Abstract
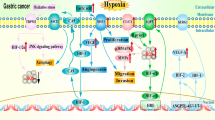
The hypoxic condition occurs in most types of solid tumors and has been shown to be associated with the metastatic ability of gastric cancer. A previous study has demonstrated that hypoxia might stimulate epithelial-to-mesenchymal transition (EMT) of gastric cancer cells. Nevertheless, the mechanism has not yet been completely understood. In the current study, the human gastric cancer cell lines HGC27 and MGC803 were presented to normoxic (21 % O2), hypoxic (1 % O2) or severe hypoxic (0.1 % O2) conditions for 24 h. We found that hypoxia exposure induced EMT of gastric cancer cells, which was promoted by severe hypoxia condition. Meanwhile, expressions of PERK, ATF4 and ATF6 proteins were elevated in cells under conditions of severe hypoxia but not by normoxia or hypoxia. Knockdown of PERK, ATF4 or ATF6 impeded EMT of gastric cancer cells induced by severe hypoxia. Furthermore, severe hypoxia exposure extremely boosted the expression of TGF-β, which was blocked by the knockdown of PERK, ATF4 or ATF6 expression. Additionally, we found that TGF-β release caused by hypoxia is facilitated by elevated UPR proteins and led to the activation of Smad2/3 and PI3K/Akt signaling. Our data suggest that UPR potentiates the EMT of gastric cancer cells under conditions of severe hypoxia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Annually, about 380,000 new cases gastric cancer are reported in China [1]. The mortality rate for gastric cancer in China has been reported as approximately 26.3 per 100,000, which is the highest in the world [2]. Multiple factors including old age, smoking, excessive alcohol intake, overweight, high salt and fat consumption, low vegetable and fruit consumption, low economic status, pernicious anemia, chronic gastric diseases and H. pylori infection contribute to the progression of gastric cancer [3, 4].
Once oxygen consumption in solid tumors exceeds its delivery, hypoxia will develop [5]. Consequently, the expression of several genes in response to hypoxia is induced; these play roles in tumor cell survival, proliferation, invasion and angiogenesis [6, 7]. Hypoxia-inducible factor-1 (HIF-1) is the best understood regulator under hypoxia exposure and is involved in many aspects of tumor biology [8–10]. Recently, other pathways have been demonstrated to independently influence gene expression in response to hypoxia [11].
Notably, hypoxia has been reported to induce epithelial-to-mesenchymal transition (EMT) of cancer cells. Hypoxia could induce EMT and promote invasion and migration in hepatocellular cancer cells via the activation of SNAI1 [12]. Morphological EMT-like changes have been triggered in ovarian cancer cells by hypoxia [13]. Moreover, hypoxia is known to have caused the activation of EMT gene expression in breast cancer cells via Period 2 (PER2) degradation [14]. In gastric carcinoma cells, hypoxia activates the autocrine TGF-β signaling pathway and might be implicated in EMT [15].
TGF-β has emerged as a potent factor that drives cancer progression and as a potent inducer of epithelial plasticity, leading to EMT via both Smad and non-Smad signaling pathways [16]. Interaction of TGF-β with TβRII and TβRI led to the activation of TβRI receptors through direct phosphorylation, which in turn phosphorylate Smad2 and Smad3 proteins at two C-terminal serines [16]. The activated Smad proteins then combine with Smad4 and translocate into the nucleus, regulating target gene expression [16]. TGF-β also initiates non-Smad signaling, leading to the activation of pathways such as PI3K/Akt, Erk and p38 MAPK, as well as the Rho-GTPases pathways [17–19].
Given the high metabolic rates and limiting supplies of glucose and oxygen, activated unfolded protein response (UPR) is found in multiple types of tumors [20]. In breast cancer cells, the PERK/ATF4/LAMP3-arm of the UPR-mediated hypoxia induced cell migration [21]. UPR also potentiated HIF-1 activity to transactivate VEGF expression [22]. In both the human and mouse models of gastric cancer, UPR is demonstrated to be activated autonomously in Helicobacter-induced metaplasia and dysplasia [23]. Although there is no direct evidence that UPR may result in EMT in tumors, activation of the PERK/eIF2a/ATF4 and ATF6 arms of the UPR led to the induction of ADAM17, which functionally controlled TGF-β signaling and modulated the EMT [24].
As hypoxia modulates the progression of gastric cancer by EMT induction and UPR is activated in gastric cancer cells [23, 25], we hypothesized that UPR may contribute to EMT of gastric cancer and result in aggressive tumor phenotypes in gastric cancer. We managed to evaluate the EMT as well as UPR of gastric cancer cells in vitro under conditions of hypoxia or severe hypoxia. We found that UPR promoted EMT, while the knockdown of UPR proteins blocked EMT induced by severe hypoxia. Furthermore, TGF-β release is facilitated by elevated UPR proteins and led to the activation of Smad2/3 and PI3K/Akt signaling. Thus, we detailed the role of UPR in the induction of EMT of gastric cancer cells.
Materials and methods
Cell line, hypoxia, thapsigargin, TGF-β mAb and LY294002 treatment
Human gastric cancer cell lines (HGC27 and MGC803) were purchased from the Cell Resource Center, Shanghai Institute of Biochemistry and Cell Biology at the Chinese Academy of Sciences (Shanghai, P.R. China). Cells were cultured in RPMI-1640 medium (Gibco, Rockville, MD, USA) supplemented with 10 % fetal bovine serum (FBS; Gibco, Rockville, MD, USA), 100 U/mL penicillin, 100 μg/mL streptomycin (Sigma Aldrich, St. Louis, MO, USA) and 1 mmol/l l-glutamine (Gibco, Rockville, MD, USA). Cells were grown at 37 °C in a humidified atmosphere containing 5 % CO2. In our study, cells were treated under hypoxic conditions [5 % CO2 and 1 % O2 (v/v)] or severe hypoxic conditions [5 % CO2 and 0.1 % O2 (v/v)], balanced with N2 gas, at 37 °C for 24 h. For thapsigargin treatment, cancer cells were incubated with 300 nmol/L thapsigargin for 16 h. For TGF-β mAb treatment, cancer cells were incubated with TGF-β mAb for 12 h. For LY294002 treatment, cancer cells were incubated with 25 μmol/L LY294002 for 12 h. The study protocol was in accordance with the Helsinki Declaration and approved by the ethics committee of The First Affiliated Hospital of Zhengzhou University.
Morphological transition observation
HGC27 and MGC803 cells were exposed to hypoxic or severe hypoxic conditions, and cell morphology was observed with a microscope (Olympus, Tokyo, Japan). EMT was determined when the polygonal or spindle shape was observed in cancer cells by microscope.
Knockdown by siRNA
Scrambled siRNA and small-interfering RNA (siRNA) targeting PERK, ATF4, ATF6, TFG-β, Smad2 and Smad3 were purchased from Santa Cruz Biotechnology. Cells were transfected with scrambled or target siRNA according to the manufacturer’s protocol. Briefly, target and scrambled siRNAs (30 pmol) were diluted in 500 μL DMEM and mixed with 5 μL Lipofectamine RNAi MAX (Invitrogen, Carlsbad, CA). After the 15-min incubation at room temperature, the complexes were added to the cells to a final volume of 3 mL, which was made up with medium. Cells were then harvested at the indicated times for further analysis. The knockdown efficiency was confirmed by Western blot analysis of Flag expression.
Western blot analysis
Cells were homogenized and lysed with RIPA lysis buffer (100 mM NaCl, 50 mM Tris–HCl pH 7.5, 1 % TritonX-100, 1 mM EDTA, 10 mM b-glycerophosphate, 2 mM sodium vanadate and protease inhibitor). Protein concentration was assayed using a micro-BCA protein kit (Pierce, Rockford, IL). Forty micrograms of protein per lane was separated by 12 % SDS–PAGE and electroblotted onto nitrocellulose (Amersham Pharmacia, Germany). Then, nonspecific binding was blocked by incubating with 5 % nonfat milk in TBST buffer at room temperature for 1 h. Detection of vimentin, E-cadherin, PERK, ATF4, ATF6, p-Smad2/3, p-Akt and β-actin was performed using mouse mAb (1:1,500, Santa Cruz, CA), and anti-β-actin (Sigma). Goat anti-mouse IgG (1:5,000, Sigma) followed by enhanced chemiluminescence (ECL, Amersham Pharmacia, NJ) was used for detection.
ELISA analysis
Measurement of autocrine TFG-β by gastric cells was processed with a commercially available enzyme-linked immunosorbent assay (ELISA; Yanjin Biotechnology Co., Shanghai, China), according to the manufacturer’s instructions. The absorbance was read at 450 nm using a 680XR Microplate reader (Bio-Rad, Hercules, CA, USA). All of the samples were analyzed in duplicate. The standard curve for protein estimation was made by linear regression analysis.
Statistical analysis
Results are expressed as mean ± SD. Statistical significance was analyzed with one-way ANOVA or Student’s two-tailed t test. A value of P < 0.05 was considered statistically significant. All analyses were conducted using SPSS software (SPSS Inc., Chicago, IL, USA).
Results
Severe hypoxia treatment promotes EMT of gastric cancer cells
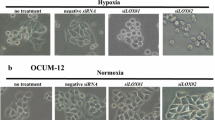
Hypoxia has been shown to be associated with the invasiveness and metastasis of gastric carcinomas [15]. In our study, the human gastric cancer cell lines HGC27 and MGC803 were treated with hypoxic (99 % N2 + 1 % O2) or severe hypoxic (99.9 % N2 + 0.1 % O2) conditions for 24 h. At the end of the treatment, a group of spindle-shaped gastric cancer cells were observed in both cell lines under hypoxia or severe hypoxia but not normoxia (Fig. 1A). Furthermore, the expression of vimentin, a protein marker of EMT, and E-cadherin in gastric cancer cell lines was analyzed by Western blot. Compared with the gastric cancer cells under normoxia, the protein levels of vimentin were markedly raised, while E-cadherin expression was inhibited in those cells under hypoxia and severe hypoxia (Fig. 1B). Meanwhile, gastric cancer cells under severe hypoxia were characterized by the higher expression of vimentin and lower expression of E-cadherin compared to cells under hypoxia (Fig. 1B). These results revealed that EMT of gastric cancer cells was induced by hypoxia and promoted by severe hypoxic conditions.
Epithelial-to-mesenchymal transition (EMT) of human gastric cancer cells was induced by conditions of severe hypoxia. A Two human gastric cancer cell lines, HGC27 and MGC803, were presented to hypoxic conditions (99 % N2 +1 % O2) or severe hypoxic conditions (99.9 % N2 + 0.1 % O2) for 24 h. After that, cell morphology was observed with a microscope. B Gastric cancer cells were treated as (A) and the expression of vimentin and E-cadherin was analyzed by Western blot
UPR proteins are involved in EMT induced under severe hypoxia condition
In a previous study, severe hypoxia treatment resulted in endoplasmic reticulum (ER) stress and UPR in gastric cancer cells [26]. To examine whether UPR is implicated in the induction of EMT, HGC27 cells were treated as above and the expressions of PERK, ATF4 and ATF6 were analyzed. Elevated PERK, ATF4 and ATF6 proteins were observed in cells under severe hypoxia but not in cells under normoxia or hypoxia (Fig. 2A). Subsequently, PERK siRNA, ATF4 siRNA and ATF6 siRNA were transfected into HGC27 cells and then exposed to severe hypoxia. Knockdown of PERK, ATF4 or ATF6 impeded increased vimentin expression and decreased E-cadherin expression after severe hypoxia exposure (Fig. 2B). These results indicated that augmented PERK, ATF4 or ATF6 expression was involved in the induction of EMT by severe hypoxia.
UPR-related proteins were involved in EMT of human gastric cancer cells induced by severe hypoxia conditions. A HGC27 cells were treated by hypoxic conditions (99 % N2 +1 % O2) or severe hypoxic conditions (99.9 % N2 + 0.1 % O2) for 24 h. Then, the expressions of PERK, ATF4 and ATF6 proteins were analyzed by Western blot. B PERK siRNA, ATF4 siRNA and ATF6 siRNA were transfected into HGC27 cells and then exposed to severe hypoxia. The expression of vimentin and E-cadherin was analyzed by Western blot
UPR proteins prompt TGF-β release induced by severe hypoxia in gastric cancer cells
It is well known that TGF-β is one of the main EMT-inducing factors and leads to the activation of ZEB1, ZEB2, Twist, Slug and Snail [27]. Therefore, we next validated whether the promotion of EMT of gastric cancer by UPR proteins was mediated by TGF-β activation. Human HGC27 cells were exposed to severe hypoxia conditions as above, and the release of TGF-β was determined by ELISA. Severe hypoxia exposure extremely boosted the expression of TGF-β in HGC27 cells compared with those under normoxia (Fig. 3A). However, the knockdown of PERK, ATF4 or ATF6 blocked elevated TGF-β release in HGC27 cells after exposure to severe hypoxia (Fig. 3A). Furthermore, thapsigargin, a pharmacological inducer of the UPR, was added to and incubated with HGC27 cells under normoxia or hypoxia conditions. Incubation with thapsigargin distinctly prompted PERK, ATF4 and ATF6 expression in HGC27 cells under either normoxic or hypoxic conditions (Fig. 3B, C). However, significant enhancement of TGF-β expression was only observed in HGC27 cells under conditions of hypoxia (Fig. 3D). Thus, our results suggested that UPR proteins were induced by severe hypoxia or that thapsigargin promoted the TGF-β release of HGC27 cells under hypoxic conditions.
UPR-related proteins contributed to TGF-β release. A HGC27 cells were treated as in Fig. 2A and the release of TGF-β was determined by ELISA. The expression of PERK, ATF4 and ATF6 was knocked-down by the transfection of PERK siRNA, ATF4 siRNA and ATF6 siRNA and the levels of TGF-β were determined by ELISA. B, C Cancer cells were incubated with 300 nmol/L thapsigargin for 16 h, and PERK, ATF4 and ATF6 expression in HGC27 cells was analyzed by Western blot. D The levels of TGF-β were determined after thapsigargin treatment
Smad2/3 and PI3K/Akt signaling is activated by autocrine TGF-β in gastric cancer cells following severe hypoxia treatment
Next, we investigated activation of the downstream part of TGF-β signaling in human gastric cancer cells. HGC27 cells were exposed to severe hypoxia as above, and the expressions of phosphorylated Smad2/3 (p-Smad2/3) and phosphorylated Akt (p-Akt) were analyzed by Western blot. Raised expressions of p-Smad2/3 and p-Akt were observed in HGC27 cells under severe hypoxia. In contrast, neutralization of TGF-β by TGF-β mAb or knockdown of TGF-β expression by specific siRNA stunted elevated p-Smad2/3 and p-Akt expression in HGC27 cells under severe hypoxia (Fig. 4A). Lastly, the effect of activated Smad2/3 and PI3K/Akt signaling on HGC27 cells was examined by the transfection of Smad2 or Smad3 siRNA or by the addition of the PI3K specific inhibitor LY294002 (Fig. 4B). As shown by the results, the knockdown of Smad2 or Smad3 or inhibition of PI3K remarkably reduced vimentin expression and up-regulated E-cadherin expression in HGC27 cells after hypoxia exposure. These results suggested the involvement of Smad2/3 and PI3K/Akt signaling in the EMT of gastric cancer cells exposed to severe hypoxia.
Smad2/3 and PI3K/Akt signaling is activated following severe hypoxia treatment. A HGC27 cells were treated as in Fig. 2A and the expression of phosphorylated Smad2/3 (p-Smad2/3) and phosphorylated Akt (p-Akt) was analyzed by Western blot. B HGC27 cells were transfected with Smad2 or Smad3 siRNA or cultured with the PI3K-specific inhibitor LY294002 and the expression of vimentin and E-cadherin was analyzed
Discussion
With the application of Eppendorf microelectrodes, it is possible to directly measure the tumor partial pressure of oxygen [28]. Ambient air is 21 % O2 (150 mm Hg) and most mammalian tissues exist at 2–9 % O2 (on average 40 mm Hg) [29]. However, tumor oxygen levels can vary from 0.01 to 3.9 % O2 (0.08–30 mmHg), which is much lower than the range for normal tissue and indicates that hypoxic conditions occur in tumors [30]. A report has shown that exposure to the conditions of hypoxia re-oxygenation led to an increased metastatic phenotype of tumor cells in mouse models [31]. In addition, studies also revealed the association between a poor clinical outcome and the low levels of oxygen within solid tumors [32].
The current study found that hypoxia exposure confers an epithelial character on human gastric cancer cells with morphological changes and transition in vimentin and E-cadherin expression. Down-regulation of E-cadherin is considered an EMT marker [33, 34]. In the process of EMT, cadherin switches from E-cadherin to N-cadherin, which is expressed in mesenchymal cells. Meanwhile, vimentin, the major component of mesenchymal cells’ cytoskeleton, is up-regulated during EMT and associated with the acquisition of a migratory phenotype and invasion [35]. Importantly, severe hypoxia potentiated the process of EMT, which implied a distinct mechanism that is not completely understood.
Our further examination found that severe hypoxia exposure but not normoxia or hypoxia exposure may promote UPR and the expression of PERK, ATF4 and ATF6 in gastric cancer cells. PERK (PKR-like ER kinase, also known as EIF2AK3)/ATF4, inositol-requiring protein 1 (IRE1) and ATF6 are three ER stress sensors that initiate UPR signaling [11, 36]. Moreover, knockdown of PERK, ATF4 and ATF6 blocked the up-regulation of vimentin and down-regulation of E-cadherin in HGC27 cells after severe hypoxia exposure, which suggested the role of UPR proteins in EMT of cancer cells.
TGF-β signaling has been shown to directly activate EMT via the promotion of transcription factors like Snail and Slug, ZEB1 and ZEB2\ and Twist [27]. In the present study, TGF-β expressions in gastric cancer cells were significantly triggered under conditions of severe hypoxia. Besides, TGF-β may also regulate the expression of MMPs such as MMP2 and MMP9, as well as components of the ECM such as fibronectin and collagen [37], whereas the knockdown of PERK, ATF4 or ATF6 blocked TGF-β release induced by severe hypoxia exposure in HGC27 cells. Furthermore, thapsigargin was added and induced UPR in HGC27 cells, in which the elevated expression of PERK, ATF4 and ATF6 subsequently enhanced TGF-β release under hypoxia.
Additionally, in our study, exposure of autocrine TGF-β of HGC27 cells under conditions of severe hypoxia led to the activation of Smad2/3 and PI3K/Akt signaling, which were stunted by the pretreatment of TGF-β mAb or TGF-β siRNA. Our data also showed that severe hypoxia-induced EMT of HGC27 cells was reversed by the knockdown of Smad2/Smad3 or inhibition of PI3K. In line with this, a study of Tanjore H demonstrated that induction of ER stress by tunicamycin results in EMT, which is mediated through Smad2/3 and Src kinase activation [38].
Overall, the current study found that severe hypoxia potentiated EMT of gastric cancer cells via the promotion of UPR and the enhancement of PERK, ATF4 and ATF6 expression. Autocrine TGF-β, as well as Smad2/3 and PI3K/Akt signaling was also triggered under severe hypoxia conditions. Hence, we clarified that severe hypoxia induced UPR and thus elevated TGF-β releasing may be implicated in the EMT of gastric cancer cells.
References
Zhu X, Li J. Gastric carcinoma in China: current status and future perspectives (Review). Oncol Lett. 2010;1:407–12.
Yan S, Li B, Bai ZZ, et al. Clinical epidemiology of gastric cancer in Hehuang valley of China: a 10-year epidemiological study of gastric cancer. World J Gastroenterol. 2014;20:10486–94.
Tkachenko MA, Zhannat NZ, Erman LV, et al. Dramatic changes in the prevalence of Helicobacter pylori infection during childhood: a 10-year follow-up study in Russia. J Pediatr Gastroenterol Nutr. 2007;45:428–32.
Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40:250–60.
Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–39.
Kunz M, Ibrahim SM. Molecular responses to hypoxia in tumor cells. Mol Cancer. 2003;2:23.
Brown JM. Tumor microenvironment and the response to anticancer therapy. Cancer Biol Ther. 2002;1:453–8.
Carmeliet P, Dor Y, Herbert JM, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–90.
Goubran HA, Kotb RR, Stakiw J, et al. Regulation of tumor growth and metastasis: the role of tumor microenvironment. Cancer Growth Metastasis. 2014;7:9–18.
Teppo S, Sundquist E, Vered M, et al. The hypoxic tumor microenvironment regulates invasion of aggressive oral carcinoma cells. Exp Cell Res. 2013;319:376–89.
Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–64.
Zhang L, Huang G, Li X, et al. Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor-1alpha in hepatocellular carcinoma. BMC Cancer. 2013;13:1471–2407.
Du J, Sun B, Zhao X, et al. Hypoxia promotes vasculogenic mimicry formation by inducing epithelial-mesenchymal transition in ovarian carcinoma. Gynecol Oncol. 2014;133:575–83.
Hwang-Verslues WW, Chang PH, Jeng YM, et al. Loss of corepressor PER2 under hypoxia up-regulates OCT1-mediated EMT gene expression and enhances tumor malignancy. Proc Natl Acad Sci USA. 2013;110:12331–6.
Matsuoka J, Yashiro M, Doi Y, et al. Hypoxia stimulates the EMT of gastric cancer cells through autocrine TGFbeta signaling. PLoS ONE. 2013;8:e62310.
Katsuno Y, Lamouille S, Derynck R. TGF-beta signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25:76–84.
Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84.
Lu L, Wang J, Zhang F, et al. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J Immunol. 2010;184:4295–306.
Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–84.
Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004;4:966–77.
Nagelkerke A, Bussink J, Mujcic H, et al. Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast Cancer Res. 2013;15:R2.
Pereira ER, Frudd K, Awad W, et al. Endoplasmic reticulum (ER) stress and hypoxia response pathways interact to potentiate hypoxia-inducible factor 1 (HIF-1) transcriptional activity on targets like vascular endothelial growth factor (VEGF). J Biol Chem. 2014;289:3352–64.
Baird M, Woon Ang P, Clark I, et al. The unfolded protein response is activated in Helicobacter-induced gastric carcinogenesis in a non-cell autonomous manner. Lab Invest. 2013;93:112–22.
Malapeira J, Esselens C, Bech-Serra JJ, et al. ADAM17 (TACE) regulates TGFbeta signaling through the cleavage of vasorin. Oncogene. 2011;30:1912–22.
Katoh M. Epithelial-mesenchymal transition in gastric cancer (Review). Int J Oncol. 2005;27:1677–83.
Rzymski T, Petry A, Kracun D, et al. The unfolded protein response controls induction and activation of ADAM17/TACE by severe hypoxia and ER stress. Oncogene. 2012;31:3621–34.
Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90.
Vaupel P, Hockel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal. 2007;9:1221–35.
Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–75.
Kizaka-Kondoh S, Inoue M, Harada H, et al. Tumor hypoxia: a target for selective cancer therapy. Cancer Sci. 2003;94:1021–8.
Cairns RA, Hill RP. Acute hypoxia enhances spontaneous lymph node metastasis in an orthotopic murine model of human cervical carcinoma. Cancer Res. 2004;64:2054–61.
Evans SM, Judy KD, Dunphy I, et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res. 2004;10:8177–84.
Bae GY, Choi SJ, Lee JS, et al. Loss of E-cadherin activates EGFR-MEK/ERK signaling, which promotes invasion via the ZEB1/MMP2 axis in non-small cell lung cancer. Oncotarget. 2013;4:2512–22.
Zheng H, Li W, Wang Y, et al. miR-23a inhibits E-cadherin expression and is regulated by AP-1 and NFAT4 complex during Fas-induced EMT in gastrointestinal cancer. Carcinogenesis. 2014;35:173–83.
Gilles C, Polette M, Mestdagt M, et al. Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res. 2003;63:2658–64.
Magagnin MG, Koritzinsky M, Wouters BG. Patterns of tumor oxygenation and their influence on the cellular hypoxic response and hypoxia-directed therapies. Drug Resist Updat. 2006;9:185–97.
Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–72.
Tanjore H, Cheng DS, Degryse AL, et al. Alveolar epithelial cells undergo epithelial-to-mesenchymal transition in response to endoplasmic reticulum stress. J Biol Chem. 2011;286:30972–80.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, X., Xue, Y., Si, Y. et al. The unfolded protein response potentiates epithelial-to-mesenchymal transition (EMT) of gastric cancer cells under severe hypoxic conditions. Med Oncol 32, 447 (2015). https://doi.org/10.1007/s12032-014-0447-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0447-0