Abstract
We performed a systematic review and meta-analysis to assess the potential of erlotinib plus platinum-based chemotherapy relative to platinum-based chemotherapy alone for advanced non-small-cell lung cancer (NSCLC). Search of PubMed, EMBASE, Web of Science, CBM, CNKI, China Wan Fang databases and the Cochrane library was performed for studies regarding erlotinib plus platinum-based chemotherapy for advanced NSCLC published between 1 January 2000 and 28 August 2014. We identified eight eligible studies including 3,363 patients with advanced NSCLC. For PFS measure, an HR of 0.73 (0.58–0.93) with statistical significance was estimated when erlotinib plus platinum-based chemotherapy compared with platinum-based chemotherapy alone; objective response rate of 32.86 versus 24.85 % was obtained for both groups, respectively. HR of 0.93 (0.86–1.00) with P of 0.170 was calculated for OS. We concluded that the erlotinib plus chemotherapy for advanced NSCLC could increase PFS and objective response rate, but not benefit OS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is one of the leading causes of cancer death in China. Approximately 1 million new lung cancer cases will be diagnosed annually by 2025 [1]. The CONCORD Working Groups aimed to initiate worldwide surveillance of cancer survival by central analysis of population-based registry data, between 1995 and 2009; more than five million populations died of lung cancer [2]. Non-small cell lung cancer (NSCLC) accounts for more than 85 % of lung cancers [3]. Approximately 60 % of NSCLCs is at the terminal stage. The median overall survival for patients with NSCLC treated by first-line chemotherapy ranges from 7 to 12 months [4]. Second- and third-line chemotherapy has been used to further increase the survival rate. Comprehensive regimes included all current therapies were used to manage NSCLC; however, patient’s survival remains unoptimistic [5].
The Food and Drug Administration (FDA), in 2013, approved erlotinib (Tarceva®) to be as the first-line agent for patients with metastatic NSCLC with EGFR mutations [6]. However, erlotinib was not recommended to be given as first therapy for patients with negative or unknown EGFR status [7]. Consequently, a pooled analysis of currently available studies restricted to patients who used erlotinib alone compared with other chemotherapy may provide relevant information for the treatment of patients with advanced NSCLC. We undertake a systematic review and meta-analysis to evaluate the potential of erlotinib plus platinum-based chemotherapy compared with platinum-based chemotherapy alone in advanced NSCLC. In addition, subgroup analysis was conducted according to the treatment period, ECOG-PS, gender and smoking status. We also comprehensively appraised the quality of the evidence by using GRADEpro to facilitate clinical decision-making.
Methods
Ethical approval and patient consent are not required due to this is a systematic review and meta-analysis of previously published studies. This study was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [8]. Two reviewers (Jian-Guo Zhou and Xue Wang) participated in citations search, study selection and data extraction independently. Divergences between reviewers were resolved through consulting with a third reviewer (Xu Tian).
Identification of studies
Electronic databases, including PubMed, EMBASE, Web of Science, China Biomedical Literature Database (CBM), Chinese National Knowledge Infrastructure (CNKI), China Wan Fang database and the Cochrane library, were searched for relevant clinical trials published between January 2000 and August 2014. The search combined key words: (“non-small-cell lung carcinoma” OR “non-small cell lung cancer”) AND (“Erlotinib” OR “Tarceva”). The PubMed search string is summarised in “Appendix”. Baidu (Chinese), Google Scholar, DXY.com (Chinese), and conference proceedings were also searched for any eligible studies. The reference lists of included studies were also manually searched to identify any relevant articles. Only articles in English and Chinese languages were eligible.
The following study selection criteria were applied: (a) population: patients were diagnosed as advanced NSCLC. No other restrictions were imposed; (b) intervention: erlotinib plus platinum-based chemotherapy; (c) comparison: platinum-based chemotherapy alone; (d) outcomes: overall survival (OS), objective response rate (ORR) and progress-free survival (PFS) will be evaluated; and (e) study design: RCTs.
Data extraction
We used predesigned data extraction sheet to obtain the following information: first author, number of patients, population, setting, intervention, control, smoking status, histology and median age, ECOG-PS, anatomic stage, trial phase, and current status of treatment. Two investigators extracted data independently; Xu Tian resolved discrepancies. We contacted the corresponding author to obtain the data when necessary. The formula recommended by Spotswood et al. [9] was adopted to calculate the corresponding HR of the missing data. Kaplan–Meier curve was read by using Engauge Digitizer version 4.1 (available at: http://sourceforge.net/) unless the adequate data can be extracted [10].
Assessing risk of bias and grading the quality of evidence
Assessment for risk of bias was performed in accordance with guideline outlined in the Cochrane handbook for systematic reviews of interventions (version 5.1.0) [11]. Two investigators (Fei Wang and Yi Wang) objectively reviewed all studies and assigned a value of “high”, “low” or “unclear” to the following domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and other bias. Trial with high risk of bias for any one or more key domains was considered as at “high risk”. Trial with low risk of bias for all key domains was considered as at “low risk”. Otherwise, it was considered as “unclear” [12].
The GRADE system identified the following four grades for rating the quality of evidence [13]: (a) high: further research is very unlikely to change our confidence in the estimate of the effect; (b) moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; (c) low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; and (d) very low: any estimate of effect is very uncertain. The GRADE profiler software (version 3.6) (available at: http://www.gradeworkinggroup.org/) was used to rate the level of evidence.
Statistical analysis
We estimated the HR or relative risk (RR) with 95 % confidence interval (CI) for dichotomous outcomes, and the weighted mean difference (WMD) with 95 % CI for continuous outcomes. A random-effects model was used regardless of heterogeneity. Level of heterogeneity (Level of variance) across studies was evaluated using I 2 statistic. I 2 of 40, 70 and 100 % was used to represent low, moderate and high variance, respectively [14]. If obvious differences for clinical characteristics and methodology were not identified and I 2 ≤ 40 %, a fixed-effects model was adopted. A random-effects model will be used if clinical characteristics and methodology were not identified to be great difference and I 2 > 40 %; in contrast, if the clinical characteristic and/or methodology across studies regardless I 2 statistic was considered as to be obvious different, qualitative analysis was adopted [15]. Meta-regression and sensitivity analyses were conducted to determine the possible causes of heterogeneity and to further identify the influence of various exclusion criteria on the overall risk estimate. The influence of individual trials was also investigated using the leave-one-out cross-validation method to test the robustness of the primary outcomes [16]. Potential publication bias was assessed by visually inspecting the Begg funnel plots in which the log RRs were plotted against their standard errors (SEs). The presence of publication bias was also evaluated by using the Begg and Egger tests [17, 18]. P < 0.05 was considered to indicate statistical significance [19]. All statistical analyses were performed by using STATA version 12.0 (Stata Corp., College Station, TX, USA) and RevMan 5.3.4(The Nordic Cochrane Centre, Copenhagen, Denmark).
Results
Study selection and characteristics
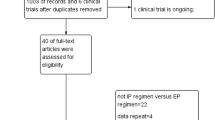
A total of 837 relevant citations were identified at the initial search stage; eight studies, involving 3,363 patients who 1,680 and 1,683 patients were divided into erlotinib plus platinum-based chemotherapy and platinum-based chemotherapy alone, respectively, were included in the meta-analysis [3, 20–26]. The flow diagram of the literature retrieval and selection was shown in Fig. 1.
The main characteristics of all eligible RCTs were presented in Table 1. These studies were published between 2005 and 2013. Of the eight studies included, two were conducted in USA [22, 26], two in East Asia [3, 24], two in Europe [21, 23], one in Germany [25] and one in America and Asia [20]. The sample size of the RCTs ranged from 95 to 1,159. Gemcitabine plus platinum-based chemotherapy [3, 22, 24, 25], paclitaxel plus platinum-based chemotherapy [21, 26] and platinum-based chemotherapy [23] were adopted to as control regime for four, two, one and one eligible trial(s), respectively. Four trials reported PFS measures [3, 20, 22, 24], and time to tumour progression (TTP) can be accessed for one study included [25]. The ORR and OS were available in six [1, 11, 13, 15–17] and eight trials, respectively.
Risk of bias and grades of evidence
The details for assessing risk of bias are shown in Fig. 2. Eight trials were all open-label. Random sequence generation and allocation concealment were performed adequately in most of the trials. However, five trials did not describe the reasons for incomplete outcome data [27]. Under the assumption that the PFS outcome might not different from the time to progression, the PFS data were used and pooled [28]. The overall methodological quality of the included trials was generally good and fair.
The quality of the evidence of each outcome is reported in Table 2. There were four efficacy outcomes in this meta-analysis. OS and PFS were critical results, and ORR was important results. GRADE Working Group grades of evidence were high for OS, and moderate for PFS and objective response rate.
PFS (five trials, 2,017 patients)
The PFS of the erlotinib plus platinum-based chemotherapy arm ranged from 1.6 to 13.1 months, and the PFS of the platinum-based chemotherapy alone arm ranged from 1.2 to 5.2 months. The heterogeneity test indicated that a random effect model could be selected (I 2 = 85.1 %, P < 0.0001). The meta-analysis showed that the pooled HR was 0.73 (95 % CI = 0.58, 0.93), P = 0.009) and without statistical significance was identified in terms of the erlotinib platinum-based chemotherapy regimen relative to the platinum-based chemotherapy alone (Fig. 3).
OS (eight trials, 3,363 patients)
A total of eight RCTs regarding OS were incorporated into this meta-analysis [3, 20–26]. The heterogeneity test indicated that a fixed effect model could be selected (I 2 = 39.6 %, P = 0.115). The pooled results showed that there was no significant difference between the two groups (HR 0.93; 95 % CI 0.86, 1.00; P = 0.170) (Fig. 4).
ORR (six trials, 2,361 patients)
A total of eight RCTs regarding ORR were incorporated into this pooled analysis [3, 20, 22, 24–26]. According to the heterogeneity test, the I 2 was equal to 78.2 % and the P value was <0.05(=P < 0.0001). Thus, a random-effects model was selected. The pooled RR for ORR showed that there was no significant difference between the erlotinib plus platinum-based chemotherapy and platinum-based chemotherapy alone groups (RR 1.51; 95 % CI 1.07, 2.11, P = 0.018) (Fig. 5).
Subgroup analyses and meta-regression
Subgroup analysis was adopted to explore the causes of heterogeneity for the analysis of PFS (Fig. 6). The effect sizes were similar between the subgroups divided by gender, smoking status, histology and patient year, ECOG-PS, anatomic stage and trial phase under the seven predefined subgroups. To investigate the effects of various study characteristics on the HR estimates, a meta-regression analysis was conducted by subgroups. No statistical significance was identified regarding the differences in treatment effects for the various subgroups, and the P values for gender, and patient year, ECOG-PS, anatomic stage and trial phase were 0.10, 0.11, 0.10, 0.99 and 0.83, respectively. However, smoking status with P of 0.01 and histology with P of 0.04 were identified to be as variance resources (Table 3).
Sensitivity analysis
Significant heterogeneity was observed among the included studies for PFS (I 2 = 85.1 %). As shown in Fig. 7, the study conducted by Gatzemeier et al. [25] showed results that were completely out of range of the others and probably contributed to the heterogeneity. After excluding this study, the results suggested that compared with platinum-based chemotherapy, erlotinib plus chemotherapy was associated with an increased PFS (HR 0.652, 95 % CI 0.546–0.759, P < 0.0001). No evidence of high heterogeneity was observed among the remaining studies (I 2 = 44.7 %).
Publication bias
For the meta-analyses of OS, PFS and ORR, there was no evidence of significant publication bias by inspection of the formal statistical tests [(1) OS: Egger’s test, P = 0.944; Begg’s test, P = 0.711); (2) PFS: Egger’s test, P = 0.519; Begg’s test, P = 0.816); (3) ORR: Egger’s test, P = 0.249; Begg’s test, P = 0.452)].
Discussion
This is a systematic review and meta-analysis to further evaluate the efficacy of erlotinib plus platinum-based chemotherapy for advanced NSCLC. The present systematic review and meta-analysis suggested that erlotinib combined with platinum-based chemotherapy was beneficial for advanced NSCLC patient with EGFR mutation compared with platinum-based chemotherapy alone regime. So the NCCN guideline recommended erlotinib as a first-line therapy in patients with sensitising EGFR mutations [7]. A great deal of RCTs on the same topic was finished by clinical oncologists [3, 20–26]; however, these conclusions were inconclusive.
The meta-analysis of eight studies comparing erlotinib plus platinum-based chemotherapy and platinum-based chemotherapy alone revealed that no significant difference existed with regard to OS, whereas PFS and ORR shown significant difference between two arms. In this review, subgroup analyses were conducted according to histology condition, smoking status, length of illness and other factors. The smoking status and histological type can explain most of the heterogeneity observed, according to the results of the sensitivity analysis and meta-regression. Several meta-analyses on EGFR-TKIs have been published in recent years, most of which employed trials with varying drug priorities. A majority of the published studies focused on efficacy, while the correlation between EGFR mutation and efficacy was reported in four meta-analyses [28–31]. Additionally, all meta-analyses that did not focus on the efficacy compare with erlotinib plus chemotherapy and chemotherapy. Importantly, the GRADE system was performed to assess the level of evidence summarised in the meta-analysis.
This meta-analysis has several potential limitations that should be taken into account. First, only English and Chinese language literature articles were considered for publication. If the search had been extended to include literature published in other languages, it is possible that additional relevant trials may have been identified. Second, ongoing studies were ineligible for inclusion. Limitations in quality, even though most of the studies were of high quality, cannot be ignored, and the pooled results of this meta-analysis may have been affected slightly. Moreover, only a small number of trials met the subgroup analysis criteria, thus reducing the power of the analyses. These factors may have a potential impact on our results. Additionally, we are unable to assess the effects of erlotinib on other clinically meaningful endpoints, such as quality of life, patient and physician satisfaction, because of sparse and inconsistent reporting across studies. Finally, because the studies included in the meta-analysis were carried out in various countries, oncologists should carefully and judiciously assess the feasibility of applying the results in the clinical setting in China.
Conclusions
In summary, the current available evidence suggests that erlotinib lacks the potential to improve OS. PFS and objective response rate could be improved by using erlotinib plus chemotherapy in patients with advanced NSCLC. Finally, smoking status and histological type are important evaluation factors that should be considered for evaluating clinical therapy and prognosis.
References
Chen WQ, Zheng RS, Zhang SW, Zeng HM, Zou XN. The incidences and mortalities of major cancers in China, 2010. Chin J Cancer. 2014;33(8):402–5. doi:10.5732/cjc.014.10084.
Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang X-S, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2014. doi:10.1016/S0140-6736(14)62038-9.
Wu YL, Lee JS, Thongprasert S, Yu CJ, Zhang L, Ladrera G, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol. 2013. doi:10.1016/S1470-2045(13)70254-7.
Bezjak A, Tu D, Seymour L, Clark G, Trajkovic A, Zukin M, et al. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2006;24(24):3831–7. doi:10.1200/jco.2006.05.8073.
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–32. doi:10.1056/NEJMoa050753.
http://www.cancer.gov/cancertopics/druginfo/fda-erlotinibhydrochloride. 19 Aug 2014.
http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#nscl,version.4,2014.55–56.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi:10.1136/bmj.b2535.
Spruance SL, Reid JE, Grace M, Samore M. Hazard ratio in clinical trials. Antimicrob Agents Chemother. 2004;48(8):2787–92. doi:10.1128/AAC.48.8.2787-2792.2004.
Tarro G, Perna A, Esposito C. Early diagnosis of lung cancer by detection of tumor liberated protein. J Cell Physiol. 2005;203(1):1–5. doi:10.1002/jcp.20195.
Julian PT Higgins SG. Cochrane handbook for systematic reviews of interventions Version 5.1.0 [updated March 2011] Chapter 8: Assessing risk of bias in included studies. 2011.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928.
Schünemann HJ, Oxman A. GRADE handbook for grading the quality of evidence and the strength of recommendations Version 3.2 [updated March 2009]. 2009.
Deeks JJ, Altman DG. Cochrane handbook for systematic reviews of interventions Version 5.1.0 [updated March 2011] Chapter 9: Analysing data and undertaking meta-analyses. 2011.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi:10.1002/sim.1186.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi:10.1136/bmj.327.7414.557.
Egger M, Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Xu YH, Lu S. A meta-analysis of STAT3 and phospho-STAT3 expression and survival of patients with non-small-cell lung cancer. Eur J Surg Oncol. 2014;40(3):311–7.
Lee DH, Lee JS, Kim SW, Rodrigues-Pereira J, Han B, Song XQ, et al. Three-arm randomised controlled phase 2 study comparing pemetrexed and erlotinib to either pemetrexed or erlotinib alone as second-line treatment for never-smokers with non-squamous non-small cell lung cancer. Eur J Cancer. 2013;49(15):3111–21.
Boutsikou E, Kontakiotis T, Zarogoulidis P, Darwiche K, Eleptheriadou E, Porpodis K, et al. Docetaxel–carboplatin in combination with erlotinib and/or bevacizumab in patients with non-small cell lung cancer. Onco Targets Ther. 2013;6:125–34.
Stinchcombe TE, Peterman AH, Lee CB, Moore DT, Beaumont JL, Bradford DS, et al. A randomized phase II trial of first-line treatment with gemcitabine, erlotinib, or gemcitabine and erlotinib in elderly patients (age>/=70 years) with stage IIIB/IV non-small cell lung cancer. J Thorac Oncol. 2011;6(9):1569–77. doi:10.1097/JTO.0b013e3182210430.
Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczesna A, Juhasz E, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11(6):521–9.
Mok TSK, Wu YL, Yu CJ, Zhou C, Chen YM, Zhang L, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2009;27(30):5080–7.
Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva lung cancer investigation trial. J Clin Oncol. 2007;25(12):1545–52.
Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23(25):5892–9.
Kelly K, Azzoli CG, Zatloukal P, Albert I, Jiang PY, Bodkin D, et al. Randomized phase 2b study of pralatrexate versus erlotinib in patients with stage IIIB/IV non-small-cell lung cancer (NSCLC) after failure of prior platinum-based therapy. J Thorac Oncol. 2012;7(6):1041–8. doi:10.1097/JTO.0b013e31824cc66c.
Lee J-K, Hahn S, Kim D-W, Suh KJ, Keam B, Kim TM, et al. Epidermal growth factor receptor tyrosine kinase inhibitors vs conventional chemotherapy in non-small cell lung cancer harboring wild-type epidermal growth factor receptor: a meta-analysis. JAMA. 2014;311(14):1430–7.
Haaland B, Tan PS, de Castro Jr G, Lopes G. Meta-analysis of first-line therapies in advanced non-small-cell lung cancer harboring EGFR-activating mutations. J Thorac Oncol. 2014;9(6):805–11.
Zhang Y, Sun Y, Wang L, Ye T, Pan Y, Hu H, et al. Sequential treatment of tyrosine kinase inhibitors and chemotherapy for EGFR-mutated non-small cell lung cancer: a meta-analysis of phase III trials. Chest. 2014;145(3 Suppl):348A.
Zhao N, Zhang X-C, Yan H-H, Yang J-J, Wu Y-L. Efficacy of epidermal growth factor receptor inhibitors versus chemotherapy as second-line treatment in advanced non-small-cell lung cancer with wild-type EGFR: a meta-analysis of randomized controlled clinical trials. Lung Cancer (Amsterdam, Netherlands). 2014;85(1):66–73.
Acknowledgments
The authors thank the reviewers for their helpful comments on this article. This work was supported by NSFC (Natural Science Foundation of China) (81360351), Start-Up Fund for Doctor of Zunyi Medical University and the Department of Science and Technology of Guizhou Province (Grant No. Qian Ke He SY [2013] 3003).
Conflict of interest
The authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jian-Guo Zhou, Xu Tian and Xue Wang have contributed equally to this work.
Appendix: PubMed search terms
Appendix: PubMed search terms
#1 Search (((((((((Carcinoma, Non Small Cell Lung[Title/Abstract]) OR Carcinomas, Non Small Cell Lung[Title/Abstract]) OR Lung Carcinoma, Non-Small-Cell[Title/Abstract]) OR Lung Carcinomas, Non-Small-Cell[Title/Abstract]) OR Non-Small-Cell Lung Carcinomas[Title/Abstract]) OR Non small Cell Lung Cancer[Title/Abstract]) OR Non-Small-Cell Lung Carcinoma[Title/Abstract]) OR Carcinoma, Non-Small Cell Lung[Title/Abstract]) OR Non-Small Cell Lung Cancer[Title/Abstract]) OR “Carcinoma, Non-Small-Cell Lung”[Mesh].
#2 Search ((((((((((((OSI-774[Title/Abstract]) OR CP-358774[Title/Abstract]) OR CP-358,774[Title/Abstract]) OR CP 358,774[Title/Abstract]) OR CP 358774[Title/Abstract]) OR Tarceva[Title/Abstract]) OR 11C-erlotinib[Title/Abstract]) OR erlotinib HCl[Title/Abstract]) OR erlotinib hydrochloride[Title/Abstract]) OR N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine[Title/Abstract]) OR erlotinib[Title/Abstract])) OR “erlotinib” [Supplementary Concept].
#3 Search (((“Controlled Clinical Trial” [Publication Type]) OR (“Randomized Controlled Trials as Topic”[Mesh] OR “Randomized Controlled Trial” [Publication Type] OR “Controlled Clinical Trials as Topic”[Mesh]))) OR ((((((Controlled Clinical Trial[Title/Abstract]) OR Controlled Clinical Trials, Randomized[Title/Abstract]) OR Clinical Trials, Randomized[Title/Abstract]) OR Trials, Randomized Clinical[Title/Abstract]) OR Controlled Clinical Trials[Title/Abstract]) OR random*[Title/Abstract]).
#4: #1 AND #2 AND #3.
Rights and permissions
About this article
Cite this article
Zhou, JG., Tian, X., Wang, X. et al. Treatment on advanced NSCLC: Platinum-based chemotherapy plus erlotinib or platinum-based chemotherapy alone? A systematic review and meta-analysis of randomised controlled trials. Med Oncol 32, 23 (2015). https://doi.org/10.1007/s12032-014-0471-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0471-0