Abstract
The expression of survivin, an inhibitor of apoptosis can be seen in most tumors and is correlated with the angiogenic factor vascular endothelial growth factor (VEGF). But little is known about their contribution in small-cell lung cancer (SCLC). This study was designed to investigate the expression of survivin and VEGF in SCLC, and to explore their correlation with clinical-pathological feature and prognosis. Forty-five patients with pathological histology of SCLC were entered into this study. Forty-five cases of matched adjacent non-tumor samples and 10 samples of operated patients with benign lung tumor were also included as control. The expression of survivin and VEGF was detected by immunohistochemistry (IHC, SP). These two sets of data were processed and tested for correlation with major patients’ characteristics, and overall survival. The correlations between survivin and VEGF expressions and the clinical-pathological features were evaluated by chi-square test. The correlation between survivin and VEGF expressions was analyzed by Spearman’s rank correlation test; the overall survival was analyzed by the Kaplan–Meier method; and the relationship between clinical and pathological features and overall survival was analyzed by the Cox proportional hazard models. Positive expression rate of survivin and VEGF was significantly higher in SCLC than those of adjacent non-tumor tissues and benign lung tumor tissues (73.3 vs. 15.6 vs. 0 %, P < 0.05) and (75.6 vs. 20 vs. 0 %, P < 0.05), respectively. Survivin and VEGF expressions were significantly associated with lymph node metastasis (P = 0.003, 0.011) and clinical stage (P = 0.006, 0.021). The expression of survivin was significantly coincident with the expression of VEGF (r = 0.644, P = 0.000). The median overall survival in survivin positive group and VEGF positive group was significantly shorter than those in survivin negative and VEGF negative group, respectively (log-rank P = 0.000). Moreover, multivariate analysis showed that survivin expression (HR 0.224; 95 % CI 0.074–0.675; P = 0.008) and VEGF expression (HR 0.172; 95 % CI 0.054–0.559; P = 0.003) were statistically independent predictive factors of poorer prognosis for SCLC patients. Our results indicated that survivin and VEGF were over-expressed in small-cell lung cancer, each of them may be an independent poor prognostic factor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Small-cell lung cancer (SCLC), which represents 13 % of all lung cancer cases, is the most malignant and lowest differentiated tumor. Although a chemotherapy and radiation-sensitive disease, SCLC recurs rapidly with an overall median survival following the treatment of 10 months and a 5-year survival of 5 % [1, 2]. The survival of SCLC patients is dismal and has not been greatly improved in the last 20 years. Early metastasis was commonly recognized as an important cause of poor clinical outcome in SCLC. So far, the prediction of SCLC prognosis is mainly based on the clinical stage; however, SCLC patients with the same clinical stage often present different clinical prognosis, which suggests that the clinical stage alone is insufficient to precisely predict the prognosis of SCLC. Some molecular markers have been found to be related to the prognosis of SCLC, including c-kit [3], MET [4], microsatellite alterations and TP53 mutations in plasma DNA [5], and so on.
Survivin [6], a recently discovered inhibitor of apoptosis protein (IAP), plays a crucial role in the regulation of cell proliferation, apoptosis, and angiogenesis in cancers [7]. Survivin is undetectable in the majority of normal adult tissues, but is over-expressed in a variety of human neoplasm, including colorectal [7], uterine, esophageal, bladder, breast [8], liver, ovarian carcinoma, lymphoma, acute lymphoblastic leukemia [9], and non-small-cell lung cancer (NSCLC) [10]. During recent years, more and more studies found that survivin over-expression is related with more aggressive behavior [11], increased tumor recurrence, and chemotherapy resistance in various tumors [12], and is inversely correlated with the prognosis [13, 14].
The vascular endothelial growth factor (VEGF) has been identified as a crucial regulator of tumor-related angiogenesis. VEGF family is comprised of VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and their three VEGF receptors (VEGFR 1–3) [15]. The VEGF signaling pathway leads to increased proliferation, migration, and invasion of endothelial cells, thus mediating tumor angiogenesis [15, 16]. Tumors may activate angiogenic inhibitors such as angiostatin and endostatin, which control growth by suppressing endothelial cell proliferation and angiogenesis and by indirectly increasing apoptosis in tumor cells [17]. Increased expression of VEGF was found in many kinds of tumors. Some studies reported that over-expression of VEGF is associated with a poor prognosis in many types of cancers such as esophageal, pancreatic, and colorectal cancer [18, 19].
Several authors already reported a relationship between survivin and VEGF in some kinds of malignant lesions. Above all, survivin and VEGF are both important regulators in tumor growth. They were reported to be over-expressed in many types of cancers, but the studies that investigate the clinical value of these factors in SCLC have not been reported. Therefore, in the present study, we performed statistical analyses to determine the expression of survivin and VEGF, and to investigate the impact of survivin and VEGF expressions on prognosis in SCLC. Moreover, the interrelationship between these factors was initially evaluated.
Methods
Ethics statement
This study was carried out in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of West China Hospital, Sichuan University. All patients signed informed consent prior to sample examination.
Patients and clinical-pathological data
This study enrolled 45 SCLC patients who underwent radical resection without either radiotherapy or chemotherapy before operation at the Department of Chest Surgery, West China Hospital, Sichuan University, from January 2000 to June 2007. All the specimens were confirmed as SCLC following pathological diagnosis. Clinical-pathological characteristics such as age, gender, tumor size, smoking history, tumor type, clinical stage, and lymph node metastasis were involved. There were 37 males (82.2 %) and 8 females (17.8 %), with a median age of 53 (ranged from 30 to 76) years. Thirty-four patients had smoking history, and 11 patients never smoked. Tumor stage was conducted according to TNM staging system, and 14 patients were at stages I–II (31.1 %); 31 were at stages III–IV (68.9 %). According to American veterans hospital team, 39 patients (86.7 %) were limited stage and 6 patients (13.3 %) were extensive stage; III–IV stage patients were mainly staged as either major blood vessels or lymph node involvement, and no distant metastasis was involved. Extensive stage patients were mainly staged as contralateral mediastinal lymph node metastases. There were 40 patients (88.9 %) with tumor size > 3 cm, and 5 patients (11.1 %) with tumor size ≤ 3 cm; 32 patients (71.1 %) had lymph node metastasis, and 13 patients (28.9 %) without. From tumor type, 29 patients (64.4 %) were assigned to central type, and 16 patients (35.6 %) were peripheral type. After surgery, 37 patients received chemotherapy and radiotherapy, while 8 patients only received chemotherapy, 41 patients received platinum-based chemotherapy, and 4 patients received non-platinum-based chemotherapy. The chemotherapies included VP-16 and cisplatin, VP-16 and carboplatin, cyclophosphamide doxorubicin and cisplatin, cyclophosphamide doxorubicin and vincristine, and so on. The average number of chemotherapy cycles was 3.9, and the average radiation dose was 4,300 cGy. Forty-five cases of adjacent non-tumor samples and 10 samples of operated patients with benign lung tumor were also included as control. Follow-up information was obtained according to patients’ medical records provided by the contact, and overall survival (OS) was recorded. The median follow-up was 11 months, forty-three patients were reported dead, and two patients were still alive. Overall survival was calculated from the date of diagnosis to death.
Immunohistochemistry (IHC) assays
All specimens were routinely processed formalin-fixed (10 %), paraffin-embedded blocks and sectioned. Serial sections (4 μm) were prepared from the cut surface of tumor blocks at the maximum cross section of the tumor. IHC staining for survivin and VEGF antigen was conducted using the standard streptavidin-peroxidase-biotin technique (SP technique) with an SP kit (purchased from Beijing Zhong Shan-Golden Bridge Biological Technology CO, LTD, China). Immunostaining results were evaluated independently by 3 pathologists. Percentage and intensity of positive cells were used to evaluate each tissue section. The mean percentage of positive tumor cells was determined in at least five strongly staining areas at × 200 magnification for survivin and VEGF. Positive cell rates of 0–10, 11–25, 26–50, 51–75, and >75 % were scored 0, 1, 2, 3, and 4, respectively. The staining intensity was graded as follows: no staining (score 0); pale yellow staining (score 1); buffy staining (score 2); and strongly brown staining (score 3). The scores for the above parameters were multiplied synchronically to calculate a weighted score for each section. Cases with weighted scores 0–1, 2–3, 4–6, and 7–12 were defined as “−”, “+”, “++”, and “+++”, respectively. Cases with weighted scores 0–1 were defined as negative expression, while the other cases with weighted scores ≥2 were defined as positive expression.
Statistical analysis
SPSS 16.0 software was used for statistical analysis. The results were considered to indicate a statistically significant difference for P values <0.05. The difference between survivin and VEGF expressions in SCLC, adjacent non-tumor, and benign lung tumor samples was assessed using Kruskal–Wallis test. Associations of survivin and VEGF expressions and clinical-pathological parameters were analyzed by the chi-square test. Spearman’s correlation analysis from ranks was used to analyze the coincident expression between survivin and VEGF. The impact of survivin and VEGF expressions on survival was assessed with the Kaplan–Meier method and determined by the log-rank test. The influence of various clinical-pathological variables on the survival was assessed with the Cox regression model.
Results
Survivin and VEGF expressions
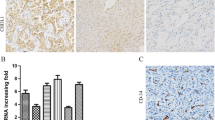
The staining of survivin and VEGF was both localized in cytoplasm (Fig. 1), of the samples, 33/45(73.3 %) SCLC, 7/45(15.6 %) adjacent non-tumor samples, and 0 benign lung tumor tissues were observed for positive survivin expression; 34/45(75.6 %) SCLC, 9/45(20 %) adjacent non-tumor samples, and 0 benign lung tumor tissues were observed for positive VEGF expression. The expression of survivin and VEGF in SCLC was significantly higher than in either adjacent non-tumor samples or benign lung tumor tissues (P < 0.05). There was no statistical difference between adjacent non-tumor samples and benign lung tumor tissues (P > 0.05).
Immunohistochemical expression of survivin and VEGF in SCLC and adjacent non-tumor sample (SP method; magnification, ×200). a Immunoreactivity was observed in the SCLC cytoplasm. The brown granules in the cytoplasm indicate survivin expression in SCLC cells. b Negative expression of survivin expression in adjacent non-tumor sample. c Immunoreactivity was observed in the SCLC cytoplasm. The brown granules in the cytoplasm indicate VEGF expression in SCLC cells. d Negative expression of VEGF expression in adjacent non-tumor sample
Survivin, VEGF, and clinical characteristics of SCLC
Survivin expression in lymph node positive metastasis group 28/32(87.5 %) was significantly higher than in lymph node negative metastasis group 5/13(38.5 %) (P = 0.003). In III–IV stage group, 27/31(87.1 %) showed survivin positive expression, while in I–II stage group, 6/14(42.9 %) expressed survivin (P = 0.006). Similarly, we found that VEGF expression in lymph node positive metastasis group 28/32(87.5 %) was significantly higher than in lymph node negative metastasis group 6/13(46.2 %) (P = 0.011). In III–IV stage group, 27/31(87.1 %) showed VEGF positive expression, while in I–II stage group, 7/14(50.0 %) expressed VEGF (P = 0.021). But survivin and VEGF expressions were not significantly associated with age, gender, tumor size, smoking history, and tumor type (P > 0.05) (Table 1).
Interrelationship between survivin and VEGF
In survivin positive group, 30/33(90.9 %) showed VEGF positive expression; meanwhile, in survivin negative group, 8/12(66.7 %) cases also detected VEGF negative. Using Spearman’s rank correlation test, the expression of survivin was significantly coincident with the expression of VEGF (r = 0.644, P = 0.000) (Table 2).
Survival analysis
With a total follow-up of 105 months, analysis of the impact of survivin expression on OS is shown in Fig. 2a. The median OS in survivin positive group and survivin negative group was 9 months and 42 months each, a significant difference can be seen (log-rank P = 0.000). Analysis of the impact of VEGF expression on OS is shown in Fig. 2b. The median OS in VEGF positive group was 9 months, which was also significantly shorter than that in VEGF negative group 65 months (log-rank P = 0.000).
Overall survival curves for patients with SCLC. a The median overall survival in survivin positive group and survivin negative group was 9 and 42 months each, and a significant difference can be seen (log-rank P = 0.000). b The median overall survival in VEGF positive group was 9 months, which was also significantly lower than that in VEGF negative group 65 months (log-rank P = 0.000)
Multivariate analysis of survival
According to multivariate analysis with Cox regression model using all clinical-pathological characteristics, including gender, age, tumor size, tumor type, clinical stage, lymph node metastasis, survivin expression, and VEGF expression, it can be seen that survivin expression (HR 0.224; 95 %CI 0.074–0.675; P = 0.008), VEGF expression (HR 0.172; 95 % CI 0.054–0.559; P = 0.003), and lymph node metastasis (HR 0.001; 95 % CI 0.030–0.423; P = 0.001) were significantly associated with OS. It was identified that survivin expression and VEGF expression were statistically independent predictive factors of poorer prognosis for SCLC patients along with lymph node metastasis (Table 3).
Discussion
SCLC represents a highly malignant and particularly aggressive form of cancer, with early and widespread metastasis and poor prognosis. Along with the development of antitumor treatment, identifying patients with high risk of poor prognosis has never been given up.
Our study referred to two molecular markers (survivin and VEGF) and found that survivin and VEGF were highly expressed in SCLC, and they were significantly associated with the clinical stage and lymph node metastasis. Survivin and VEGF expressions were significantly associated with each other, and each of them implied poor prognosis in patients with SCLC after surgery. Moreover, in multivariate analysis, over-expression of survivin and VEGF was an independent prognostic factor for OS of SCLC.
Survivin, an inhibitor of apoptosis, plays a crucial role in regulating the cell proliferation, apoptosis, and angiogenesis [7, 20, 21]. Over-expression of survivin has been implicated in tumor progression in various human cancers, such as colorectal cancer [7], breast cancer [8], liver cancer [6], ovarian carcinoma, and NSCLC [10]. However, the expression of survivin in SCLC and its correlation with the clinical-pathological factors have not been studied. In this study, we examined the expression of survivin in SCLC and normal lung tissues by IHC. Our results showed that positive expression of Survivin was frequently observed in SCLC tissues. In contrast, only a small population of adjacent non-tumor tissues showed positive expression for survivin. These findings suggest that over-expression of survivin may play a crucial role in the SCLC progression.
In previous studies, survivin expression was found to be elevated and significantly associated with more aggressive behavior, increased tumor recurrence, chemotherapy resistance, and decreased survival in many human cancers such as colorectal, ovarian, liver [22], breast carcinomas [8], NSCLC [10], and so on. A clinical study involving 210 NSCLC patients showed that survivin could serve as an independent predictor for OS of NSCLC patients in stage III [10]. Another clinical study involving 102 cases of esophageal squamous cell carcinoma demonstrated that positive expression of survivin was observed in 60.8 % of the cases, and it was a poor prognostic predictor of the patients [23]. This was also consistent with our studies. In our study, further correlation analysis revealed that positive expression of survivin was significantly correlated with lymph node metastasis and late clinical stage. Importantly, positive expression of survivin was a strong and independent predictor of short OS of SCLC patients. This result suggests that survivin expression reflects more aggressive clinical behavior in SCLC, and detection of survivin protein expression in the tumor tissue could predict the prognosis of SCLC patients; survivin could be a potential biomarker to evaluate prognosis and a promising target to treat SCLC.
VEGF is a potent growth factor for endothelial cells. In normal adult tissues, VEGF is undetectable, while in many types of carcinomas, VEGF is over-expressed. In certain types of carcinomas, VEGF expression is significantly associated with poorer survival such as malignant pleural mesothelioma [24].
Our results showed that positive expression of VEGF was frequently observed in SCLC tissues. In contrast, only a small population of adjacent non-tumor tissues showed positive expression for VEGF. Further correlation analysis revealed that positive expression of VEGF was correlated closely with lymph node metastasis and late clinical stage. Importantly, it can also be seen that patients with positive VEGF expression had a statistically poorer prognosis in comparison with patients with negative VEGF expression. Thus, VEGF expression appears to reflect more aggressive clinical behavior in SCLC, and VEGF may be significant in local recurrence and metastasis through angiogenesis in SCLC; VEGF expression could be a valuable marker for prognosis prediction in SCLC. This was consistent with previous reports. One article studied survivin and VEGF in small adenocarcinoma of the lung concluded that [25] multiple increased expression of oncogene is a poor prognostic factor.
Previous studies have shown that the expression of VEGF is correlated with the expression of survivin in many cancers such as hepatocellular cancer, nasopharyngeal carcinoma (NPC), NSCLC, and breast cancer [26]. It has been known that survivin expression was regulated by many agents such as cytokines, kinase inhibitors, and growth factors. Some articles revealed that survivin is a novel growth factor-inducible protective gene expressed by endothelial cells during angiogenesis [27], and survivin was inducted by VEGF via a PI3 K/Akt pathway [28]. In our study, the results demonstrated a significantly positive correlation between survivin and VEGF expressions in SCLC. Therefore, the examination of survivin expression and VEGF expression could be used in identifying the prognosis of patients with SCLC.
Conclusion
In this study, we referred to survivin and VEGF expressions in SCLC and normal lung tissues, and our results described that over-expression of survivin and VEGF may be important in tumorigenesis of SCLC and can be used to indicate the poorer prognosis, and each of them could be used as an important molecular marker for shortened survival time in patients with SCLC.
Abbreviations
- SCLC:
-
Small-cell lung cancer
- VEGF:
-
Vascular endothelial growth factor
- IHC:
-
Immunohistochemistry
- IAP:
-
Inhibitors of apoptosis
- NSCLC:
-
Non-small-cell lung cancer
- SP technique:
-
Streptavidin-peroxidase-biotin technique
- OS:
-
Overall survival
- NPC:
-
Nasopharyngeal carcinoma
References
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277–300.
Yip D, Harper PG. Predictive and prognostic factors in small cell lung cancer: current status. Lung Cancer. 2000;28:173–85.
Batus M, Myint R, Coon J, Basu S, Kaiser K, Fidler M, Bonomi P. N-cadherin, E-cadherin, ERCC1, and c-kit expression in small cell lung cancer (SCLC) and potential for new therapeutic targets. J Clin Oncol. 2009; 27(Suppl 15s):(abstract e22157).
Cañadas I, Arumi M, Lema L, Martinez A, Grande E, Bellosillo B, Rojo F, Rovira A, Albanell J, Arriola E. MET in small cell lung carcinoma (SCLC): effects of a MET inhibitor in SCLC cell lines and prognostic role of MET status in patients. J Clin Oncol. 2009; 27 (Suppl 15s):(abstract e14617).
Gonzalez R, Silva JM, Sanchez A, Dominguez G, Garcia JM, Chen XQ, Stroun M, Provencio M, España P, Anker P, Bonilla F. Microsatellite alterations and TP53 mutations in plasma DNA of small-cell lung cancer patients: follow-up study and prognostic significance. Ann Oncol. 2000;11:1097–104.
Srinivasula SM, Ashwell JD. IAPs: what’s in a name? Mol Cell. 2008;30:123–35.
Hernandez JM, Farma JM, Coppola D, Hakam A, Fulp WJ, Chen DT, Siegel EM, Yeatman TJ, Shibata D. Expression of the antiapoptotic protein survivin in colon cancer. Clin Colorectal Cancer. 2011;10:188–93.
Adamkov M, Halasova E, Kajo K, Machalekova K, Vybohova D, Varga I, Rajcany J. Survivin: a promising biomarker in breast carcinoma. Neoplasma. 2010;57:572–7.
Park E, Gang EJ, Hsieh YT, Schaefer P, Chae S, Klemm L, Huantes S, Loh M, Conway EM, Kang ES, Hoe Koo H, Hofmann WK, Heisterkamp N, Pelus L, Keerthivasan G, Crispino J, Kahn M, Müschen M, Kim YM. Targeting survivin overcomes drug resistance in acute lymphoblastic leukemia. Blood. 2011;118:2191–9.
Wang M, Liu BG, Yang ZY, Hong X, Chen GY. Significance of survivin expression: prognostic value and survival in stage III non-small cell lung cancer. Exp Ther Med. 2012;3:983–8.
Salz W, Eisenberg D, Plescia J, Garlick DS, Weiss RM, Wu XR, Sun TT, Altieri DC. A survivin gene signature predicts aggressive tumor behavior. Cancer Res. 2005;65:3531–4.
Trabulo S, Cardoso AM, Santos-Ferreira T, Cardoso AL, Simões S, Pedroso de Lima MC. Survivin silencing as a promising strategy to enhance the sensitivity of cancer cells to chemotherapeutic agents. Mol Pharm. 2011;8:1120–31.
Nassar A, Lawson D, Cotsonis G, Cohen C. Survivin and caspase-3 expression in breast cancer: correlation with prognostic parameters, proliferation, angiogenesis, and outcome. Appl Immunohistochem Mol Morphol. 2008;16:113–20.
Kawasaki H, Toyoda M, Shinohara H, Okuda J, Watanabe I, Yamamoto T, Tanaka K. Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer. 2001;91:2026–32.
Tanno S, Ohsaki Y, Nakanishi K, Toyoshima E, Kikuchi K. Human small cell lung cancer cells express functional VEGF receptors, VEGFR-2 and VEGFR-3. Lung Cancer. 2004;46:11–9.
Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med. 2005;9:777–94.
Lizasa T, Chang H, Suzuki M, Otsuji M, Yokoi S, Chiyo M, Motohashi S, Yasufuku K, Sekine Y, Iyoda A, Shibuya K, Hiroshima K, Fujisawa T. Overexpression of collagen XVIII is associated with poor outcome and elevated levels of circulating serum endostatin in non-small cell lung cancer. Clin Cancer Res. 2004;10:5361–6.
Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61.
Giatromanolaki A, Sivridis E, Koukourakis MI. Angiogenesis in colorectal cancer: prognostic and therapeutic implications. Am J Clin Oncol. 2006;29:408–17.
Pavlyukov MS, Antipova NV, Balashova MV, Vinogradova TV, Kopantzev EP, Shakhparonov MI. Survivin monomer plays an essential role in apoptosis regulation. J Biol Chem. 2011;286:23296–307.
Kim K, Chie EK, Wu HG, Kim SG, Lee SH, Kang GH, Hyun CL, Ha SW. High survivin expression as a predictor of poor response to preoperative chemoradiotherapy in locally advanced rectal cancer. Int J Colorectal Dis. 2011;26:1019–23.
Yang Y, Zhu J, Gou HF, Cao D, Jiang M, Hou M. Clinical significance of Cox-2, Survivin and Bcl-2 expression in hepatocellular carcinoma (HCC). Med Oncol. 2011;28:796–803.
Zhu HX, Wang QF, Hu CF, Zhang WC, Quan LP, Liu M, Xu NZ, Xiao ZF. High expression of survivin predicts poor prognosis in esophageal squamous cell carcinoma following radiotherapy. Tumor Biol. 2011;32:1147–53.
Yasumitsu A, Tabata C, Tabata R, Hirayama N. Clinical significance of serum vascular endothelial growth factor in malignant pleural mesothelioma. J Thorac Oncol. 2010;5:479–83.
Oshita F, Ito H, Ikehara M, Ohgane N, Hamanaka N, Nakayama H, Saito H, Yamada K, Noda K, Mitsuda A, Kameda Y. Prognostic impact of surviving, cyclin D1, integrin beta 1, and VEGF in patients with small adenocarcinoma of stage I lung cancer. Am J Clin Oncol. 2004;27:425–8.
Ryan BM, Konecny GE, Kahlert S, Wang HJ, Untch M, Meng G, Pegram MD, Podratz KC, Crown J, Slamon DJ, Duffy MJ. Survivin expression in breast cancer predicts clinical outcome and is associated with HER2, VEGF, urokinase plasminogen activator and PAI-1. Ann Oncol. 2006;17:597–604.
O’Connor DS, Schechner JS, Adida C, Mesri M, Rothermel AL, Li F, Nath AK, Pober JS, Altieri DC. Control of apoptosis during angiogenesis by survivin expression in endothelial cells. Am J Pathol. 2000;156:393–8.
Beierle EA, Nagaram A, Dai W, Iyengar M, Chen MK. VEGF-mediated survivin expression in neuroblastoma cells. J Surg Res. 2005;127:21–8.
Acknowledgments
The authors would like to thank all their colleagues who participated in this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ping Chen and Jiang Zhu have been equally contributed to this work.
Rights and permissions
About this article
Cite this article
Chen, P., Zhu, J., Liu, Dy. et al. Over-expression of survivin and VEGF in small-cell lung cancer may predict the poorer prognosis. Med Oncol 31, 775 (2014). https://doi.org/10.1007/s12032-013-0775-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0775-5