Abstract
The androgens and androgen receptor (AR) play key roles in the prostate cancer (PCa) development and progression via epithelium-stroma cross talk. Prostate cancer-associated fibroblasts (CAFs) are dominant components in PCa stroma and are essential in the malignant progression by supporting tumorigenesis and metastasis. However, the AR roles in CAFs are still obscure. We isolated and immortalized the CAFs from human PCa tissues and found the CAFs are AR positive. We then knocked down their AR with siRNA and co-cultured the resultant CAFs with PCa cell line PC3. The MTT, invasion, and colony formation assays were performed to study the PC3 biological behavior. The results showed that the PCa epithelial growth, invasion, and colony formation abilities decreased when knocking down the CAFs AR. By using the real-time quantitative polymerase chain reaction, we found the IGF1, FGF7, FGF10, SDF1, HGF, and TGFb2 expression levels decreased in the AR knocked down CAFs. These results suggested that the AR in CAFs promoted PCa epithelial growth and invasion via regulating a series of growth factors. Targeting the AR in CAFs might be a potential therapeutic option for PCa in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of prostate cancer (PCa) is high in the western world, and increasing in Asian countries [1, 2]. The androgens, through their receptor (androgen receptor, AR), play central roles in the prostate normal development and cancer progression via the epithelium-stroma cross talk [3].
Since 1941, androgen deprivation therapy (ADT) has become the first therapeutic option for advanced PCa, and could lead to tumor regression in most of the cases in the first 1–2 years [4, 5]. Previously, people mostly focused on the AR roles in PCa cells [6, 7]. However, the PCa tissue contains not only epithelial cancer cells but stromal cells. PCa-associated fibroblasts (CAFs) are key determinants in the malignant progression of PCa by supporting tumorigenesis and metastasis [8]. However, the AR roles in CAFs are still not clear.
In this paper, we isolated and immortalized the CAFs from the human PCa tissues, knocked down their AR and then co-cultured the CAFs with PCa cell line PC3 to study the CAFs AR roles in PCa progression. For mechanism studies, we screened some popular growth factors by using real-time quantitative polymerase chain reaction (Q-PCR).
Materials and methods
Cell culture and plasmid transfection
PCa specimen were obtained from a PCa patient (67-year old, primary PCa, Gleason score = 3 + 3) at the Urology Department of Yantai Yuhuangding Hospital. The sample collection and primary cell culture were approved by the Yantai Yuhuangding Hospital Ethics Committee. The specimen was cut into small pieces and suspended in RPMI-1640 medium containing collagenase (1 mg/ml) and DNase (1 mg/ml) in 37 °C for 5 h. The suspension was filtered and separated by percoll gradient technique [9]. The upper layer of the cells was washed and cultured in RPMI-1640 medium containing 20 % fetal bovine serum (FBS). At passage 3, the plasmid PBabe-SV40-T (neo) was transfected into the cells by electroporation (280 V, 960μF) for the cell immortalization. The immortalized CAFs are maintained in RPMI-1640 medium with the supplement of 10 % FBS.
To knock down the AR in CAFs, we performed the lentivirus infection strategy. The plasmids (pLVTHM-GFP-ARsi/sc:PAX2:pMD2G = 4:3:2) were co-transfected in the 293T cells by Lipofectamine™ 2000 (Invitrogen). After 48 h, we collected and filtered the culture medium to infect the CAFs. After sorted by flow cytometry, the CAF-ARsi and the control CAF-sc cells were purified.
Immunofluorescence assay (I.F.)
The CAF-ARsi/sc cells (104) were seeded in the 4-well chamber slides and cultured overnight. The chamber slides with attached cells were fixed and blocked routinely, then incubated with the following antibodies: anti-vimentin (Sigma, 1:200), anti-smooth muscle α-actin (SMA) (Sigma, 1:200), and IgG control, then incubated with fluorescence-labeled secondary antibodies, and mounted by medium containing 4′,6-diamidino-2-phenylindole (DAPI).
Western blot analysis
The CAFs, CAF-ARsi/sc, and LNCaP cells were cultured to 90 % confluence and then lysed in RIPA lysis buffer for Western blot analysis. The rabbit anti-AR (N20), rabbit anti-vimentin, mouse anti-SMA, and mouse anti-tubulin primary antibody were from Santa Cruz Biotechnology. The secondary antibodies were from Santa Cruz Biotechnology. The Substrate Kit was from BIO-RAD Inc.
Reporter gene assay
The cells seeded in 48-well plates were cultured in the medium RPMI 1640 with 10 % charcoal-deprived FBS (CDS-FBS) before transfection. MMTV plasmids (containing the androgen-response element, ARE) at 0.5 μg per well were transfected into cells using Lipofectamine™ 2000 (Invitrogen). Five nanogram of TK-PRL (Promega) was used in each well for internal control. Eight hours after transfection, the cells were treated with 10 nM DHT or ethanol (ETOH) for 16 h. Then the cells were lysed, and the luciferase activity was analyzed using Dual-Luciferase Reporter Assay System (Promega).
Real-time quantitative polymerase chain reaction (Q-PCR)
Total RNA was extracted and purified using Trizol (Invitrogen, Carlsbad, CA). Three microgram RNA was subjected to reverse transcription using Superscript III (Invitrogen) [10]. The Q-PCR was performed on iCycler iQ Multi-Color Real-Time PCR Detection System (Bio-Rad) as described previously [10]. All samples were run in triplicate. Primer sequences were as follows: AR: sense, 5′-TGTCCATCTTGTCGTCTTC-3′; antisense, 5′-CCTCTCCTTCCTCCTGTAG-3′; insulin-like growth factor 1 (IGF1): sense, 5′-CCTCCTCGCATCTCTTCTAC-3′; antisense, 5′-AATACATCTCCAGCCTCCTTAG-3′;epidermal growth factor (EGF): sense, 5′-TACCGAGACCTGAAGTGG-3′; antisense, 5′-TCTGAGTCCTGTAGTAGTGGG-3′; fibroblast growth factor 2 (FGF2): sense, 5′-GCCTTCTCTTTCAGCATTCAC-3′; antisense, 5′-CCAACTCGTAACAATCCATCAG-3′; fibroblast growth factor 7 (FGF7): sense, 5′-CCCTGAGCGACACACAAG -3′; antisense, 5′-CACAATTCCAACTGCCACTG-3′; fibroblast growth factor 10 (FGF10): sense, 5′-CCTCCTTCTCCTCTCCTTCC-3′; antisense, 5′-GGCAGTTCTCCTTCTTGGTC-3′; stromal cell-derived factor 1 (SDF1): sense, 5′-CTGTGCCCTTCAGATTGTT-3′; antisense, 5′-GGCGGAGTGTCTTTATGC-3′; hepatocyte growth factor (HGF): sense, 5′-AGGGGCACTGTCAATACCATT-3′; antisense, 5′-CGTGAGGATACTGAGAATCCCAA-3′; transforming growth factor β 1 (TGFβ1): sense, 5′-CTAATGGTGGAAACCCACAACG-3′; antisense, 5′-TATCGCCAGGAATTGTTGCTG-3′; transforming growth factor β 2 (TGFβ2): sense, 5′-CCATCCCGCCCACTTTCTAC-3′; antisense, 5′-AGCTCAATCCGTTGTTCAGGC-3′; transforming growth factor β 3 (TGFβ3): sense, 5′-CACCCAGGAAAACACCGAGTC-3′; antisense, 5′-GCGGAAAACCTTGGAGGTAAT-3′; β-actin: sense, 5′-CATGTACGTTGCTATCCAGGC-3′; antisense, 5′-CTCCTTAATGTCACGCACGAT-3′.
Methyl thiazolyl tetrazolium (MTT) assay
The CAF-ARsi and CAF-sc cells were seeded in 24-well plates (1 × 104 cells per well) and incubated in RPMI 1640 with 10 % FBS. The cell culture medium was changed every 2 days. The plates were stained with MTT (Sigma) at day 0, 2, 4, 6 for 3 h. The absorbency was read at a wave length of 575 nm.
Transwell co-culture and invasion assay
For the cell co-culture MTT assay, we used the 1-μm transwell system from BD Biosciences following the manufacturer’s suggested protocol. Briefly, 1 × 105 CAF-ARsi/sc cells in 0.5 ml RPMI medium (with 2 % FBS) were seeded into the lower chamber, and 2 × 104 PC3 cells in 0.75 ml same medium were put into the upper chamber. The lower chambers containing CAF-ARsi/sc cells were refreshed every 2 days. The upper chamber was stained with MTT.
For the invasion assay, 5 × 104 PC3 cells in serum-free RPMI 1640 medium were added to upper chamber with microporous (8 μm) membrane with Matrigel coating or without Matrigel coating, 1 × 105 CAF-ARsi/sc cells in 0.5 ml RPMI medium (with 2 % FBS) were seeded into the lower chamber (BD Biosciences) according to the manufacturer’s suggested protocol.
Soft agar assay
Put 0.5 ml 1 % base agar (DNA grade) into each well of the six-well plate. After cooling down, melt 1 % agar (DNA grade agarose) in microwave, cooling to 40 °C in a water bath, then make a suspension of agar, 3 × RPMI 1640 medium, and PC3 cells at a ratio of 1:1:1 to prepare a cell concentration of 104 cells/ml. Add 1 ml of the mixture on the top of the base agar. After 2 weeks culture in 37 °C incubator, the colonies were stained by 1 mg/ml INT (Sigma) solution.
Statistical analysis
The value of data was presented as the mean ± standard deviation. To compare data between groups, we used a two-sided Student’s T test. *P < 0.05, **P < 0.01.
Results
Establishment and characterization of the CAFs
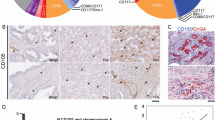
We isolated CAFs from human PCa tissues and immortalized the cells with SV40-T. The morphology of immortalized CAFs is elongated or stellated (Fig. 1a). We co-stained the vimentin (fibroblast marker) and smooth muscle α-actin (SMA, smooth muscle marker) using I.F. staining, and found most of the CAFs are double positive for vimentin and SMA (Fig. 1b). This indicates that the CAFs are myofibroblasts, consistent with the previous report [11]. To assure the AR expression in CAFs, we performed Western blotting assay. The CAFs could express AR, but at a lower level than LNCaP cells (Fig. 1c). As expected, the SV40-T expressed in CAFs, but not in LNCaP cells.
Establishment and characterization of CAFs: a The morphology of CAFs is elongated or stellated. The CAFs are maintained in RPMI-1640 medium with the supplement of 10 % FBS. b The I.F. staining of vimentin (green) and SMA (red) in the CAFs. The nuclei are stained by DAPI. Most of the cells are vimentin and SMA double positive. c The AR and SV40-T expression was determined by Western blot. The expression of tubulin was used as an internal control for protein loading. LNCaP cells were used as positive control (for AR) and negative control (for SV40-T)
Establishment of the AR knockdown CAFs cells
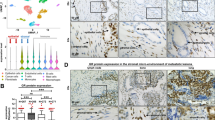
To study the AR roles in the CAFs, we knocked down the AR by lentivirus (containing AR siRNA) to obtain the AR knockdown cells (CAF-ARsi) and control cells (CAF-sc). The AR knockdown efficiency in CAF-ARsi was around 80 % (Fig. 2a). We further determined the AR expression level with Western blot (Fig. 2b). To confirm the AR functions in CAF-ARsi/sc, we performed androgen-response element (ARE) reporter gene assay (MMTV-LUC) [12]. After treating with 10 nM DHT and ETOH for control, the luciferase activity was analyzed by Dual-Luciferase Reporter Assay System. When treated with DHT, the luciferase activity was increased significantly in CAF-sc cells (P < 0.01), but not in CAF-ARsi cells (Fig. 2c). Furthermore, the CAF-ARsi grew slower than the CAF-sc cells in the MTT assay (Fig. 2d).
Establishment and characterization of the CAF-sc/ARsi cells: a Q-PCR for the AR expression level: the AR mRNA expression level is much lower (21.42 %) in the CAF-ARsi cells than the control cells, **P < 0.01 (n = 3). b Western blot for the AR expression level: the AR protein expression level is much lower in the CAF-ARsi cells than the control cells. The expression of tubulin was used as an internal control for protein loading. c MMTV-LUC was used to detect the AR trans-activity in CAF-ARsi/sc cells. The MMTV plasmid and TK-PRL plasmid were co-transfected into CAF-ARsi/sc cells. After treating with 10 nM DHT or ethanol for control, the luciferase activity was analyzed by Dual-Luciferase Reporter Assay System. When treated with DHT, the luciferase activity was increased significantly in CAF-sc cells, but not in CAF-ARsi cells, **P < 0.01 (n = 3). d MTT assay: CAF-ARsi/sc cells were cultured in 24-well plates for 0, 2, 4, and 6 days. Cellular growth was performed by MTT assay. At Day 4 and 6, the CAF-sc cells had higher O.D values than CAF-ARsi cells, **P < 0.01 (n = 3)
The AR in CAFs plays positive roles in PCa progression
To study whether the AR in CAFs could influence PCa progression, we performed the stromal–epithelial co-culture strategy (Fig. 3a). In MTT assay, the PCa cell line PC3 co-cultured with CAF-ARsi grew slower than the control cells (Fig. 3b). In the transwell invasion assay, the PC3 cells co-cultured with CAF-ARsi had fewer invaded cells than the cells co-cultured with CAF-sc (Fig. 3c). In the colony formation assay, the PC3 colonies in CAF-ARsi medium were fewer and smaller than those in CAF-sc medium (Fig. 3d).
Co-culture of CAF-sc/ARsi and PC3 in vitro: a The transwell co-culture model: CAF-ARsi/sc cells were seeded into the lower chamber, and PC3 cells were put into the upper chamber. b MTT assay in the co-culture system: PC3 cells co-cultured with CAF-sc cells grew slower than those co-cultured with CAF-ARsi cells at Day 4 (*P < 0.05, n = 3) and Day 6 (**P < 0.01, n = 3). c Invasion assay: The left panel shows the invaded PC3 cells co-cultured with CAF-ARsi (upper) and CAF-sc (lower). The right panel shows the ratio of invaded cells versus migrated cells: PC3 co-cultured with CAF-sc (39.23 ± 4.28 %) versus PC3 co-cultured with CAF-ARsi (23.34 ± 2.82), **P < 0.01 (n = 3). d Anchor-independent colony formation assay (soft agar assay): the PC3 cells seeding in soft agar were cultured in the CAF-ARsi/sc conditional medium. After 2 weeks culture, the colonies were stained by 1 mg/ml INT solution. The left panel shows the PC3 colonies growing in CAF-ARsi (upper) or CAF-sc (lower) conditional medium. The right panel shows quantified PC3 colonies: PC3 cultured in CAF-sc medium grew more colonies than those cultured in CAF-ARsi medium (28.54 ± 2.60 vs. 18.88 ± 1.69), **P < 0.01 (n = 3)
Decreased expression of growth factors when knocking down the AR
To investigate the mechanism of how the CAFs AR influenced the PCa epithelial cells, we screened the growth factors in CAF-ARsi/sc by using Q-PCR. The expression levels of IGF1, FGF7, FGF10, SDF1, HGF, and TGFb2 decreased significantly in the CAF-ARsi cells (Fig. 4). These data suggested that the AR might regulate a series of growth factors secreted by CAFs and then further regulate the biological behavior of PCa epithelial cells.
The expression levels of growth factors in CAFs when knocking down the AR Q-PCR were performed to detect the expression level of grow factors in CAF-ARsi/sc cells. The expression levels of IGF1, FGF7, FGF10, SDF1, HGF, and TGFb2 decreased significantly in the CAF-ARsi cells. **P < 0.01, *P < 0.05 (n = 3). The expression levels of EGF, FGF2, TGFb1, and TGFb3 did not decrease, or even increased slightly (FGF2, TGFb1) in the CAF-ARsi cells. *P < 0.05 (n = 3)
Discussion
AR plays central roles in the prostate normal development and cancer progression [3]. However, the epithelial AR itself could not support the prostate’s normal development. The stromal AR, but not epithelial AR, is critically involved in the prostate early development [13, 14].
For the PCa progression, the AR also plays key roles. Niu et al. [15] used transgenic strategy to knock out the AR in TRAMP mice, and found knocking out the AR both in epithelium and stroma cells resulted in small primary prostate tumors, but knockout of AR only in epithelium resulted in larger primary prostate tumors with higher proliferation rates, which suggested that the prostate stromal AR might play more dominant roles than the epithelial AR. However, the PCa cell type in TRAMP mice (neuroendocrine carcinoma) is different from most of the PCa patients (adenocarcinoma), which might not reflect the human PCa accurately [16].
In PCa tissue, the microenvironment of stroma has changed with significant alterations in growth factor, signal transduction pathways, etc. [17]. The most common marker of PCa stroma is the appearance of activated stromal cells with myofibroblastic characteristics, that is the CAFs [18]. The CAFs are more active than normal prostate fibroblasts and could transform a non-tumorigenic human prostatic epithelial cell line (BPH-1) to malignancy [19], and induce EMT and stemness of PCa cells [8, 20]. Because of its property in promoting the cancer progression and aggression, the CAFs have been regarded as a potential novel targets in anti-cancer therapy [21].
However, the AR roles in CAFs are still elusive. In this study, we isolated and immortalized the CAFs from human PCa tissue. The CAFs have elongated or stellated morphology and have myofibroblasts properties. We knocked down the AR in CAFs and co-cultured the CAFs-ARsi/sc cells with PCa epithelial cell line PC3, and found the AR in CAFs could promote the growth, invasion, and anchor-independent colony formation abilities. To probe the possible mechanism, we used Q-PCR to screen some growth factors, and found the AR could regulate the IGF1, FGF7, FGF10, SDF1, HGF, and TGFb2 expression levels.
Overall, by using primary culture and in vitro co-culture strategy, we found the CAFs AR play key roles in the PCa progression via regulating a series of growth factors. Our data suggested targeting the CAFs AR might be a potential therapeutic option for PCa in future.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29.
Ian LH, Li H, Yang Y, Ho CF. Comparisons of the incidence and pathological characteristics of prostate cancer between Chinese and Portuguese in Macau. Chin Med J (Engl). 2008;121:292–4.
Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308.
Miyamoto H, Messing EM, Chang C. Androgen deprivation therapy for prostate cancer: current status and future prospects. Prostate. 2004;61:332–53.
Gruca D, Bacher P, Tunn U. Safety and tolerability of intermittent androgen deprivation therapy: a literature review. Int J Urol. 2012;19:614–25.
Lin Y, Lu Z, Kokontis J, Xiang J. Androgen receptor primes prostate cancer cells to apoptosis through down-regulation of basal p21 expression. Biochem Biophys Res Commun. 2013;430:289–93.
Yu SQ, Lai KP, Xia SJ, Chang HC, Chang C, Yeh S. The diverse and contrasting effects of using human prostate cancer cell lines to study androgen receptor roles in prostate cancer. Asian J Androl. 2009;11:39–48.
Giannoni E, Bianchini F, Calorini L, Chiarugi P. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid Redox Signal. 2011;14:2361–71.
Ilio KY, Nemeth JA, Lang S, Lee C. The primary culture of rat prostate basal cells. J Androl. 1998;19:718–24.
Yu S, Zhang C, Lin CC, Niu Y, Lai KP, Chang HC, et al. Altered prostate epithelial development and IGF-1 signal in mice lacking the androgen receptor in stromal smooth muscle cells. Prostate. 2011;71:517–24.
Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–11.
Hartig PC, Bobseine KL, Britt BH, Cardon MC, Lambright CR, Wilson VS, et al. Development of two androgen receptor assays using adenoviral transduction of MMTV-luc reporter and/or hAR for endocrine screening. Toxicol Sci. 2002;66:82–90.
Cooke PS, Young P, Cunha GR. Androgen receptor expression in developing male reproductive organs. Endocrinology. 1991;128:2867–73.
Cunha GR. Mesenchymal-epithelial interactions: past, present, and future. Differentiation. 2008;76:578–86.
Niu Y, Altuwaijri S, Yeh S, Lai KP, Yu S, Chuang KH, et al. Targeting the stromal androgen receptor in primary prostate tumors at earlier stages. Proc Natl Acad Sci USA. 2008;105:12188–93.
Chiaverotti T, Couto SS, Donjacour A, Mao JH, Nagase H, Cardiff RD, et al. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am J Pathol. 2008;172:236–46.
Dakhova O, Ozen M, Creighton CJ, Li R, Ayala G, Rowley D, et al. Global gene expression analysis of reactive stroma in prostate cancer. Clin Cancer Res. 2009;15:3979–89.
Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166:2472–83.
Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61:8135–42.
Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70:6945–56.
Micke P, Ostman A. Tumour-stroma interaction: cancer-associated fibroblasts as novel targets in anti-cancer therapy? Lung Cancer. 2004;45(Suppl 2):S163–75.
Acknowledgments
This study was supported by Shandong Provincial Natural Science Foundation (ZR2011HQ047); Shandong Provincial Medicine and Health Technological Development Project (2011QZ029), China; NIH Grant: DK60912 and CA122840, USA.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Shengqiang Yu and Shujie Xia contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yu, S., Xia, S., Yang, D. et al. Androgen receptor in human prostate cancer-associated fibroblasts promotes prostate cancer epithelial cell growth and invasion. Med Oncol 30, 674 (2013). https://doi.org/10.1007/s12032-013-0674-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0674-9