Abstract
Abnormal tumor vasculature and subsequent tumor hypoxia contribute to immune tolerance of tumor cells by impeding the homing of cytotoxic T cells into tumor parenchyma and inhibiting their antitumor efficacy. These obstacles might explain why the promising approach of adoptive cell immunotherapy does not exert significant antitumor activity. Hypoxia contributes to immune suppression by activating hypoxia-inducible factor (HIF-1) and the vascular endothelial growth factor pathway, which plays a determining role in promoting tumor cell growth and survival. Tumor hypoxia creates an immunosuppressive microenvironment via the accumulation and subsequent polarization of inflammatory cells toward immune suppression phenotypes, such as myeloid-derived suppressor cells, tumor-associated macrophages, and dendritic cells. Antiangiogenic therapy could normalize tumor vasculature and decrease hypoxic tumor area and thus may be an effective modality to potentiate immunotherapy. Adoptive cell immunotherapy alone is not efficient enough to decrease tumor growth as its antitumor effect is inhibited by the immunosuppressive hypoxic tumor microenvironment. This review describes that combination of antiangiogenic therapy with adoptive cell immunotherapy can exert synergistic antitumor effect, which will contribute to improve strategies for future anticancer therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tumor microenvironment is a complex and highly dynamic environment, which is closely tied to tumor development and progression [1]. The cells in the tumor microenvironment consist of tumor cells and non-tumoral cells, which include fibroblasts, endothelial cells, supporting pericytes, bone marrow-derived cells, and immune and inflammatory cells [2]. Six hallmark capabilities, required for tumorigenesis, were defined in 2000. These hallmarks are, sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis [3]. In 2011, two additional criteria were put forward: deregulating cellular energetics and avoiding immune destruction [4]. There are two major obstacles for the antitumor activity of the immune system: first, reduced homing of immune cells to the tumor site and, second, hampering of the antitumor immune functions due to tumor microenvironment or immunomodulatory properties of suppressive cells. At present, a lot of research is focused on adoptive cell immunotherapies, including tumor-infiltrating lymphocytes (TIL), cytokine-induced killer (CIK) cells, lymphokine-activated killer (LAK) cells, and tumor-associated antigen (TAA)-specific cytotoxic T cell (CTL) among others [5]. Despite the renewed hope for cancer immunotherapy, survival benefits from adoptive cell immunotherapy alone remain modest. One of the critical challenges that adoptive cell immunotherapy faces is the immunosuppressive tumor microenvironment, as such, combining the adoptive cell immunotherapy with an agent that reprograms the tumor microenvironment is an attractive therapeutic strategy. It has been indicated that the tumor vasculature could be normalized, and the hypoxic tumor area could be reduced by a vascular normalizing dose of antiangiogenic therapy, which would reengineer the tumor microenvironment toward a more immunosupportive profile.
This review aims to give an overview of the current knowledge of the main tolerance and immunosuppression mechanisms elicited by the hypoxic tumor microenvironment, with the focus on combining antiangiogenic therapy with adoptive immunotherapy to facilitate the homing and activity of immune effectors cells to tumors.
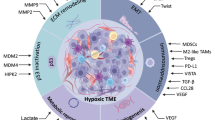
Tumor hypoxia and immune tolerance
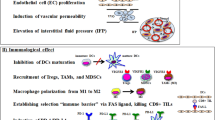
When a tumor attains a volume greater than 2 mm3, it requires neo-vascularization to provide an adequate supply of oxygen and nutrients for the rapidly proliferating tumor cells. However, the vasculature in tumor microenvironment is structurally and functionally abnormal. In certain regions of tumors, the vessels are leaky, twisted, and lack stabilizing pericytes and basement membrane. The structurally abnormal tumor vessels do not provide nutritive blood flow. In addition, proliferating cancer cells could generate solid pressure, which compresses vessels and reduces intratumoral blood and lymphatic flow [6]. The immature tumor vessels contribute to an abnormal tumor microenvironment with the characteristics of hypoxia, high intratumoral hydrostatic pressures, and acidosis. Tumor hypoxia plays a critical role in tumor cells’ resistance to radiotherapy, chemotherapy, and immunotherapy, and it is an adverse prognostic factor in patients with cancer. Hypoxia contributes to immune suppression by activating HIF-1α and vascular endothelial growth factor (VEGF) [7]. It has been reported that hypoxia-inducible factor (HIF-1α) overexpression is associated with poor treatment response and outcome [8]. Accumulating evidence demonstrates that hypoxia contributes to tumor immune tolerance by the recruitment of immunosuppressive cells [7].
Tumor hypoxic microenvironment impairs dendritic cell maturation
Dendritic cells (DCs), which are generated in the bone marrow and migrate as precursor cells to sites of potential pathogens, are essential for anti-tumor immunity. Immature DCs are able to capture antigens, including tumor antigens, but cannot stimulate T cell activation efficiently. Mature DCs are characterized by expression of high levels of major histocompatibility complex (MHC) molecules and costimulatory molecules (CD40, CD80, CD86, and CD83). Mature DCs are primed by pathogens, present antigen to T cells for activation and initiate adaptive immune responses [9, 10]. Yang et al. [11] showed that when human monocyte-derived DCs were cultured in hypoxic (1 % O2) conditions for 5 days, the maturation process was inhibited compared with those cultured in normoxic (21 % O2) conditions. Hypoxia not only reduced the expression of CD40 and MHC molecules on DCs, but also inhibited DCs producing Th1-type cytokines (i.e., IFN-γ, TNF-γ, and IL-12). Hypoxia-skewed DCs to a T help2 (Th2)-stimulating phenotype which could, in turn, promote the development of naive T cells into Th2 cells. CD4+ T cells, also known as mature T helper cells, cannot exert a direct antitumor effect due to their lack of cytotoxic and phagocytic properties. However, T helper cells play an important role in the initiation and activation of the antitumor immune response. T helper cells are divided into Th1 and Th2 types according to their characteristics and functions. Th1 cells are protective effector cells which could improve the antitumor effect of tumor-specific CTL, and they are characterized by expressing high levels of IFN-γ. However, hypoxia-skewed Th2 cells could potentially compromise antitumor immunity by producing IL-4, IL-5, and IL-13. Ryzhov et al. [12] found that activation of adenosine receptors at hypoxic sites skews the differentiation of DCs to a tumor-promoting phenotype. DCs differentiated under the influence of adenosine express high levels of proinflammatory-, angiogenic-, and immune-suppressive effector molecules, and they have been shown to improve tumor growth when they are injected into Lewis lung carcinoma xenografts. To further demonstrate the mechanisms by which hypoxia modulates DCs differentiation, Yang et al. [13] further evaluated the ability of adenosine- or adenosine-receptor-specific antagonist-treated normoxic or hypoxic DCs to stimulate the primary allogeneic T cell response. They found that adenosine-treated normoxic DCs polarize naive T cells toward a Th2 phenotype, while adenosine-receptor-specific antagonist-treated hypoxic DCs produced higher amount of the Th1 cytokine IFN-γ and lower levels of IL-4. These results implied that hypoxia can skew DCs to a T help2 (Th2)-stimulating phenotype by activating the adenosine A2b receptor.
Hypoxia inhibits the proliferation and function of T cells and impedes accumulation of tumor-infiltrating T lymphocytes
Activation of tumor antigen-specific T cells is an important event in antitumor immunity. High level of intratumoral infiltration of lymphocytes, especially T lymphocytes, has been reported to be associated with good prognosis in many cancers such as melanoma, non-Hodgkin’s lymphoma, head and neck cancer, non-small cell lung cancer, breast cancer, ovarian cancer, esophageal cancer, and urothelial carcinoma [14]. In addition, it has been reported that the density of tumor-infiltrating lymphocytes (TILs) was related to the clinical outcome of patients with colorectal cancer (CRC). Patients with high intratumoral density of CD3+, CD8+ lymphocytes, and CD45RO+ memory T cells have a lower incidence of tumor metastasis and a better prognosis [15, 16] CD8+ cytotoxic T cells (CTLs) are important effectors in the immune response against virally infected cells and tumor cells, and increased CTL infiltration is correlated with a better follicular lymphoma (FL) prognosis [17].
T cells experience different levels of oxygen tension, including hypoxia, during their development and their migration from blood into tissues. A hypoxic tumor microenvironment has inhibitory effects on T cell behavior. Hypoxia has been reported to impair T cell growth and survival by decreasing the expression of IL-2 in T cells [18]. Kim et al. [19] engineered CTLs with hypoxia-inducible human IL-2 genes (HRE-IL-2) and found that the proliferation and survival of CTLs was sustained and that CTL cytotoxicity was improved by the increased IL-2 production under hypoxic conditions. Hypoxia could potentially impair the antitumor activity of T cells by increasing the production of extracellular adenosine in the hypoxic tumors. Adenosine activates A2 adenosine receptors (A2AR) expressed on T cells and triggers elevation of intracellular cAMP which induces protein kinase A-mediated phosphorylation and activation of COOH-terminal Src kinase (Csk) and ultimately diminishes TCR signaling and IFN-γ production. The genetic deletion of A2AR in the host resulted in rejection of established immunogenic tumors in 60 % of A2AR-deficient mice, while no rejection was observed in control WT mice. The use of an antagonist or the pretreatment of T cells with siRNA could inhibit tumor growth and destroys metastases [20].
Cytokine-induced killer cells (CIK cells) are a heterogeneous subset of ex vivo expanded T lymphocytes which are characterized by a MHC-unrestricted tumor-killing activity and a mixed T-NK phenotype. In our previous study, we cultured CIKs in vitro in normoxic or hypoxic conditions to test the effects of hypoxia on CIKs. We found that both the proliferation and cytotoxicity of CIK cells were inhibited by low oxygen levels. Our findings also showed that hypoxia reduced the migration of CIK cells. Our research revealed that suppression of hypoxia could be a mechanism of modulation of tumor-induced immunosuppression [21].
Tumors are able to reduce the activity of lymphocytes and to limit the migration of lymphocytes from tumor vessels into the tumor parenchyma because they possess an immunosuppressive microenvironment and morphologically abnormal and functionally defective tumor vasculature [22, 23]. It has been found that tumors could impede leukocyte–vessel wall interactions by down-regulation of vascular adhesion molecules, such as intercellular cell adhesion molecule-1/2 (ICAM-1/2), vascular endothelial cell adhesion molecule-1 (VCAM-1), and CD34. The expression of adhesion molecules was decreased when endothelial cells were exposed to angiogenic factors such as vascular endothelial cell growth factors (VEGFs) and fibroblast growth factors (FGFs) [24]. However, little has been reported about the effect of hypoxia on the infiltration of cytotoxic T cells. Palazon has demonstrated that T cells tend to infiltrate into the vascularized tumor area in subregions that ought to be less hypoxic [25]. In our previous study, CIK cells were adoptively transfused to non-small cell lung cancer-bearing mice. Immunohistochemistry was conducted to test the spacial relationship between tumor hypoxia and intratumoral infiltrating CIK cells. We found that fewer CIK cells accumulated in hypoxic areas are compared with normoxic areas, indicating impaired CIK cell recruitment to hypoxic tumor tissue [21].
Hypoxia modulates the differentiation and function of myeloid-derived suppressor cells (MDSCs)
MDSCs are a heterogeneous family of immature myeloid cells that do not fully differentiate under conditions of chronic inflammation and are typically found in the tumor microenvironment. MDSCs have been demonstrated to directly promote angiogenesis and efficiently inhibit T cell-mediated antitumor reactivity [26]. The presence of increased immunosuppressive circulating and intratumoral MDSCs in patients with colorectal cancer is closely correlated with clinical cancer stage and metastasis [27]. The proportion of MDSCs was also found to be significantly increased in primary cancer tissues and peripheral blood of patients with breast cancer. STAT3-dependent IDO expression mediates the immunosuppressive effects of MDSCs in breast cancer [28]. Higher frequencies of T regulatory cells (Tregs) and myeloid-derived suppressor cells were found in hepatocellular carcinoma patients, and these high levels are associated with impaired T cell functionality. Combined regimens to deplete Tregs and MDSC in advanced HCC patients restored the function of CD8+ T cells and CD4+ T cells [29]. The immunosuppressive effects of MDSCs have also been found in other cancers such as neuroblastoma, multiple myeloma, chronic myeloid leukemia, and prostate cancer [30, 31].
Hypoxia could regulate the function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment via HIF-1α. HIF-1α up-regulates the expression of arginase and NO, two factors which are responsible for conversion of MDSC in the tumor microenvironment to antigen-non-specific suppressor of T cell function. In addition, hypoxia via HIF-1α helps MDSCs differentiate into immunosuppressive TAMs and further aggravates tumor immune tolerance [32].
Hypoxia polarizes tumor-associated macrophages (TAMs)
Tumor-associated macrophages represent a prominent component of the immune infiltrate in most malignant tumors [7]. Macrophages are mainly divided into two phenotypes: classically activated macrophages (M1) and alternatively activated macrophages (M2). M1-like TAMs exert antitumor activities and have high expression of pro-inflammatory factors, such as interleukin (IL)-12, nitric oxide synthase 2 (NOS2), and tumor necrosis factor (TNF)-α. On the other hand, M2-like TAMs have been reported to be able to facilitate tumor growth, angiogenesis, and metastasis by producing abundant anti-inflammatory factors [33, 34].
It has been indicated that high TAM infiltration into the tumor microenvironment is associated with a poor prognosis in patients with breast cancers [35]. Clinical data of patients with stages III–IV epithelial ovarian cancer was retrospectively reviewed, and the correlation between TAM density and patient survival was analyzed. The results showed that the infiltration of M2 macrophages, as well as activation of macrophages toward the M2 phenotype, may contribute to poor survival in advanced ovarian cancer [36]. In a meta-analysis of 55 studies (n = 8,629 patients), a high density of TAMs was associated with poor prognosis in patients with gastric cancer, breast cancer, bladder cancer, ovarian cancer, oral cancer, urogenital caner, head and neck cancer, and thyroid cancer. However, TAMs were associated with better overall survival in patients with colorectal cancer [37].
Hypoxia has been found to be involved in the accumulation of TAMs in the tumor microenvironment [38]. TAMs are found to be preferentially located in hypoxic areas of the tumor, and the underlying mechanisms have been studied. Hypoxia could up-regulate the expression of chemoattractants and generate a chemoattractant gradient, which the monocytes follow as they migrate into the hypoxic area. One cytokine implicated in monocyte recruitment is VEGF. More TAMs are present in poorly vascularized, VEGF-positive areas of breast carcinomas compared with VEGF-negative, well-vascularized areas, indicating that VEGF may play a role in guiding the migration of TAMs into hypoxic tumor sites [38]. Craig Murdoch et al. [39] have reported that endothelins 1 (ET-1) and endothelins 2 (ET-2) are associated with TAMs located in hypoxic tumor areas. ET-1 helps to recruit monocytes into tumors, and the subsequent localization of these monocytes within the tumor is controlled by ET-2.
Antiangiogenic therapy normalizes tumor vasculature
Tumor angiogenesis contributes to the abnormalities of the tumor microenvironment which can inhibit therapeutic outcomes. Normalizing tumor vessels and thus reversing the abnormal tumor microenvironment is therefore an intuitively attractive proposition.
Systemic antiangiogenic therapy has been developed to inhibit tumor vessel formation, induce vascular regression, and starve tumor cells to death. This rationale was supported by preclinical trials which demonstrated that mouse tumor growth was delayed after treatment with anti-VEGF antibodies. Despite the promising preclinical results, the effect of such agents in clinical trials has been disappointing, with only modest response rates and a lack of prolonged survival in solid tumor patients [40]. Clinical data suggest that anti-VEGF monotherapy is unable to induce significant tumor shrinkage. However, the combination of anti-VEGF therapy with chemotherapy has demonstrated significant antitumor effect with prolonged overall survival in cancer patients compared with chemotherapy alone [41].
Jain et al. [42] proposed the hypothesis of vascular normalization in 2001. Judicious antiangiogenic therapy can revert the abnormal structure of tumor vessels toward a more normal state, improve tumor vessel perfusion, reduce tumor hypoxia, and in turn normalize tumor microenvironment [40, 43]. Normalized tumor vessels are more orderly arranged and allow for increased homogeneity in blood flow in different regions within a tumor with a subsequent reduction in hypoxic tumor area. In addition, normalized tumor vessels have increased pericyte coverage and reduced vascular permeability, which in turn results in a drop in intratumoral IFP [40, 44]. By targeting pro-angiogenic factors, such as VEGF, angiopoietins (Ang), platelet-derived growth factor (PDGF) and placenta growth factor (PlGF), and integrins, along with antiangiogenic antibodies and small molecule inhibitors, vascular normalization may be achieved [43].
Huang et al. [45] reported that lower doses of anti-VEGFR2 antibody therapy induced breast tumor vascular normalization in two murine cancer models. The anti-VEGFR2 antibody induced a more homogeneous distribution of perfused tumor vessels, improved pericytes coverage, and reduced hypoxic tumor area. A lot of preclinical research has provided evidence that anti-angiogenic therapy could induce improvement in tumor oxygenation [40]. In our previous study, we found that recombinant human endostatin (rh-endostatin), an endogenous angiogenesis inhibitor, could normalize tumor vasculature in a murine lung cancer model. Rh-endostatin significantly decreased tumor microvascular density, increased pericytes coverage and collagen coverage and subsequently reduced hypoxic fraction. The antitumor efficacy of paclitaxel was significantly improved 7 days after the treatment of rh-endostatin [46–48]. We also found that rh-endostatin could not only normalize the abnormal structure of tumor vessels, but it could also normalize their functional abnormalities by decreasing tumor vascular hyperpermeability [21].
Antiangiogenic therapy improves the antitumor activity of adoptive cell immunotherapy
Effective adoptive cell transfer is facing numerous challenges, such as systemic immune tolerance and tumor local immune escape. The homing of immune cells to the tumor site is reduced, and the antitumor immune functions of these immune cells are inhibited by tumor microenvironment and immunosuppressive cell populations [49]. A successful adoptive cell immunotherapy requires both the access of adoptive T cells to malignant cells and an immunosupportive environment to maintain T cell function and proliferation. Thus, it is critical to find an effective therapy to enhance the adoptive cell transfer efficacy if we are to improve the clinical effect in cancer patients. Normalizing tumor vasculature could achieve these goals by improving the accumulation of T cells to tumor tissue and by alleviating the hypoxic and immunosuppressive tumor microenvironment [40, 43, 50, 51]. Recently, a lot of studies have been focused on combining a “vascular normalizing” dose of antiangiogenic therapy with adoptive cell immunotherapy in the treatment of cancers.
Rajeev et al. [52] have reported that the administration of an antibody against mouse VEGF synergized with adoptive cell transfer in the treatment of B16 melanoma and improved survival. In addition, synergistic antitumor efficacy has been shown when DC101, an antibody against VEGFR-2, was combined with adoptive cell transfer in this model. Antiangiogenic therapy has been found to enhance the infiltration of T cells into B16 tumors. In another study, the combination of anti-VEGFR2 antibody with T cell activation, induced by a whole cancer cell vaccine, enhanced the anticancer efficacy in murine breast cancer models. One possible mechanism for this effect is that the anti-VEGFR2 antibody normalizes the tumor vasculature, which improves overall tissue perfusion and reprograms the tumor microenvironment away from immunosuppression by polarizing tumor-associated macrophages from an immune inhibitory M2-like phenotype toward an immune-stimulatory M1-like phenotype and, ultimately, enhancing CD4+ and CD8+ T cell tumor infiltration [45].
G-protein signaling 5 (Rgs5) is a master gene responsible for the abnormal tumor vasculature in mice. In Rgs5-deficient mice, the loss of Rgs5 results in pericyte maturation, vascular normalization, and consequently marked reductions in tumor hypoxia and vessel leakiness. In addition, the infiltration of CD8+ T cells into tumor parenchyma was increased in Rgs5-deficient mice after adoptive transfer [53]. Antiangiogenic therapy could significantly improve the antitumor efficacy of adoptive T cells transfer by overcoming endothelial cell anergy and enhancing leukocytes infiltration into tumor [54].
In our studies, we combine recombinant human endostatin (rh-endostatin) and CIK cells for the treatment of lung carcinoma murine models. The Rh-endostatin we use is a modified form of endostatin, which is an endogenous pro-angiogenic factor. CIK cells mediate potent MHC-unrestricted cytotoxicity against a variety of tumor cells, and they can recognize and kill tumor cells without prior exposure or priming [55]. Our results indicate that rh-endostatin synergizes with adoptive CIK cells to inhibit the growth of lung carcinoma. Rh-endostatin normalized tumor vasculature and reduced hypoxic area in the tumor microenvironment. We found that fewer CD3+ T cells accumulated in the hypoxic tumor area compared with the non-hypoxic area. In addition, rh-endostatin significantly increased the homing of CIK cells and decreased the accumulation of myeloid-derived suppressor cells (MDSCs) in the tumor parenchyma [21].
Conclusion
Abnormal tumor vasculature plays an important role in the formation of a hypoxic immunosuppressive tumor microenvironment. Hypoxia itself is a factor that could benefit the growth of tumor cells as well as promote tumor cells into a more aggressive phenotype. In addition, hypoxic tumor microenvironments facilitate the production of immunosuppressive factors and cells, such as TAMs and MDSCs. The infiltration of cytotoxic T cells into tumor parenchyma is inhibited by the tumor microenvironment which impedes the efficacy of adoptive cell immunotherapy. Further, the few transferred cytotoxic T cells that accumulate in tumor parenchyma cannot fully exert their function because of the immunosuppressive tumor microenvironment, which can inhibit the proliferation and cytotoxicity of transferred T cells. Normalization of tumor vasculature by antiangiogenic agents has been reported to improve the antitumor efficacy of adoptive cell immunotherapy by decreasing hypoxic tumor area and improving the homing of adoptive T cells into tumor parenchyma. In our study, we observed that the abnormal tumor vasculature was normalized, hypoxic tumor area was decreased, and the infiltration of adoptive CIK cells was increased after the administration of recombinant human endostatin. Encouraging results have been seen from experiments in animal tumor models that combine antiangiogenic agents with adoptive cell immunotherapy. However, there are still two main challenges that hinder the successful clinical use of combination therapies. The first challenge is that it is difficult to generalize the optimal dose of anti-angiogenic agent for vascular normalization, as it may vary by patient or by disease status. Thus, it is urgent to develop imaging technology that will help to monitor the vascular normalization in patients. The second challenge is to identify optimal combination schedules. The efficacy of cancer therapies that combine antiangiogenic and adoptive cell immunotherapy depends on the dose and delivery schedule of each drug.
Although these challenges will take time to overcome, the combination of vascular normalizing doses of anti-angiogenic agents with adoptive cell immunotherapy offers a hope for developing new approaches that could enhance treatment efficacy in several malignancies.
References
Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–47.
Hiscox S, Barrett-Lee P, Nicholson RI. Therapeutic targeting of tumor-stroma interactions. Expert Opin Ther Targets. 2011;15:609–21.
Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL. The immune hallmarks of cancer. Cancer Immunol Immunother. 2011;60:319–26.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Zheng YW, Li RM, Zhang XW, Ren XB. Current adoptive immunotherapy in non-small cell lung cancer and potential influence of therapy outcome. Cancer Invest. 2013;31:197–205.
Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427(6976):695.
Chouaib S, Messai Y, Couve S, Escudier B, Hasmim M, Noman MZ. Hypoxia promotes tumor growth in linking angiogenesis to immune escape. Front Immunol. 2012;3:21.
Benito J, Shi Y, Szymanska B, Carol H, Boehm I, Lu H, Konoplev S, Fang W. Pronounced hypoxia in models of murine and human leukemia: high efficacy of hypoxia-activated prodrug PR-104. PLoS ONE. 2011;6(8):e23108.
Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–93.
Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205.
Yang M, Ma C, Liu S, Sun J, Shao Q, Gao W, Zhang Y, Li Z, Xie Q, Dong Z, Qu X. Hypoxia skews dendritic cells to a T helper type 2-stimulating phenotype and promotes tumor cell migration by dendritic cell-derived osteopontin. Immunology. 2009;128:e237–49.
Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–31.
Yang M, Ma C, Liu S, Shao Q, Gao W, Song B, Sun J, Xie Q, Zhang Y, Feng A, Liu Y, Hu W, Qu X. HIF-dependent induction of adenosine receptor A2b skews human dendritic cells to a Th2-stimulating phenotype under hypoxia. Immunol Cell Biol. 2010;88:165–71.
Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–102.
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4.
Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66.
Laurent C, Müller S, Do C, Al-Saati T, Allart S, Larocca LM, Hohaus S, Duchez S, Quillet-Mary A, Laurent G, Brousset P, Valitutti S. Distribution, function, and prognostic value of cytotoxic T lymphocytes in follicular lymphoma: a 3-D tissue-imaging study. Blood. 2011;118:5371–9.
Zuckerberg AL, Goldberg LI, Lederman HM. Effects of hypoxia on interleukin-2 mRNA expression by T lymphocytes. Crit Care Med. 1994;22:197–203.
Kim H, Peng G, Hicks JM, Weiss HL, Van Meir EG, Brenner MK, Yotnda P. Engineering human tumor-specific cytotoxic T cells to function in a hypoxic environment. Mol Ther. 2008;16:599–606.
Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–7.
Shi S, Wang R, Chen Y, Song H, Chen L, Huang G. Combining antiangiogenic therapy with adoptive cell immunotherapy exerts better antitumor effects in non-small cell lung cancer models. PLoS ONE. 2013;. doi:10.1371/journal.pone.0065757.
Chinnasamy D, Yu Z, Theoret MR, Zhao Y, Shrimali RK, Morgan RA, Feldman SA, Restifo NP, Rosenberg SA. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Invest. 2010;120:3953–68.
Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74:72–84.
Dirkx AE, oude Egbrink MG, Castermans K, van der Schaft DW, Thijssen VL, Dings RP, Kwee L, Mayo KH, Wagstaff J, Bouma-ter Steege JC, Griffioen AW. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 2006;20:621–30.
Palazón A, Aragonés J, Morales-Kastresana A, de Landázuri MO, Melero I. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin Cancer Res. 2012;18:1207–13.
Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–79.
Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding J, Zhu J, Wei H, Zhao K. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS ONE. 2013;8:e57114.
Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, Shen C, Liu J, Ren X. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190:3783–97.
Suresh KG, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP + CTLA-4 + Foxp3 + T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T cell functionality. Cancer Res. 2013;73:2435–44.
Xu J, Escamilla J, Mok S, David J, Priceman SJ, West BL, Bollag G, McBride WH, Wu L. CSF1R signaling blockade improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–94.
Christiansson L, Söderlund S, Svensson E, Mustjoki S, Bengtsson M, Simonsson B, Olsson-Strömberg U, Loskog AS. Increased level of myeloid-derived suppressor cells, programmed death receptor ligand 1/programmed death receptor 1, and soluble CD25 in Sokal high risk chronic myeloid leukemia. PLoS ONE. 2013;. doi:10.1371/journal.pone.0055818.
Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–53.
Mukhtar RA, Nseyo O, Campbell MJ, Esserman LJ. Tumor-associated macrophages in breast cancer as potential biomarkers for new treatments and diagnostics. Expert Rev Mol Diagn. 2011;11:91–100.
Laoui D, Movahedi K, Van Overmeire E, Van den Bossche J, Schouppe E, Mommer C, Nikolaou A, Morias Y, De Baetselier P, Van Ginderachter JA. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol. 2011;55:861–7.
Tang X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett. 2013;332:3–10.
Lan C, Huang X, Lin S, Huang H, Cai Q, Wan T, Lu J, Liu J. Expression of M2-polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technol Cancer Res Treat. 2013;12:259–67.
Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS ONE. 2012;7:e50946.
Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–8.
Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–34.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62.
Geva R, Vecchione L, Tejpar S, Piessevaux H, Van Cutsem E, Prenen H. Bevacizumab plus chemotherapy as salvage treatment in chemorefractory patients with metastatic colorectal cancer. Onco Targets Ther. 2013;6:53–8.
Jain RK. Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J Natl Cancer Inst. 1989;81:570–6.
Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–121.
Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–9.
Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F, Leblanc P, Munn LL, Huang P, Duda DG, Fukumura D, Jain RK, Poznansky MC. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109:17561–6.
Huang G, Chen L. Recombinant human endostatin improves anti-tumor efficacy of paclitaxel by normalizing tumor vasculature in Lewis lung carcinoma. J Cancer Res Clin Oncol. 2010;136:1201–11.
Huang G, Chen L. Tumor vasculature and microenvironment normalization: a possible mechanism of antiangiogenesis therapy. Cancer Biother Radiopharm. 2008;23:661–7.
Huang G, Chen L. Discrepancies between antiangiogenic and antitumor effects of recombinant human endostatin. Cancer Biother Radiopharm. 2009;24:589–96.
Rosenberg, SA. Overcoming obstacles to the effective immunotherapy of human cancer. Proc Natl Acad Sci U S A. 2008;105:12643–4.
Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, Costa S, Vinckier S, Dresselaer T, Åkerud P, De Mol M, Salomäki H, Phillipson M, Wyns S, Larsson E, Buysschaert I, Botling J, Himmelreich U, Van Ginderachter JA, De Palma M, Dewerchin M, Claesson-Welsh L, Carmeliet P. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44.
Manning EA, Ullman JG, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, Hicklin DJ, Jaffee EM, Emens LA. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res. 2007;13:3951–9.
Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–80.
Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, Rabie T, Kaden S, Gröne HJ, Hämmerling GJ, Arnold B, Ganss R. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–4.
Dings RP, Vang KB, Castermans K, Popescu F, Zhang Y, OudeEgbrink MG, Mescher MF, Farrar MA, Griffioen AW, Mayo KH. Enhancement of T-cell-mediated antitumor response: angiostatic adjuvant to immunotherapy against cancer. Clin Cancer Res. 2011;17:3134–45.
Rettinger E, Kuçi S, Naumann I, Becker P, Kreyenberg H, Anzaghe M, Willasch A, Koehl U, Bug G, Ruthardt M, Klingebiel T, Fulda S, Bader P. The cytotoxic potential of interleukin-15-stimulated cytokine-induced killer cells against leukemia cells. Cytotherapy. 2012;14:91–103.
Acknowledgments
We are grateful for the review by Ross Smith.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, S., Chen, L. & Huang, G. Antiangiogenic therapy improves the antitumor effect of adoptive cell immunotherapy by normalizing tumor vasculature. Med Oncol 30, 698 (2013). https://doi.org/10.1007/s12032-013-0698-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0698-1