Abstract
Gastric cancer (GC) is a highly aggressive malignant tumor. Its high mortality rate prompts the urgent need for novel therapeutic agents. The aim of this study is to detect the expression of CRM1 in GC, which has not been reported to date. The expression of CRM1 in GC and adjacent noncancerous tissues (ANCT) of gastrectomy specimens from 120 GC patients was measured by immunohistochemistry. In addition, correlations between the CRM1 staining and the clinicopathologic features as well as survival were analyzed. Positive expression rates of CRM1 in GC and ANCT were 57.8 and 6.7 %, respectively. High expression of CRM1 was significantly associated with increased serum level of carcinoma embryonic antigen (CEA, P = 0.02) but not associated with that of carbohydrate antigen 19–9 (P = 0.38). CRM1 levels were correlated with more advanced tumor stages (P = 0.01), positive Her2 status (P = 0.01), and distant metastasis (P = 0.02). Univariate analysis showed that CEA (P = 0.0076), TNM stage (P = 0.0001), metastasis (P = 0.027), and CRM1 expression (P = 0.0019) were significant risk factors affecting overall survival of GC patients. The multivariate analysis indicated that the CRM1 was an independent indicator for GC survival (P = 0.0048). The current results indicated that CRM1 expressed in a subpopulation of GC with aggressive behavior and could serve as a prognosis marker for poor outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
About one million people are diagnosed with gastric cancer (GC) each year globally, which makes it the fourth most common cancer and the second leading cause of cancer-related death [1, 2]. Despite the rapid development of several anticancer drugs, the prognosis for gastric adenocarcinoma patients remains poor. Currently, except for the cancer staging, which adjuvant treatment depends on, there are few predictive factors for the prognosis of GC [3, 4]. A few previous studies demonstrated that in GC, pre-treatment serum carcinoma embryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19–9) levels have a prognostic value, and they should be adopted when different therapeutic protocols are evaluated [5]. Although many patients with GC have high serum CEA and CA19–9 levels, the exact role of these factors still remains controversial. Therefore, seeking new prognostic and predictive factors is extremely urgent at present.
The chromosomal region maintenance (CRM1)/exportin1/Xpo1 has been demonstrated to be involved in controlling chromosomal structures and mediating the subcellular distribution of important molecules such as epidermal growth factor receptor, P53, and protein kinase 1 [6, 7]. Additionally, CRM1 overexpression has been detected in several cancers (glioblastoma [8], osteosarcoma [9], pancreas [10], ovarian [11], and cervical cancer [12]) and has been associated with worse outcome. However, data are missing for other cancer types to further evaluate the prognostic value of CRM1 expression.
In this study, we sought to measure the expression of CRM1 in human GC specimens and gauged its prognostic value by determining the correlation between CRM1 and GC survival. The correlation between CRM1 expression and the clinicopathological parameters of GC was also analyzed.
Materials and methods
Patient samples
Surgical specimens from 135 GC patients who had undergone curative surgery at the Affiliated Hospital of Medical College, Qingdao University between November 2007 and March 2009 were reviewed. The mean age of the patients was 63.5 years (range 28–85 years). The median follow-up period was 39 months (range 3–55 months). Ten normal gastric tissues were also investigated. Of the 195 cases, 101 were males (74.8 %) and 34 were females (25.2 %). According to the American Joint Committee on Cancer (AJCC) standard, there were 39 cases in stage I, 65 cases in stage II, 31 cases in stage III, and no stage IV cases. The 3-year postsurgical survival was 57.8 %. The Her2/neu overexpression was detected in 31 (15.9 %) cases by postoperative fluorescence in situ hybridization (FISH) examination. All specimens were routinely fixed in 10 % formalin and embedded in paraffin. All had no radiotherapy and chemotherapy before operation. Progression-free survival (PFS) was calculated as the period from surgery until the date of the first recurrence. All the cases were reviewed by a pathologist to confirm the malignancy. The investigation was approved by the Ethics Committee of Affiliated Hospital of Medical College, Qingdao University. The written informed consents were obtained from all the patients.
Immunohistochemistry
Immunohistochemistry was performed according to the protocol procedures. Briefly, the paraffin-embedded tissue blocks were sectioned in 3- to 4-um slices and placed on slides. After de-waxing and hydration, the slides were rinsed in phosphate-buffered saline (PBS), blocked for 10 min with 3 % hydrogen peroxide to quench the endogenous peroxidase activity, then washed in PBS, and incubated with the rabbit polyclonal anti-CRM1 antibody (ab77977; Abcam; 1:33 dilution) overnight at 4 °C. On the following day, the sections were re-warmed at 37 °C for 45 min. Subsequently, they were rinsed 3 times with PBS for 10 min followed by incubation with a PV9000 2-step plus Poly-HRP Anti-mouse/rabbit IgG Detection System (Beijing fir Jinqiao) for a total of 40 min at 37 °C in a humid chamber. The sections were washed 3×3 min with PBS, followed by the addition of diaminobenzidine (DAB) as a chromogen. Antibodies were optimized using positive control tissue according to the manufacturer’s instructions. In negative controls, the primary antibody was replaced with PBS. Each slide was scored in a blinded fashion by two pathologists according to the manufacturer’s recommended criteria at 100× and 200× magnifications. The overall percentage of positive cells on an immunostained section was determined according to the pattern of intracellular localization. The staining intensity of the eIF3f-specific within a tissue section was determined by the percentage of cells with cytoplasm staining. The immunostaining was read in a semiquantitative manner. Three visual fields were examined randomly and the rates of positive cells were divided into less than 5 % (score 0), 6–25 % (score 1), 26–50 % (score 2), 51–75 % (score 3), and more than 75 % (score 4). The staining intensity can be divided into four grades: no staining (score 0), slightly yellowish (score 1), brownish yellow (score 2), and dark brown (score 3). The multiplication of the two was graded as follows: 0 (score 0), 1+ (score 1–4), 2+ (score 5–8), and 3+ (score 9–12). Intensity scores of 0 or 1+ were designated as low expression, and 2+ and 3+ were designated as high expression.
Statistical analysis
Student’s t test was used to analyze the differences between groups for continuous variables and a x 2 test or Fisher’s exact test for proportions. The overall survival rate was estimated by the Kaplan–Meier method. Survival analyses (Cox proportional hazard regression models) were also performed to assess the prognostic value of CRM1 expression and other clinicopathological features. We utilized SPSS 17.0 software system for statistical analysis (SPSS, Inc., Chicago, IL).
Results
CRM1 expression in GC tissues, ANCT, and normal tissues
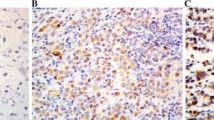
GC tissues, ANCT, and normal tissues all showed CRM1 expression (Fig. 1a–h). CRM1 was located in the nucleus and/or cytoplasm, and the intensity of the immunohistochemical staining was variable: 30 cases of GC, 83 cases of ANCT, 6 cases of normal tissues exhibit negative staining intensity (grade 0); 27 cases of GC, 43 cases of ANCT, 4 cases of normal tissues exhibit weak staining intensity (grade 1+); 48 cases of GC and 6 cases of ANCT exhibit moderate staining intensity (grade 2+); and 30 cases of GC and 3 cases of ANCT exhibit strong staining intensity (grade 3+). There are no grade 2+/3+ expression in normal tissues (Fig. 1i). As shown in Table 1, compared with the low CRM1 expression in ANCT and normal tissues, the CRM1 expression in GC tissues showed obviously increased levels (both P < 0.0001).This significant difference suggested that CRM1 might be a prognostic factor in GC.
Representative immunohistochemical staining of CRM1 in gastric cancer (GC), adjacent noncancerous tissues (ANCT) and normal tissues. a–d show different staining intensities of CRM1 in GC: negative staining/grade 0 (a); weak staining/grade 1+ (b); moderate staining/grade 2+ (c); strong staining/grade 3+ (d) (magnification of a ×400; b–d ×200). e and f show CRM1 staining in ANCT: low expression (e) and high expression (F) (magnification ×200). g and h show CRM1 staining in normal tissues: negative (g) and positive (h) (magnification of g ×200; h ×100). i shows the distribution of GC patients with different staining intensity
Correlation between CRM1 expression and clinicopathologic factors
We also analyzed the correlation between high expression of CRM1 and clinicopathological factors that can affect the prognosis of GC patients, including serum CEA and CA19–9. Our data demonstrated that the increased expression of CRM1 was significantly associated with increased serum level of CEA (P = 0.02) but not associated with that of CA19–9 (P = 0.38). High expression of CRM1 was associated with TNM stage (P = 0.01), Her2 status (P = 0.01), and metastasis (P = 0.02), while expression of CRM1 was not correlated with gender (P = 0.59), age (P = 0.18), and differentiation status (P = 0.06) (Table 2).
Correlation between CRM1 expression and survival
In Kaplan–Meier survival analysis, high expression of CRM1 was significantly associated with poor prognosis. Remarkably, the 4-year survival rates in CRM1—high and low groups—were 46.9 and 67.5 %, respectively (P < 0.001), indicating that CRM1 was a crucial factor on clinical outcome in GC patients. In addition, univariate analyses were conducted to estimate the clinical significance of other prognostic factors that might influence the survival of the studied population. As shown in Table 3, serum CEA (P = 0.0076), TNM stage (P = 0.0001), metastasis (P = 0.027), and CRM1 expression (P = 0.0019) were statistically significant risk factors affecting overall survival of GC patients. Multivariate analyses showed that CRM1 expression [HR 2.07; 95 % confidence interval (CI) 1.15–4.31; P = 0.0048] was an independent risk factor predicting GC patients (Table 3). Besides, serum CEA (P = 0.019) and TNM stage (P = 0.039) are also independent risk factors based on statistical data analysis. To sum up, our findings indicate that CRM1 may be a useful predictor of the survival of GC patients (Fig. 2).
Discussion
Despite advances in diagnosis and treatment, the progress on the long-term prognosis of gastric cancer (GC) is limited. Therefore, finding a new prognostic marker and exploring new therapeutic opportunities become particularly important.
In the past decades, we have studied various clinical parameters related to prognosis, such as tumor size [13], lymph node status [14], and serum tumor markers [15], which might reflect the frequency of advanced disease at diagnosis and predict tumor recurrence and progression of GC. Due to the development in biotechnology, recent research emphasis has turned to identifying biomarkers that can predict survival or recurrence of GC. However, they all lack sensitivity and specificity to facilitate the early detection, so searching for other predictive biomarkers is warranted.
Several studies have demonstrated that the chromosomal region maintenance (CRM1)/exportin1/Xpo1 is important in nuclear–cytoplasmic transportation and has been determined to be involved in chromosomal structural control [16, 17]. Regarding the transporting function, CRM1 is the main nuclear export receptor in humans and has been reported to control several processes during cellular mitosis [18]. Mechanistic investigations have proved the importance of the CRM1 nuclear export pathway to many signaling molecules, including epidermal growth factor receptor, P53, protein kinase 1, among others [19–21]. Given the key roles of these exported molecules in the proliferation and survival of cancer cells, CRM1 may play important roles in carcinogenesis and tumor progression [19, 22]. Recently, CRM1 overexpression has been detected in several cancers, such as glioblastoma, osteosarcoma, pancreas cancer, ovarian, and cervical cancer and has been associated with worse outcome [23].
The present study was the first to investigate the expression of CRM1 in human GC with immunohistochemistry technology. CRM1 expression was increased significantly in malignant tumors compared with the expression in adjacent noncancerous tissues (ANCT) and normal tissues. This significant difference of CRM1 expression in GC and normal tissues suggested that CRM1 might be a prognostic factor in GC.
Next, this study was discussed to determine whether CRM1 may be used as a prognostic marker in GC. Our analyses showed the positive correlation between high expression of CRM1 and clinicopathological factors, including serum CEA and CA19–9. Our results also showed that CRM1 overexpression is associated with advanced TNM stage, Her2 status, and distant metastasis, which can be used as an assisted method for evaluating recurrence and prognosis of GC. In addition, our data demonstrated that the increased expression of CRM1 was significantly associated with increased serum level of CEA but not associated with that of CA19–9. The cellular mechanisms responsible for the poor prognosis of tumors with high expression of CRM1 are still unclear. Our statistical analysis suggests a link between high expression of CRM1 and increased serum level of CEA. Our data also support that there is an association between high CRM1 expression and advanced TNM stage. Since CEA expression is reported to correlate with tumor size and TNM stage [24], it is possible that CEA may be a target gene through which CRM1 executes its function.
In Kaplan–Meier survival analysis, high expression of CRM1 was significantly associated with a poor prognosis. The 4-year survival rates in the CRM1—high and low groups—were obviously lower than that in CRM1, indicating that CRM1 was a crucial factor on clinical outcome in GC patients. In addition, univariate analyses showed that serum CEA, TNM sage, metastasis, and CRM1 expression were significant risk factors affecting overall survival of GC patients. Multivariate analyses showed that besides serum CEA and TNM stage, CRM1 expression was also an independent risk factor predicting survival of GC patients. To sum up, our findings indicate that CRM1 may be a useful predictor to the survival of GC patients.
It cannot be denied that our present study has limitation. For example, all immunohistochemical-based studies have observer bias, because it cannot be eliminated when determining the grade and type of tumors during analysis. Furthermore, the interpretation concerning the prognostic role of CRM1 expression in GC is rather preliminary. Additionally, large-scale prospective and retrospective studies will be needed to investigate whether CRM1 expression is indeed of practical utility as a prognostic predictor.
References
Lee J, Lee SH, Hur KY, Woo SY, Kim SW, Kang WK. Statins and the risk of gastric cancer in diabetes patients. BMC Cancer. 2012;12:596.
Syrios J, Sougioultzis S, Xynos ID, Kavantzas N, Kosmas C, Agrogiannis G, Griniatsos J, Karavokyros I, Pikoulis E, Patsouris ES, et al. Survival in patients with stage IV noncardia gastric cancer—the influence of DNA ploidy and Helicobacter pylori infection. BMC Cancer. 2012;12:264.
Kwon KJ, Shim KN, Song EM, Choi JY, Kim SE, Jung HK, Jung SA. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. 2013 [Epub ahead of print].
Liu D, Zhang L, Shen Z, Tan F, Hu Y, Yu J, Li G. Clinicopathological significance of NMIIA Overexpression in Human Gastric Cancer. Int J Mol Sci. 2012;13:15291–304.
Wang JH, Mai C, Hong J, Zhang Q, Tang HS, Tang YQ, Cui SZ. Predicting value of serum CEA and CA19-9 in neoadjuvant chemotherapy for advanced gastric carcinoma. Zhonghua Wei Chang Wai Ke Za Zhi. 2012;15:1273–6.
Camara Mde L, Bouvier LA, Canepa GE, Miranda MR, Pereira CA. Molecular and functional characterization of a Trypanosoma cruzi nuclear adenylate kinase isoform. PLoS Negl Trop Dis. 2013;7:e2044.
Zhang KJ, Wang M. Potential effects of CRM1 inhibition in mantle cell lymphoma. Chin J Cancer Res. 2012;24:374–87.
Shen A, Wang Y, Zhao Y, Zou L, Sun L, Cheng C. Expression of CRM1 in human gliomas and its significance in p27 expression and clinical prognosis. Neurosurgery. 2009;65:153–9 (discussion 159–160).
Yao Y, Dong Y, Lin F, Zhao H, Shen Z, Chen P, Sun YJ, Tang LN, Zheng SE. The expression of CRM1 is associated with prognosis in human osteosarcoma. Oncol Rep. 2009;21:229–35.
Huang WY, Yue L, Qiu WS, Wang LW, Zhou XH, Sun YJ. Prognostic value of CRM1 in pancreas cancer. Clin Invest Med. 2009;32:E315.
Noske A, Weichert W, Niesporek S, Roske A, Buckendahl AC, Koch I, Sehouli J, Dietel M, Denkert C. Expression of the nuclear export protein chromosomal region maintenance/exportin 1/Xpo1 is a prognostic factor in human ovarian cancer. Cancer. 2008;112:1733–43.
van der Watt PJ, Maske CP, Hendricks DT, Parker MI, Denny L, Govender D, Birrer MJ, Leaner VD. The Karyopherin proteins, Crm1 and Karyopherin beta1, are overexpressed in cervical cancer and are critical for cancer cell survival and proliferation. Int J Cancer. 2009;124:1829–40.
Jiang CG, Xu Y, Wang ZN, Sun Z, Liu FN, Yu M, Xu HM. Clinicopathological analysis and prognostic significance of peritoneal cytology in Chinese patients with advanced gastric cancer. ANZ J Surg. 2011;81:608–13.
Nesi G, Basili G, Girardi LR, Manetti A, Biliotti G, Barchielli A. Pathological predictors of lymph node involvement in submucosal gastric carcinoma: a retrospective analysis of long-term outcome. In Vivo. 2009;23:337–41.
Jiexian J, Xiaoqin X, Lili D, Baoguo T, Ting S, Xianwen Z, Cunzhi H. Clinical assessment and prognostic evaluation of tumor markers in patients with gastric cancer. Int J Biol Mark. 2013;28:e192–200.
Zhang X, Hakata Y, Tanaka Y, Shida H. CRM1, an RNA transporter, is a major species-specific restriction factor of human T cell leukemia virus type 1 (HTLV-1) in rat cells. Microbes Infect. 2006;8:851–9.
Smulevitch S, Michalowski D, Zolotukhin AS, Schneider R, Bear J, Roth P, Pavlakis GN, Felber BK. Structural and functional analysis of the RNA transport element, a member of an extensive family present in the mouse genome. J Virol. 2005;79:2356–65.
Li J, Shiraki T, Igarashi K. Bach1 as a regulator of mitosis, beyond its transcriptional function. Commun Integr Biol. 2012;5:477–9.
He W, Wang X, Chen L, Guan X. A crosstalk imbalance between p27(Kip1) and its interacting molecules enhances breast carcinogenesis. Cancer Biother Radiopharm. 2012;27:399–402.
Fukumoto M, Sekimoto T, Yoneda Y. Proteomic analysis of importin alpha-interacting proteins in adult mouse brain. Cell Struct Funct. 2011;36:57–67.
Wang YC, Zhang DM, Shen AG, Lu JX, Shao XY, He S, Cheng C. Expression and relationship of p27(kip1) and its related molecules Jab1 and CRM1 during proliferation of lymphoma cells U937. Zhonghua Zhong Liu Za Zhi. 2007;29:657–61.
Budhu AS, Wang XW. Loading and unloading: orchestrating centrosome duplication and spindle assembly by Ran/Crm1. Cell Cycle. 2005;4:1510–4.
Siddiqui N, Borden KL. mRNA export and cancer. Wiley Interdiscip Rev RNA. 2012;3:13–25.
Dearling JL, Flynn AA, Qureshi U, Whiting S, Boxer GM, Green A, Begent RH, Pedley RB. Localization of radiolabeled anti-CEA antibody in subcutaneous and intrahepatic colorectal xenografts: influence of tumor size and location within host organ on antibody uptake. Nucl Med Biol. 2009;36:883–94.
Acknowledgments
This study is supported by Shandong Natural Science Foundation (2009HW024), Shandong Excellent Young Scientist Research Award Fund Project (2006BSB14114), and Shandong Tackle Key Problems in Science and Technology (2010GSF10245).
Conflict of interest
We have no conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Zhou and W. Qiu have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Zhou, F., Qiu, W., Yao, R. et al. CRM1 is a novel independent prognostic factor for the poor prognosis of gastric carcinomas. Med Oncol 30, 726 (2013). https://doi.org/10.1007/s12032-013-0726-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0726-1