Abstract
Optimal treatment strategies for localized extranodal natural killer/T cell lymphoma (ENKTL) have not been fully defined. We retrospectively compared the efficacy and safety of combined gemcitabine, l-asparaginase, and oxaliplatin (GELOX) (n = 38), continuous infusion of etoposide, vincristine and doxorubicin, with cyclophosphamide and prednisone (EPOCH) (n = 54), or combined cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) (n = 135) as induction chemotherapy in patients who were newly diagnosed with stage I/II ENKTL. After induction chemotherapy, the complete response (CR) rate and overall response rate (ORR) for the GELOX group were significantly higher than those in the EPOCH group (68.4 vs. 42.6 %, P = 0.011 for CR and 86.8 vs. 68.5 %, P = 0.038 for ORR). Both EPOCH and GELOX groups can attain much higher CR rates than CHOP group (CR rate was 31.8 %, P < 0.05). The 3-year overall survival (OS) and progression-free survival (PFS) rate were significantly better in GELOX group than in EPOCH or CHOP group (87.0 vs. 54.0 vs. 54.0 % for OS, P < 0.05; 72.0 vs. 50.0 vs. 43.0 % for PFS, P < 0.05). However, no significant differences were found between EPOCH and CHOP groups in OS or PFS (P = 0.765 for OS, and 0.421 for PFS). The safety profiles were acceptable in all three groups. In conclusion, GELOX is superior to EPOCH or CHOP in the treatment of patients with stage I/II ENKTL. Further clinical trials of ENKTL should use asparaginase-based regimens as the standard chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the World Health Organization classification of lymphoid tumors, extranodal natural killer/T cell lymphoma (ENKTL) is a distinct entity of non-Hodgkin lymphoma (NHL) [1], which is featured by angiodestruction, obvious necrosis, and cytotoxic phenotypes [2]. There is a racial and geographical predisposition of ENKTL, and although uncommon in western countries, ENKTL accounts for 5–10 % of all malignant lymphomas in China [3]. Most patients of ENKTL are diagnosed as stage IE/IIE disease, but the prognosis was still poor due to rapid disease progression and recurrence in the past decades [4]. Because ENKTL is a recently recognized entity, optimal treatment strategies for ENKTL have not been fully defined. For patients with localized disease, radiation therapy (RT) is widely administered due to the rapid response. However, approximately, 50 % of patients who receive RT alone experience local relapse, and systemic failure reportedly occurs in about 25 % of patients [5]. Therefore, the addition of chemotherapy is usually incorporated to reduce the risk of recurrence. Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) are the most commonly used regimen for NHL in the past decades, which did not work well in ENKTL as in B cell NHL, and more than 60 % patients of ENKTL are resistant to CHOP due to overexpression of the multidrug-resistant (MDR) gene, with an increase in the amount of P-glycoprotein in NK/T-cell lymphoma cells [6]. Several studies have demonstrated that changing the schedule of drug delivery to continuous infusion might partially overcome drug resistance caused by MDR induction of P-glycoprotein [7]. It was reported that continuous infusion of etoposide, vincristine, and doxorubicin, with cyclophosphamide and prednisone (EPOCH) followed by RT yielded promising results [8]. Recently, more and more l-asparaginase-based regimens such as combined dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide (SMILE) [9], combined asparaginase, methotrexate, and dexamethasone (AspaMetDex) [10], and combined gemcitabine, l-asparaginase, and oxaliplatin (GELOX) have emerged with promising results in the treatment of patients with ENKTL [11]. However, no prospective studies have been conducted to compare the efficacy of those regimens in the treatment of ENKTL. Here, we reported a retrospective study comparing the efficacy of GELOX, EPOCH, and CHOP in a cohort of 227 patients with long-term follow-up and found that GELOX is superior to EPOCH and CHOP in the treatment of ENKTL with acceptable toxicities.
Methods and materials
Patients
Two hundred and eighty one patients were diagnosed as ENKTL in Sun Yat-sen University Cancer Center between January 1995 and July 2012, among whom 227 patients of stage IE/IIE received CHOP, EPOCH, or GELOX as induction therapy. The inclusion criteria of this retrospective study were as follows: (1) pathologically confirmed diagnosis of ENKTL based on the WHO classification of Tumours of Haematopoietic and Lymphoid Tissues [1]; (2) Ann Arbor stage IE to IIE; (3) previously untreated patients; (4) no previous or concomitant malignancies; and (5) a complete set of clinical information and follow-up data. Approval to review, analyze, and publish the data in this study was given by the Sun Yat-sen University Cancer Center Research Ethics Board. Informed consent for the collection of medical information was obtained from all patients at their first visit.
Patients were staged based on the Ann Arbor staging system and international prognostic scores (IPI) were calculated based on demographic characteristics, physical examination, routine blood tests, computed tomography (CT) scan of the chest, abdomen, and pelvis, magnetic resonance imaging (MRI) of the head and neck, and bilateral bone marrow aspiration or biopsy. Positron emission tomography (PET)-CT, bone marrow EBER stain, or EBV DNA blood levels were not routine staging investigations in the study. Follow-up information was obtained from patients’ medical records or by telephone.
Treatment
Patients of stage IE/IIE ENKTL received CHOP, EPOCH, or GELOX as induction therapy according to the treating physicians, and the dosage and schedule of these three regimens were as previously reported [8, 11, 12]. In brief, CHOP (cyclophosphamide 750 mg/m2 on day 1; doxorubicin 50 mg/m2 on day 1; vincristine 1.4 mg/m2, maximal dose 2.0 mg, on day 1; and prednisolone 100 mg daily p.o. on day 1–5; repeated at 21-day intervals), EPOCH (24 h of continuous infusion of etoposide 50 mg/m2/day, vincristine 0.4 mg/m2/day and doxorubicin 10 mg/m2/day administered on day 1–4, followed by cyclophosphamide 750 mg/m2/day over 15 min intravenously on day 5 and prednisone 60 mg/m2/day on day 1–5 orally; repeated at 21-day intervals), or GELOX (gemcitabine 1,000 mg/m2/day on day 1 and 8, oxaliplatin 130 mg/m2/day on day 1 and l-asparaginase 6,000 IU/m2/day on day 1–7, administered intravenously, repeated every 3 weeks. Pegaspargase was used instead of l-asparaginase for patients who experienced hypersensitivity to l-asparaginase, and in accordance with the dose intensity, the GELOX regimen was modified to: gemcitabine 1,250 mg/m2/day, oxaliplatin 85 mg/m2/day, administered intravenously, and pegaspargase 2,500 IU/m2/day injected intramuscularly on day 1, repeated every 2 weeks) were administered before radiation therapy (RT).
After at least 2 cycles of induction chemotherapy, patients who had achieved stable disease (SD) following 2 cycles, partial response (PR) after four cycles or complete response (CR) after 6 cycles of chemotherapy were referred to primary involved-field radiation therapy (IFRT). A maximum of 6–8 chemotherapy cycles were administered. Second-line chemotherapy was administered to patients who suffered from progressive disease during chemotherapy. Primary IFRT was delivered using 6-MeV linear accelerator and three-dimensional conformal treatment planning was used. The IFRT dose was 56 Gy in 28 fractions, with 2 Gy a day and 5 fractions each week. We defined the clinical target volume (CTV) of limited stage IE disease as the bilateral nasal cavity, bilateral ethmoid sinuses, and ipsilateral maxillary sinus, and the CTV extended to involved tissues for patients with extensive stage IE disease. As to stage IIE disease, CTV also included the bilateral cervical lymph node area.
Response and toxicity criteria
The treatment response was assessed after every 2 cycles of chemotherapy or before and after radiotherapy in accordance with standard response criteria for non-Hodgkin lymphoma. After completion of treatment, patients were followed up by their oncologist in the outpatient department. Each follow-up visit consisted of a physical examination, complete blood count, serum biochemistry including LDH levels, and either a CT scan, MRI of the involved regions, or PET-CT scan. Follow-up visits were conducted every 3 months for the first 2 years, every 6 months for the next 3 years, and annually after the initial 5 years. All adverse events following treatment were graded based on the National Cancer Institute Common Terminology Criteria of Adverse Events v3.0.
Statistical analysis
Overall response rate (ORR) was defined as complete response (CR) rate plus partial response (PR) rate. Overall survival (OS) was calculated from the time of diagnosis until death from any cause or until the time of the last follow-up visit for the surviving patients. Progression-free survival (PFS) was defined as the interval from the time of diagnosis to the time of first documented disease progression or relapse or to the time of the last follow-up visit. The chi-square test was used to calculate statistical group comparisons of categorical variables. Survival analysis was performed using the Kaplan–Meier method, and comparisons were calculated using the log-rank test. Multivariate analysis was used to estimate the prognostic impact of different variables in OS and PFS using the Cox regression model. P < 0.05 was considered statistically significant, and all P values correspond to two-sided significance tests. Statistical analyses were performed using SPSS 16.0 software.
Results
Patient characteristics
The patient characteristics were shown in Table 1. In this cohort of 227 patients with ENKTL, the mean age was 44 years (range 17–80 years), with 40 patients (17.2 %) being older than 60 years. The distribution of IPI score included 215 patients (94.7 %) with IPI scores of 0–1 (low-risk group) and 12 (5.3 %) with IPI scores of 2–3 (high-risk group). Before 2005, most patients received CHOP as induction therapy (59.5 % of patients in this cohort), and in recent years, more and more patients were given EPOCH or GELOX as induction therapy (23.8 and 16.7 % of patients in this cohort, respectively). There were no significant differences in the rate of these characteristics among these three treatment groups (P > 0.05).
Response and treatment outcomes
Tables 2 and 3 summarized the overall results of these three groups. In the GELOX, EPOCH, and CHOP groups, there were 11 (28.9 %), 17 (32.1 %), and 37 (27.4 %) patients, respectively, not receiving radiotherapy due to high treatment expenses, disease progression, or other personal reasons. After first-line induction chemotherapy, the CR and ORR rates for the GELOX group were significantly higher than those in the EPOCH group (68.4 vs. 42.6 %, P = 0.011 for CR and 86.8 vs. 68.5 %, P = 0.038 for ORR). Both EPOCH and GELOX can attain much higher CR rates after chemotherapy than CHOP (CR rate after chemotherapy was 31.8 % in CHOP group, P < 0.05). After chemoradiotherapy, the CR rates for GELOX, EPOCH, and CHOP groups were 92.6, 89.2, and 80.6 %, respectively, and there was no significant difference among these three groups (P > 0.05). Furthermore, there was no significant difference in the treatment outcomes for patients receiving either type of asparaginase within the GELOX group (l-asparaginase vs. pegaspargase, data not shown).
Toxicity
Treatments were tolerable in most patients, and the most common toxicity developed during induction chemotherapy was bone marrow suppression. The toxicity profile for CHOP group was reported previously [12]. As was shown in Table 4, grade 3/4 bone marrow suppression was more common in EPOCH group than in GELOX group (28.3 vs. 13.2 %). However, non-hematologic toxicities were more common in GELOX group, though most of which were mild in severity.
Survival and prognostic analysis
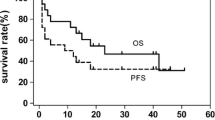
By the end of July 2013, 21 patients were lost to follow up, and complete survival data were available for the other 206 patients. The median follow-up time for GELOX, EPOCH, and CHOP groups was 25.2 (range 9.9–59.8) months, 20.7 (range 4.4–102.6) months, and 25.7 (range 0.5–156.6) months, respectively. There were 94 deaths (88 deaths due to tumor progression, 1 death due to treatment complication, and the other 5 deaths due to unknown reasons), and the other 112 patients were still alive. The 88 deaths of tumor progression occurred within a median of 11.53 months (range 0.63–127.43 months) after diagnosis of ENKTL. As was shown in Fig. 1, the 3-year OS rate was significantly better in GELOX group than in EPOCH or CHOP group (87.0 vs. 54.0 vs. 54.0 %, respectively, P = 0.001 and 0.001), and the 3-year PFS was also significantly better in GELOX group than in EPOCH or CHOP group (72.0 vs. 50.0 vs. 43.0 %, P = 0.01 and 0.001). However, no significant differences were found between EPOCH and CHOP groups in OS or PFS (P = 0.765 for OS, and 0.421 for PFS). Moreover, OS and PFS were significantly correlated with age, ECOG score, LDH level, stage, and IPI score (data was not shown, and part of the data was reported previously [12]). Moreover, the patients who got CR after treatment had significantly better survival outcome than those who got PR or stable disease. As was shown in Table 5, in a multivariate Cox regression model that included IPI score, induction chemotherapy regimen, and treatment response, it was found that all these three factors were independent prognostic factors for ENKTL patients.
Discussion
Most patients of ENKTL are resistant to CHOP chemotherapy due to overexpression of the multidrug-resistant (MDR) gene [6]. As we previously reported, the CR rate after CHOP induction therapy was only 31.8 %, and the 2-year OS rates was 60 %, which was very unsatisfactory [12]. Recently, several small cohort phase II studies have shown better results by changing the schedule of drug delivery to continuous infusion, which might partially overcome drug resistance caused by P-glycoprotein [7]. Huang et al. [8] reported a CR rate of 57.7 % after EPOCH induction therapy, and the 3-year OS rate for those localized patients was 75.0 % after combined chemoradiation therapy. Nowadays, more and more asparaginase-based chemotherapy regimens have demonstrated promising results [9, 10]. We previously reported the results of a phase II clinical trial that evaluating the efficacy and safety of GELOX as induction therapy in early stage ENKTL, and the 3-year OS was 78.0 % with acceptable safety profiles [11]. The GELOX regimen exploits the combination of three non-anthracycline drugs, gemcitabine, oxaliplatin, and asparaginase. It was proved that gemcitabine and oxaliplatin are both attractive drugs in non-Hodgkin lymphoma (NHL) because of their significant single-agent activity and favorable toxicity profile [13]. Asparaginase has a special anticancer mechanism. It can hydrolyze and exhaust serum asparagines in NK/T-cell lymphoma cells, which cannot synthesize l-asparagines by themselves, and finally produces an anticancer effect [14]. Moreover, these three drugs were rarely affected by P-glycoprotein. However, due to the rarity of ENKTL, no randomized phase III trials have been conducted so far to clarify which regimen is the best for ENKTL.
In this study, we retrospectively compared the results of these three commonly used regimens (GELOX, EPOCH, and CHOP) in our center, and this was the largest cohort so far to compare different induction regimens. Thirty-eight patients with stage I/II ENKTL received GELOX as induction therapy, and got a CR rate of 68.4 % after induction chemotherapy and 3-year OS rate of 87.0 %, which was consistent with our previous report [11]. Fifty-four patients received EPOCH as induction therapy, and got a CR rate of 42.6 % and 3-year OS rate of 54.0 %, which seems worse than Huang et al. [8] reported, and this discordance may be related to the sample size and the retrospective nature of this study. As was shown in Table 2, GELOX was significantly superior to EPOCH in the CR rate after induction chemotherapy; however, no difference existed between GELOX and EPOCH groups in the response rate after radiation therapy, indicating a better local control that attributed to radiotherapy. Both GELOX and EPOCH groups attained much higher CR rate after induction therapy than CHOP group, indicating the application of asparaginase and continuous infusion schedule of doxorubicin and vincristine can overcome the drug resistance caused by MDR of ENKTL. When the survival data were compared, it was surprising to find that there was no difference between EPOCH and CHOP in 3-year OS rate and PFS rate, which suggested the ability to overcome the P-glycoprotein-mediated MDR by EPOCH regimen is still limited, indicating anthracycline-based regimens were no longer the best choice for ENKTL. However, Lin et al. [15] recently reported the result of asparaginase plus CHOP in the treatment of ENKTL, and got a CR rate of 71.5 % and 2-year OS rate of 80.1 %, suggesting that synergistic effect may exist between asparaginase and CHOP regimen or asparagines may partially reverse the drug resistance to CHOP regimen caused by P-glycoprotein. Although the overall response rate and CR rate were similar between EPOCH and GELOX groups after radiation therapy, the survival analysis indicated that the rates of 3-year PFS and 3-year OS in the GELOX group were significantly higher than those in the EPOCH group (72.0 vs. 50.0 %, P = 0.01 for 3-year PFS rate; 87.0 vs. 54.0 %, P = 0.001 for 3-year OS rate). The advantages of PFS and OS in the GELOX group might be partly explained by the better potential minimal distant disease eradication of GELOX regimen, which needed to be validated and explored further.
As we previously reported [11, 12], IPI and treatment responses were independent prognostic factors for ENKTL. In a multivariate Cox regression model that included IPI score, induction chemotherapy regimen, and treatment responses, it was found that all these three factors were independent prognostic factors for ENKTL patients, suggesting that GELOX can improve the outcome of ENKTL patients not only by short-term efficacy, but also by eradicating the minimal residual disease or reducing relapsed disease, the mechanism of which warranted further investigation.
Nowadays, most centers recommend the combination of chemotherapy and radiotherapy in the treatment of early stage ENKTL. However, there is no consensus concerning whether concurrent chemoradiation therapy or sequential chemotherapy followed by radiation therapy is better. Japan Clinical Oncology Group Study JCOG0211 conducted a phase I/II study of concurrent chemoradiotherapy for localized ENKTL [16], and got a CR rate of 77 % and 2-year OS rate of 78 %, which was consistent with what we previously reported. However, the safety profile of sequential GELOX followed by radiotherapy is much better than JCOG0211 regimen, especially grade 3/4 bone marrow suppression. At present, we are conducting a phase II trial of concurrent chemoradiotherapy using GELOX regimen for localized ENKTL, trying to figure out whether this strategy can improve the outcomes further without killing the safety profiles.
In conclusion, we demonstrated that the GELOX regimen significantly improved the outcomes in patients of localized ENKTL with acceptable toxicities, compared with EPOCH or CHOP. Further clinical trials of ENKTL should use asparaginase-based regimens as the standard chemotherapy.
References
Chan JK, Quintanilla-Martinez L, Ferry JA, Peh S-C. Extranodal NK/T-cell lymphoma, nasal type. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. p. 285–8.
Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–30.
Li YX, Liu QF, Fang H, et al. Variable clinical presentations of nasal and Waldeyer ring natural killer/T-cell lymphoma. Clin Cancer Res. 2009;15(8):2905–12.
Huang MJ, Jiang Y, Liu WP, Li ZP, Li M, Zhou L, et al. Early or up-front radiotherapy improved survival of localized extranodal NK/T-cell lymphoma, nasal-type in the upper aerodigestive tract. Int J Radiat Oncol Biol Phys. 2008;70:166–74.
Kim GE, Cho JH, Yang WI, et al. Angiocentric lymphoma of the head and neck: patterns of systemic failure after radiation treatment. J Clin Oncol. 2000;18:54–63.
Wang B, Li XQ, Ma X, Hong X, Lu H, Guo Y. Immunohistochemical expression and clinical significance of P-glycoprotein in previously untreated extranodal NK/T-cell lymphoma, nasal type. Am J Hematol. 2008;83:795–9.
Lai G-M, Chen Y-N, Mickley LA, et al. P-glycoprotein expression and schedule dependence of adriamycin cytotoxicity in human colon carcinoma cell lines. Int J Cancer. 1991;49:696–703.
Huang H, Lin Z, Lin X, Cai Q, Xia Z, Jiang W. Long-term outcomes of patients with newly diagnosed extranodal natural killer/T-cell lymphoma treated by etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin regimen: a single-institution experience. Leuk Lymphoma. 2011;52:1041–8.
Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29(33):4410–6.
Jaccard A, Gachard N, Marin B, et al. Efficacy of l-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117(6):1834–9.
Wang L, Wang ZH, Chen XQ, Li YJ, Wang KF, Xia YF, Xia ZJ. First-line combination of gemcitabine, oxaliplatin, and l-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer. 2013;119:348–55.
Wang L, Xia ZJ, Huang HQ, Lu Y, Zhang YJ. Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) in the treatment of stage IE/IIE extranodal natural killer/T cell lymphoma, nasal type: 13-year follow-up in 135 patients. Int J Hematol. 2012;96(5):617–23.
El Gnaoui T, Dupuis J, Belhadj K, et al. Rituximab, gemcitabine and oxaliplatin: an effective salvage regimen for patients with relapsed or refractory B-cell lymphoma not candidates for high-dose therapy. Ann Oncol. 2007;18(8):1363–8.
Ando M, Sugimoto K, Kitoh T, et al. Selective apoptosis of natural killer-cell tumours by l-asparaginase. Br J Haematol. 2005;130(6):860–8.
Lin N, Song Y, Zheng W, Tu M, Xie Y, Wang X, et al. A prospective phase II study of l-asparaginase-CHOP plus radiation in newly diagnosed extranodal NK/T-cell lymphoma, nasal type. J Hematol Oncol. 2013;6(1):44.
Yamaguchi M, Tobinai K, Oguchi M, Ishizuka N, Kobayashi Y, Isobe Y, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol. 2009;27(33):5594–600.
Acknowledgments
We thank all of the physicians at Sun Yat-sen University Cancer Center for allowing us to include their patients. We also appreciate the cooperation of all the pathologists at Sun Yat-sen University Cancer Center for their support. This work received grant support from Young Teachers’ Cultivation Project of Sun Yat-sen University (No. 12ykpy54) and National Natural Science Foundation of China (No. 81272620).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, W., Wei-da, W., Zhong-jun, X. et al. Combination of gemcitabine, l-asparaginase, and oxaliplatin (GELOX) is superior to EPOCH or CHOP in the treatment of patients with stage IE/IIE extranodal natural killer/T cell lymphoma: a retrospective study in a cohort of 227 patients with long-term follow-up. Med Oncol 31, 860 (2014). https://doi.org/10.1007/s12032-014-0860-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0860-4