Abstract
HER2 amplification and/or expression occurs in gastric carcinoma (GC), but the role of HER2 in the prognosis of GC remains unclear. The dysregulation of transforming acidic coiled coil 1 (TACC1), a downstream gene of HER2, is thought to be involved in the development of GC. The aim of this study was to investigate the role and relationship of HER2 and TACC1 in GC. The expression of HER2 and TACC1 was analyzed using immunohistochemistry on 129 primary resected GC patients, and HER2 amplification was additionally determined by FISH. The data on clinicopathological features and relevant prognostic factors in these patients were analyzed. The expression (3+, 2+ and 1+) and the amplification of HER2 was observed in 57 cases (44.2 %) and 25 cases (19.4 %), respectively, and the correlation between HER2 expression and amplification was strong (p < 0.001). According to the FDA criteria, 24 cases (18.6 %) would have been considered as HER2 positive. A total 62 (48.1 %) GC tissues showed positive cytoplasmic staining of TACC1. There was a significant and positive association between TACC1 and HER2. HER2 positive was significantly associated with TNM stage (p = 0.019), and TACC1 expression was significantly associated with lymph node metastasis (p = 0.004) and TNM stage (p = 0.004). TNM stage, TACC1 expression and co-positive of both HER2 and TACC1 were independent prognostic factors. TACC1 expression is an independent prognostic indicator of GC. The correlation between TACC1 expression and HER2-positive status indicated a possible synergistic regulation of the two molecules and co-positive of both HER2 and TACC1 maybe a more valuable prognostic marker.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the fourth most common cancer worldwide [1], and the incidence of GC is much higher in China than in any other country. In 2009, 3,621,000 people were diagnosed and the morbidity rate ranked third with the proportion of 14.33 % [2]. Despite the developments of surgical technique and improvements of anticancer agents recently, GC continues to pose a major challenge for health care professionals. The TNM stage, which is determined by the depth of invasion, involvement of the lymph nodes and distant metastasis, is the most important prognostic factor now established for GC [3]. However, prognosis varies between patients in the same stage. Therefore, it is necessary to seek new prognostic factors other than the TNM stage.
The human epidermal growth factor receptor 2 (HER2/neu) gene, also called ErbB-2, is a 185-kDa transmembrane tyrosine kinase (TK) receptor and a member of the epidermal growth factor receptors (EGFRs) family [4]. In breast cancer, HER2 has been most intensively studied and been used as both prognostic and predictive markers [5]. HER2 amplification and/or protein expression has been implicated in the development of gastric adenocarcinoma [6], and HER2-targeted therapy with trastuzumab has been introduced in the treatment of metastatic GC [7]. However, unlike in breast cancer, the prognostic significance of HER2 status in patients with GC remains unclear with some studies suggesting that HER2 positivity is associated with poor prognosis [6, 8–10] and others showing that it is not an independent prognostic factor of patient outcome [11–13].
The transforming acidic coiled coil 1(TACC1), a member of TACC family, is centrosome and microtubule-binding protein and is essential for mitotic spindle formation and function as well as the other currently known TACC proteins (designated TACC2–3) [14–16]. TACC1 gene was first discovered as an oncogene amplified in breast cancer and was correlated with significantly shorter relapse-free survival [17].Using cDNA microarrays, Wilson et al. [18] identified that TACC1 is one of some genes associated with the aggressive phenotype of HER2 positive both in breast cancer cell lines and in carcinomas tissues. Our previous study had also provided proof that efficient downregulation of HER2 induced TACC1 upregulation at both mRNA and protein levels in breast cancer cell lines [19]. Increasing evidence indicates that deregulation of TACC1 is linked to carcinogenesis of various human cancer types except for breast cancer, including ovarian, gastric, colonic, prostate and hepatic carcinoma [20–24]. The expression of both TACC1 and HER2 and any possible relationship between them, however, has not been investigated in GC entity to date.
In this study, we examined both expression and amplification of HER2 and the expression of TACC1 in tumors of radical resected GC to evaluate prognostic value of HER2 and TACC1 and to investigate any possible relationship between them in patients with gastric cancer.
Materials and methods
Patients and tissues
A total of 129 GC tissues with histologically confirmed adenocarcinoma were obtained from the Department of Pathology, the Affiliated Hospital of Qingdao University, from January 2007 to January 2009, and 80 para-cancer normal tissues which were 5 cm far away from the edge of tumor were randomly selected as control. The study protocol was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (Qingdao, China). All patients underwent radical gastrectomy and D2 lymphadenectomy but not received any preoperative therapy including chemotherapy and (or) radiotherapy, were available for follow-up data and signed an informed consent. Clinicopathological information obtained from patient’s operative and pathological reports included gender, age (≤60 or >60 years), depth of tumor invasion (T1–T2 or T3–T4), lymph nodes metastasis (N), histologic grade (G1 G2 or G3, G4 not included), venous invasion, neural invasion, Lauren’s classification (intestinal, diffuse or mixed) and the 7th American Joint Committee on Cancer (AJCC) TNM stage (I–II or III) (Table 1).
Immunohistochemical staining
The expression of TACC1 and HER2 was detected using immunohistochemical (IHC) staining. Briefly, consecutive 4-μm-thick sections were cut from formalin-fixed, paraffin-embedded tissues. After dewaxing and rehydration, the slides were rinsed in phosphate-buffered saline (PBS). Endogenous peroxidase was quenched with aqueous 3 % H2O2 for 20 min and washed with PBS–Tween20 (PBS/T). Non-specific antibody binding was blocked with 10 % normal serum in Tris-HCl buffer. After antigen retrieval with the use of the microwave, the slides were incubated with primary anti-TACC1 (ab17915, diluted 1:100, Abcam, US) or anti-HER2 (dilution 1:200, Abcam, US) overnight at 4 °C. After washing, antibody staining was detected with the avid in-biotin-peroxidase complex (PV9005, mouse hypersensitivity, Beijing fir Jinqiao, China). Finally, sections were incubated in 3, 3-diaminobenzidine (DAB) chromogen solution and counterstained with 0.1 % hematoxylin. In every case, PBS instead of the primary TACC1/HER2 antibody was used on a duplicate slide as a negative control, while the known positive-stained section was used as the positive control. The results were evaluated by two pathologists independently with no knowledge of clinicopathological features.
The expression of TACC1 was scored in a semi-quantitative manner according both the extent and the intensity of the staining [25]. Briefly, the extent of staining was divided into 0–4 score according to the percentage of the staining cells (<5 %; 6–25 %; 26–50 %; 51–75 %; more than 75 %), and the intensity of staining was categorized into four grades (0, no staining; 1, slightly yellowish; 2, brownish yellow; 3, dark brown). After multiplying the two scores, the composite scores were divided into four grades: negative (0–1 scores), weakly positive (2–4 scores), moderately positive (5–8 scores) and strongly positive (9–12 scores). The scores 2 and more than 2 were designated as TACC1-positive expression.
The score of HER2 was determined according to the staining pattern adopted by Hoffmann in Toga trial [26]: high (IHC3+), strong intensity in 10 % or more of cancer cells; medium (IHC2+), weak moderate intensity in 10 % or more; low (IHC1+), faint intensity in 10 % or more; absent (IHC0). IHC3+ was considered to be positive and IHC0 or IHC1+ was considered to be negative for HER2 expression. IHC2+ was defined as equivocal and should be verified with fluorescence in situ hybridization (FISH).
Fluorescence in situ hybridization (FISH)
Fluorescence in situ hybridization analysis was performed using the PathVysion HER2 probe kit. Paraffin-embedded specimens were cut at 2 μm, using conventional histological techniques, and transferred to slides. Sections were deparaffinized and rehydrated in two changes of xylene for 30 min each, followed by two 10-min washes in absolute ethanol and dried at room temperature. Sodium thiocyanate was applied to each section. They were then placed in a thermostat bath for 30 min at 80 °C and rinsed in deionized water, pretreated with sodium thiocyanite and protease solution for 15 min and dehydrated. The probe was denatured at 80 °C for 5 min, was added to each slide and sealed under a small glass coverslip, before overnight hybridization at 42 °C. The procedure was followed by washing in 0.4 × SSC (sodium saline citrate)/0.3 % Nonidet (NP40) and 2 × SSC. After hybridization, the nuclei counterstaining was achieved with 4′, 6-diamidino-2-phenylindole (DAPI). A specimen with an HER2/CEP17 ratio of 2.0 or more in invasive cells was classified as HER2 amplification [27].
Follow-up
All the patients were performed a 5-year retrospective cohort research after gastrectomy from January 2007 to January 2014. The median duration of follow-up was 45.3 months (range, 9–60 months). The information about survival was obtained from the Oncology Department of the Affiliated Hospital of Qingdao University.
Statistical analysis
Documentation and statistical analysis were performed using the SPSS software version 22.0. The relationship between TACC1 and HER2 and clinicopathological factors was analyzed using chi-square statistical test or Fisher’s exact test, while survival analysis was carried out using the Kaplan–Meier method and compared by the Log-rank test. Any parameter can be discovered to be significant on univariate analysis by the Cox’s proportional hazards model was then went into multivariate analysis. p < 0.05 was considered as statistically significant.
Results
HER2 expression and amplification
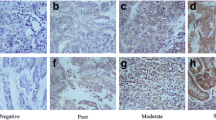
After IHC in GC tissues, 57 of 129 cases (44.2 %) showed HER2 staining, which was strong in 12 cases (3+; 9.3 %), moderate in 20 cases (2+; 15.5 %) and weak in 25 cases (1+; 19.4 %) (Fig. 1). The other 72 cases had no staining (0, 55.8 %). FISH analysis showed that 25 of 129 cases (19.4 %) were HER2 amplification (Fig. 2) and the correlation between HER2 expression and amplification was strong (p < 0.001, Table 2). All amplification cases showed IHC staining, which was strong (3+) in 11 cases, moderate (2+) in 12 cases and weak (1+) in 2 cases. By the contrast, none of the 72 cases without IHC staining showed amplification. According to the Food and Drugs Administration (FDA) criteria, which defined HER2 status positive as either IHC3+ or IHC2+ and validated by FISH amplification [28], 24 cases (18.6 %) would have been considered as HER2 status positive and 105 cases (81.4 %) as negative.
TACC1 expression
A total 62 of 129 (48.1 %) gastric adenocarcinoma tissues and 9 of 80 (11.3 %) para-cancer normal tissues showed positive staining of TACC1, respectively. Tumor staining of TACC1 expression was observed in the cytoplasmic staining pattern, and strong immunoreactivity was mostly found around nucleus (Fig. 3).In the para-cancer normal gastric epithelium, otherwise, only negative or weak immunoreactivity of TACC1 was detected (not showed), which indicated that the TACC1 was more common positive expression in human GC than para-cancer normal tissues (p < 0.001).
Association between HER2 and TACC1
There was a significant and positive association between TACC1 expression and HER2 status according to FDA (p = 0.013, Table 3). However, neither HER2 expression nor amplification had significant association with TACC1 expression (p = 0.102 and p = 0.376, respectively, Table 3).
Clinicopathological parameters and survival analysis
HER2-positive status according to FDA was significantly associated with TNM stage (p = 0.019, Table 1), and the expression of TACC1 was significantly associated with lymph node metastasis (p = 0.004, Table 1) and TNM stage (p = 0.004, Table 1). However, other clinicopathologic factors, including gender, age, depth of tumor invasion, histologic grade, venous/neural invasion and Lauren’s classification, had no significant relationship with HER2 status or TACC1 expression in this study (Table 1).
The analysis of prognostic factors for overall survival is summarized in Fig. 4. Log-rank analysis revealed that histologic grade 1 (G1: well differentiated, Fig. 4a) and TNM stages I–II (Fig. 4b) were associated with better prognosis, but the positive of TACC1 expression (Fig. 4c) was associated with worse prognosis. Additional prognostic factor was HER2 status positive according to FDA criteria (Fig. 4d), but not HER2 expression or amplification positive alone. Furthermore, GC patients whose primary tumors showed both TACC1 expression and HER2 status positive (co-positive) tended to have worse outcome than those with just one positive or co-negative (Fig. 4e). Other clinicopathological parameters had no impact on overall survival.
However, only TNM stage, TACC1 expression and co-positive of TACC1 and HER2 rather than histologic grade and HER2 status positive alone were independent prognostic factors in multivariate analysis. (Table 4).
Discussion
According to the FDA criteria of HER2 status positive, just as used in ToGA trial, we observed that HER2 positive was associated with shorter survival and turned out to be an independent significant negative prognostic marker in the patients with gastric carcinoma, which was similar with some reports [10, 29] but was faced by a considerable amount of papers which demonstrated no or opposite association with prognosis [8, 11, 30, 31]. That is at least partly caused by different detection methods and definition of HER2 status positive. IHC is the most widely used primary test for the assessment of HER2 expression, and only a score of 3+ is considered as positive [28]. A score of 2+ is considered as equivocal, and a score of 1+ and 0 is considered as negative. FISH is another method for detecting amplification of HER2. Many institutions routinely perform both IHC and FISH on all patients; otherwise, a FISH-alone screening strategy has also been suggested [32]. Data from ToGA trial demonstrated that those patients with tumors that had HER2 strong positive (IHC3+ or IHC2+/FISH validated) derived the greatest benefit from treatment with trastuzumab. Therefore, FDA recommends that IHC should be the initial testing method and FISH should be used to retest IHC2+ samples. In this study, we performed both IHC and FISH on all the samples of 129 curatively resected GC patients and found that 11 of 12 cases of IHC3+ showed amplification, 12 of 20 cases of IHC2+ exhibited amplification, but none of the IHC-negative cases showed amplification. Interestingly, 2 of 25 cases with a weak IHC staining (1+) also showed HER2 amplification, and the two cases would have been missed following the FDA criteria. The correlation between IHC and FISH amplification in our study is similar with recently literatures [7, 33], suggesting that not only HER2 IHC2+ but also 1+ maybe should be verified with FISH. But according to ToGA data, there was no significant benefit for patients whose tumors were IHC0 or IHC1+ and FISH amplification and NCCN panel therefore recommends FISH only for patients with a score of IHC2+.
The reported rates of HER2 gene amplification and protein overexpression in GC ranged from 12–27 % and 9–23 %, respectively [8, 12, 13, 29, 34, 35]. The positive rate of HER2 amplification (19.4 %) in our study was in that range; however, the rate of protein overexpression (IHC2+ and 3+) (24.8 %) was higher than the literature data. Some studies showed that HER2-positive rate varied with the Lauren’s classification subtype (intestinal > diffuse) and histologic grade (moderately differentiated > poorly differentiated) [8, 12, 13, 34]. In this study, the proportion of patients with intestinal-type cancer (81.4 %) was higher than in ToGA trial (75 %), and the proportion of well and moderately differentiated patients was also relatively higher (68.2 %). That may partly explain the higher rate of HER2 overexpression. The positive rate of HER2 status, which was according to FDA criteria, was associated with TNM stage other than Lauren’s classification or histologic grade in this study. The possible reason for the difference may be as follows: tumor heterogeneity, geographic variations and the employment of different antibodies, and the criteria for HER2 status positive may be included.
Increasing evidence indicated that TACC1, the first member of the TACCs family, played an important role in cell growth and differentiation, gene regulation by interacting with molecules involved in centrosome/microtubule dynamics, transcription and mRNA processing [36–38]. The deregulation of TACC1 was implicated in the development of various human malignancies. In our study, the expression of TACC1 in GC tissues was upregulated than para-cancer normal gastric tissues, was associated with more aggressive phenomenon such as lymph nodes metastasis and high TMN stages and then was validated as an independent negative prognostic marker in GC patients, which was similar with Devilard’s report [39], which showed that the overexpression of TACC1 mRNA and protein had been found in prostate carcinoma tissues compared with benign prostate tissues and its expression level appeared to be associated with hormone-independent growth and advanced prostate carcinoma [39]. In addition, TACC1 was significantly upregulated and correlated with shorter relapse-free survival and was an independent prognostic marker in breast cancer [40]. However, the question of whether TACC1 was up- or downregulated in ovarian cancer remained controversial because one study identified TACC1 as a possible prognostic marker using microarray analysis [41]; otherwise, another report revealed that both TACC1 and TACC3 were lost or mislocalized in ovarian tumors using IHC analysis [21]. The possible explanations for these different results may be tissue specialist and different methods, the other explanation has been proposed by Gergely et al. [16], who investigated the function of the D-TACC protein in the regulation of the interactions between centrosomes and microtubules, that because an exquisite control was required in this sensitive area, either an increase or a decrease in TACC activity could have deleterious effects on genetic ability and induce oncogenicity.
Wilson et al. [3] used cDNA microarray technology that allows for the simultaneous evaluation of expression of thousands of genes at the mRNA level. A limited number of genes were found to be up- or downregulated by high HER2 protein levels. These included TACC1 that was downregulated both in HER2-positive breast cancer cell lines and in HER2 overexpressing breast carcinomas tissues. Our previous study also indicated TACC1 expression was significantly upregulated by HER2 silencing both at the mRNA and protein levels in breast cancer cell line [19]. Similarly, our data from this study showed that HER2-positive status according to FDA criteria was positive associated with TACC1 expression in GC and co-positive of both HER2 and TACC1 tended to have worst outcome among the relationship between the two factors. These findings indicated that there might be some molecular and biological relationships between HER2 and TACC1 in breast and gastric cancer.
In summary, our results suggest that TACC1, co-positive of both HER2 and TACC1 and TNM stage are potential prognostic markers in gastric cancer. Moreover, the correlation between the expression of TACC1 and the positive status of HER2 indicated a possible synergistic regulation of the two molecules and co-positive of both HER2 and TACC1 may be more valuable for the prognosis of GC.
References
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J clin oncol. 2006;24(14):2137–50. doi:10.1200/JCO.2005.05.2308.
Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu L, et al. The incidences and mortalities of major cancers in China, 2009. Chin J Cancer. 2013;32(3):106–12. doi:10.5732/cjc.013.10018.
Qiu MZ, Wang ZQ, Zhang DS, Liu Q, Luo HY, Zhou ZW, et al. Comparison of 6th and 7th AJCC TNM staging classification for carcinoma of the stomach in China. Ann Surg Oncol. 2011;18(7):1869–76. doi:10.1245/s10434-010-1542-x.
Natali PG, Nicotra MR, Bigotti A, Venturo I, Slamon DJ, Fendly BM, et al. Expression of the p185 encoded by HER2 oncogene in normal and transformed human tissues. Int J Cancer. 1990;45(3):457–61.
Cooke T, Reeves J, Lanigan A, Stanton P. HER2 as a prognostic and predictive marker for breast cancer. Ann Oncol. 2001;12(Suppl 1):S23–8.
Hechtman JF, Polydorides AD. HER2/neu gene amplification and protein overexpression in gastric and gastroesophageal junction adenocarcinoma: a review of histopathology, diagnostic testing, and clinical implications. Arch Pathol Lab Med. 2012;136(6):691–7. doi:10.5858/arpa.2011-0168-RS.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97. doi:10.1016/S0140-6736(10)61121-X.
Gomez-Martin C, Garralda E, Echarri MJ, Ballesteros A, Arcediano A, Rodriguez-Peralto JL, et al. HER2/neu testing for anti-HER2-based therapies in patients with unresectable and/or metastatic gastric cancer. J Clin Pathol. 2012;65(8):751–7. doi:10.1136/jclinpath-2012-200774.
Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19(9):1523–9. doi:10.1093/annonc/mdn169.
Jorgensen JT, Hersom M. HER2 as a prognostic marker in gastric cancer—a systematic analysis of data from the literature. J Cancer. 2012;3:137–44. doi:10.7150/jca.4090.
Grabsch H, Sivakumar S, Gray S, Gabbert HE, Muller W. HER2 expression in gastric cancer: rare, heterogeneous and of no prognostic value—conclusions from 924 cases of two independent series. Cell Oncol. 2010;32(1–2):57–65. doi:10.3233/CLO-2009-0497.
Kunz PL, Mojtahed A, Fisher GA, Ford JM, Chang DT, Balise RR, et al. HER2 expression in gastric and gastroesophageal junction adenocarcinoma in a US population: clinicopathologic analysis with proposed approach to HER2 assessment. Appl Immunohistochem Mol Morphol. 2012;20(1):13–24. doi:10.1097/PAI.0b013e31821c821c.
Janjigian YY, Werner D, Pauligk C, Steinmetz K, Kelsen DP, Jager E, et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann Oncol. 2012;23(10):2656–62. doi:10.1093/annonc/mds104.
Lee MJ, Gergely F, Jeffers K, Peak-Chew SY, Raff JW. Msps/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behaviour. Nat Cell Biol. 2001;3(7):643–9. doi:10.1038/35083033.
Gergely F, Karlsson C, Still I, Cowell J, Kilmartin J, Raff JW. The TACC domain identifies a family of centrosomal proteins that can interact with microtubules. Proc Natl Acad Sci USA. 2000;97(26):14352–7. doi:10.1073/pnas.97.26.14352.
Gergely F, Kidd D, Jeffers K, Wakefield JG, Raff JW. D-TACC: a novel centrosomal protein required for normal spindle function in the early Drosophila embryo. EMBO J. 2000;19(2):241–52. doi:10.1093/emboj/19.2.241.
Nguyen HG, Chinnappan D, Urano T, Ravid K. Mechanism of Aurora-B degradation and its dependency on intact KEN and A-boxes: identification of an aneuploidy-promoting property. Mol Cell Biol. 2005;25(12):4977–92. doi:10.1128/MCB.25.12.4977-4992.2005.
Wilson KS, Roberts H, Leek R, Harris AL, Geradts J. Differential gene expression patterns in HER2/neu-positive and -negative breast cancer cell lines and tissues. Am J Pathol. 2002;161(4):1171–85. doi:10.1016/S0002-9440(10)64394-5.
Xiang J, Qiu W, Wang X, Zhou F, Wang Z, Liu S, et al. Efficient downregulation of ErbB-2 induces TACC1 upregulation in breast cancer cell lines. Oncol Rep. 2013;29(4):1517–23. doi:10.3892/or.2013.2253.
Partheen K, Levan K, Osterberg L, Claesson I, Fallenius G, Sundfeldt K, et al. Four potential biomarkers as prognostic factors in stage III serous ovarian adenocarcinomas. Int J Cancer. 2008;123(9):2130–7. doi:10.1002/ijc.23758.
Lauffart B, Vaughan MM, Eddy R, Chervinsky D, DiCioccio RA, Black JD, et al. Aberrations of TACC1 and TACC3 are associated with ovarian cancer. BMC Womens Health. 2005;5:8. doi:10.1186/1472-6874-5-8.
Line A, Slucka Z, Stengrevics A, Li G, Rees RC. Altered splicing pattern of TACC1 mRNA in gastric cancer. Cancer Genet Cytogenet. 2002;139(1):78–83. doi:10.1016/S0165-4608(02)00607-6
Lauffart B, Howell SJ, Tasch JE, Cowell JK, Still IH. Interaction of the transforming acidic coiled-coil 1 (TACC1) protein with ch-TOG and GAS41/NuBI1 suggests multiple TACC1-containing protein complexes in human cells. Biochem J. 2002;363(Pt 1):195–200.
Ding A, Zhao W, Shi X, Yao R, Zhou F, Yue L, et al. Impact of NPM, TFF3 and TACC1 on the prognosis of patients with primary gastric cancer. PLoS One. 2013;8(12):e82136. doi:10.1371/journal.pone.0082136.
Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Der Pathologe. 1987;8(3):138–40.
Hofmann M, Stoss O, Shi D, Buttner R, van de Vijver M, Kim W, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52(7):797–805. doi:10.1111/j.1365-2559.2008.03028.x.
Perez EA, Suman VJ, Davidson NE, Martino S, Kaufman PA, Lingle WL, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol. 2006;24(19):3032–8. doi:10.1200/JCO.2005.03.4744.
Ruschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25(5):637–50. doi:10.1038/modpathol.2011.198.
Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes–a systematic review. Int J Cancer. 2012;130(12):2845–56. doi:10.1002/ijc.26292.
Berezowska S, Novotny A, Bauer K, Feuchtinger A, Slotta-Huspenina J, Becker K, et al. Association between HSP90 and Her2 in gastric and gastroesophageal carcinomas. PLoS One. 2013;8(7):e69098. doi:10.1371/journal.pone.0069098.
Gu J, Zheng L, Wang Y, Zhu M, Wang Q, Li X. Prognostic significance of HER2 expression based on trastuzumab for gastric cancer (ToGA) criteria in gastric cancer: an updated meta-analysis. Tumour Biol. 2014;35(6):5315–21. doi:10.1007/s13277-014-1693-7.
Tubbs RR, Pettay JD, Roche PC, Stoler MH, Jenkins RB, Grogan TM. Discrepancies in clinical laboratory testing of eligibility for trastuzumab therapy: apparent immunohistochemical false-positives do not get the message. J Clin Oncol. 2001;19(10):2714–21.
Grillo F, Fassan M, Ceccaroli C, Giacometti C, Curto M, Zagonel V, et al. The reliability of endoscopic biopsies in assessing HER2 status in gastric and gastroesophageal junction cancer: a study comparing biopsies with surgical samples. Transl Oncol. 2013;6(1):10–6.
Tanner M, Hollmen M, Junttila TT, Kapanen AI, Tommola S, Soini Y, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16(2):273–8. doi:10.1093/annonc/mdi064.
Yan B, Yau EX, Bte Omar SS, Ong CW, Pang B, Yeoh KG, et al. A study of HER2 gene amplification and protein expression in gastric cancer. J Clin Pathol. 2010;63(9):839–42. doi:10.1136/jcp.2010.076570.
Ha GH, Kim JL, Breuer EK. Transforming acidic coiled-coil proteins (TACCs) in human cancer. Cancer Lett. 2013;336(1):24–33. doi:10.1016/j.canlet.2013.04.022.
Conte N, Charafe-Jauffret E, Delaval B, Adelaide J, Ginestier C, Geneix J, et al. Carcinogenesis and translational controls: TACC1 is down-regulated in human cancers and associates with mRNA regulators. Oncogene. 2002;21(36):5619–30. doi:10.1038/sj.onc.1205658.
Guyot R, Vincent S, Bertin J, Samarut J, Ravel-Chapuis P. The transforming acidic coiled coil (TACC1) protein modulates the transcriptional activity of the nuclear receptors TR and RAR. BMC Mol Biol. 2010;11:3. doi:10.1186/1471-2199-11-3.
Devilard E, Bladou F, Ramuz O, Karsenty G, Dales JP, Gravis G, et al. FGFR1 and WT1 are markers of human prostate cancer progression. BMC Cancer. 2006;6:272. doi:10.1186/1471-2407-6-272.
Ghayad SE, Vendrell JA, Bieche I, Spyratos F, Dumontet C, Treilleux I, et al. Identification of TACC1, NOV, and PTTG1 as new candidate genes associated with endocrine therapy resistance in breast cancer. J Mol Endocrinol. 2009;42(2):87–103. doi:10.1677/JME-08-0076.
Partheen K, Levan K, Osterberg L, Horvath G. Expression analysis of stage III serous ovarian adenocarcinoma distinguishes a sub-group of survivors. Eur J Cancer. 2006;42(16):2846–54. doi:10.1016/j.ejca.2006.06.026.
Acknowledgments
This work was supported by National Natural Scientific Foundation of China (NSFC) (No: 81472338); Shandong Excellent Young Scientist Research Award Fund Project (2006BSB14114).
Conflict of interest
We declare that we have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lv, J., Yao, Ys., Zhou, F. et al. Prognosis significance of HER2 status and TACC1 expression in patients with gastric carcinoma. Med Oncol 31, 280 (2014). https://doi.org/10.1007/s12032-014-0280-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0280-5