rapamycin inhibiting the growth of K562 cells through regulating mTOR signaling pathway. J Exp Clin Cancer Res. 2008;27:87.
Han S, Polizzano C, Nielsen GP, Hornicek FJ, Rosenberg AE, Ramesh V. Aberrant hyperactivation of akt and mammalian target of rapamycin complex 1 signaling in sporadic chordomas. Clin Cancer Res. 2009;15:1940–6.
Wang L, Yue W, Zhang L, Zhao X, Wang Y, Xu S. mTOR and PTEN expression in non-small cell lung cancer: analysis by real-time fluorescence quantitative polymerase chain reaction and immunohistochemistry. Surg Today. 2012;42:419–25.
Acknowledgments
We would like to thank Yongping Gu for technical guidance during the immunohistochemical analysis.
Conflict of interest
None.
Ethical standard
This study was approved by the Committee on Medical Ethics of the First affiliated Hospital of SooChow University.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Kangwu Chen and Jianqiang Mo have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, K., Mo, J., Zhou, M. et al. Expression of PTEN and mTOR in sacral chordoma and association with poor prognosis. Med Oncol 31, 886 (2014). https://doi.org/10.1007/s12032-014-0886-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0886-7