Abstract
Previous studies provided inconclusive evidence for the effectiveness of gonadotropin-releasing hormone analogue on ovarian function protection against chemotherapy-induced genotoxicity in premenopausal patients. This study was designed to examine the efficacy of leuprolide acetate on ovarian function preservation in patients with breast cancer. A total of 220 patients were recruited in this prospective clinical trial and were assigned randomly to receive cyclophosphamide–doxorubicin-based chemotherapy only or chemotherapy plus leuprolide acetate. Resumption of menses or premenopausal levels of both follicle-stimulating hormone (FSH) and estradiol (E 2) within 12 months after the end of chemotherapy were considered as effective ovarian preservation. A total of 183 patients were considered evaluable (94 in chemotherapy-only group and 89 in chemotherapy plus leuprolide acetate group). At the end of follow-up, 27 patients in chemotherapy group and 15 in chemotherapy plus leuprolide acetate group resumed menses; seven patients in chemotherapy group and 14 in chemotherapy plus leuprolide acetate group restored premenopausal levels of FSH and E 2. The median time to resume menses was 9.2 months for patients in chemotherapy plus leuprolide acetate group and was not reached in chemotherapy-only group. In addition, our results demonstrated that age and chemotherapy doses made no significant difference in the occurrence of premature menopause. The leuprolide acetate treatment simultaneously with cyclophosphamide–doxorubicin-based chemotherapy reduced the risk of developing premature menopause in premenopausal patients with breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common invasive gynecological malignancy. More than 1,000,000 cases are diagnosed each year worldwide, and approximately 15 % are detected in reproductive ages [1, 2]. Particularly, young patients with breast cancer are associated with higher incidence of hormone-insensitive, undifferentiated and human epidermal growth factor receptor 2-overexpressing tumors [3]. Although adjuvant systemic chemotherapy treatment offers significant clinical benefits to these young patients in terms of survival, the majority of these patients eventually develop premature ovarian failure (POF) [4]. The incidence rate of POF highly correlates with patient ages and chemotherapy regimen [5]. Commonly used cyclophosphamide-based chemotherapy, including cyclophosphamide, methotrexate, 5-fluorouracil (CMF) and cyclophosphamide, epirubicin, 5-fluorouracil (CEF), causes early menopause in nearly 60 % of patients [6]. Cumulative dose of chemotherapy has been reported to be a risk factor for menopause; however, the incidence of iatrogenic menopause seems to be not dose-dependent in the patients undergoing CEF regimen [6–8]. In contrast, increasing age is associated with the occurrence of chemotherapy-induced menopause with 22–61 % of incidence rate in women under 40 years versus 61–79 % in those over 40 years [9]. POF can cause infertility, which is the major concern for young patients. Apart from loss of fertility, POF is also associated with vasomotor symptoms and sexual dysfunction [10]. Therefore, preventing chemotherapy-induced POF could improve the quality of life of patients.
No standard method for preventing chemotherapy-induced POF has been established yet [11]. Several approaches have been attempted to preserve fertility in young patients who must undergo chemotherapy. One of the most effective strategies has been retrieval and fertilization of oocytes before chemotherapy followed by cryopreservation of embryos [12]. Other experimental approaches include removal and cryopreservation of unfertilized eggs [13], or whole tissues [14] have also been used. However, all of these methods are complicated and costly. Preclinical studies showed that ovarian suppression by gonadotropin-releasing hormone (GnRH) analogue could protect ovarian against chemotherapy-induced toxicity [2, 15]. Observational studies also demonstrated that GnRH analogue protects the ovaries of patients with breast cancer undergoing chemotherapy [16–18]. However, due to the lack of randomized controlled trials, the role of GnRH analogue in ovarian protection is under debate [19, 20]. This study is designed to evaluate the protective effect of leuprolide acetate, a GnRH analogue, on the premenopausal breast cancer patients undergoing chemotherapy. This is the first trial on this subject in China, where incidence of breast cancer in the past two decades has increased by 80 % in young women [21].
Patients and methods
Eligibility criteria
Premenopausal patients between 18 and 45 years with proven histological stage I–III breast cancer were eligible for this study. Premenopausal status was defined as a history of normal ovarian function [basal follicle-stimulating hormone (FSH) < 10 mIU/ml] and regular menstrual cycle before study entry [22]. All patients received primary surgical therapy, including modified mastectomy or breast-conserving surgery plus ipsilateral node dissection or sentinel lymph node biopsy before adjuvant chemotherapy. Other eligibility criteria were as follows: no history of prior chemotherapy or hormone therapy; no distant metastases or localized cancer relapse; no prior or concomitant malignancy; no history of primary or secondary ovarian insufficiency; and no pregnancy or nursing. Written consent forms were obtained from all participating patients.
Study design
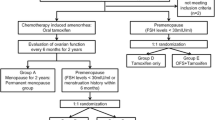
This study is a single-center, prospective randomized phase II study on leuprolide acetate in premenopausal patients with breast cancer. FSH and estradiol (E 2) levels were recorded before treatment and at 3, 6, 9 and 12 months after the end of chemotherapy. Menstrual activity was also recorded during the chemotherapy and 12-month follow-up after the end of chemotherapy. Early menopause was defined as FSH level > 40 mIU/ml and E 2 < 20 pg/ml in the absence of resumption of menstrual activity within 12 months after the end of chemotherapy. Effective treatment was defined as resumption of menstrual activity (regardless of FSH level or E 2 level) or occurrence of FSH level < 40mIU/ml and E 2 level > 20 pg/ml in the absence of resumption of menstrual activity during the 12-month follow-up after the end of chemotherapy.
This prospective randomized trial was approved by the Medical Ethics Committee of Jiangyin Hospital Affiliated to Nanjing University of Traditional Chinese Medicine.
Treatment regimen
Patients were randomly allocated to receive chemotherapy only or chemotherapy plus leuprolide acetate. The patients allocated to receive leuprolide acetate were given a subcutaneous injection of leuprolide acetate (3.75 mg, Leuplin, Takeda Pharmaceutical Company Limited, Osaka, Japan), and the serum E 2 level was measured 2 weeks after injection. If ovarian suppression was confirmed (E 2 level < 30 pg/ml), patients started to receive chemotherapy; otherwise, chemotherapy was not started until ovarian suppression was proved. During chemotherapy, patients were given leuprolide acetate at same dosage every 4 weeks. All patients received cyclophosphamide–doxorubicin-based chemotherapy as determined by oncologist before randomization. The number of patients receiving adjuvant chemotherapy including taxol was balanced between two groups. For all the patients with hormone-sensitive tumors, tamoxifen was administrated at the end of chemotherapy. If a patient resumed menstrual activity during follow-up, she restarted leuprolide acetate treatment and continued for 24 months to induce therapeutic ovarian suppression [16].
Toxicity evaluation
Clinical toxicity was evaluated every week during chemotherapy by measuring hematological and biochemical marks. Adverse events were graded based on NCI-CTC version 3.0.
Statistical methods
Assuming that 50 % of patients experienced early menopause following chemotherapy, at least 62 patients were required (31 in each group) to detect a 30 % absolute reduction in early menopause rate with leuprolide treatment [23], with a power of 80 % and a one-sided α error of 5 %. The sample size was determined using nQuery Adviser (Saugus, MA, USA).
All analyses were performed according to the intention-to-treat principle. Patients were considered as valuable if they completed at least one cycle of chemotherapy and had been followed up for 12 months after the end of chemotherapy. In the analyses for each time point, menses self-reported as regular or irregular were considered resumption of menstrual activity. The baseline characteristics, occurrence of menstrual activity resumption and early menopause were performed by using Fisher’s exact test. Mean values were compared using Student’s t test. A P value of <0.05 was considered significant. Time to resume menses was estimated by Kaplan–Meier survival analysis, according to the methods previously described by Brookmeyer and Crowley [24].
Results
Patients’ characteristics
Between 2010 February and 2012 March, 220 patients were recruited for this study of efficacy of leuprolide acetate on ovarian protection. Thirty-seven patients were considered unevaluable: 16 in chemotherapy-alone group (2 patients failed to complete at least one cycle of chemotherapy and 14 patients were lost to follow-up) and 21 in chemotherapy plus leuprolide acetate group (4 patients failed to complete at least one cycle of chemotherapy and 17 patients were lost to follow-up). The main patients’ characteristics at study entry were listed in Table 1. Chemotherapy plus leuprolide acetate group tended to be younger than those in chemotherapy-only group (mean age 40.3 vs. 42.1). However, the difference in age is not statistically significant (P > 0.05).
Treatment regimens
Two chemotherapy regimens were scheduled for patients according to the recommendations of oncologists: 1) 600 mg/m2 cyclophosphamide and 60 mg/m2 doxorubicin (CA) every 3 weeks for six cycles and 2) 600 mg/m2 cyclophosphamide, 60 mg/m2 doxorubicin and 175 mg/m2 paclitaxel (CA + Taxol) every 3 weeks for four cycles. A total of 12 patients did not complete scheduled chemotherapy due to adverse effects: five in chemotherapy-only group (1 stopped after 2 cycles and 4 patients stopped after 4 cycles) and seven in chemotherapy plus leuprolide acetate group (2 patients stopped after 2 cycles, 2 patients stopped after 4 cycles and 3 patients stopped after 5 cycles). The mean dose of cyclophosphamide and doxorubicin between two groups was not significantly different. Seven patients did not complete scheduled leuprolide acetate treatment due to chemotherapy interruption. Tamoxifen was given to all patients with hormonal receptor-positive tumor. Data about treatment adherence were summarized in Table 2.
Early menopause in patients
In our study, early menopause was defined as FSH level > 40mIU/ml and E 2 < 20 pg/ml in the absence of resumption of menstrual activity within 12 months after the end of chemotherapy. According to this definition, 27 patients (28.7 %) in chemotherapy-only group and 15 patients (16.9 %) in chemotherapy plus leuprolide acetate group had early menopause (Table 3). This result showed that leuprolide acetate treatment was associated with significant reduction in early menopause occurrence (P < 0.01). Next, we examined the effect of paclitaxel, dose of CA treatment, age and tamoxifen treatment on early menopause occurrence and found that only paclitaxel treatment significantly affected the risk of developing early menopause (0.01 < P < 0.05). Further analysis showed that patients with CA + Taxol treatment had a significantly lower occurrence of early menopause in chemotherapy plus leuprolide acetate group (0.01 < P < 0.05), whereas no significant difference in the occurrence of early menopause was observed between patients treated with CA and CA + Taxol regimens in chemotherapy group.
Menstrual activity resumption, FSH and E 2 levels
As shown in Table 4, resumption of menses was reported by 39 patients in chemotherapy-only group and 53 patients in chemotherapy plus leuprolide acetate group (0.01 < P < 0.05). Premenopausal level of FSH and E 2 without resumption of menses was observed in seven patients in chemotherapy-only group and 14 patients in chemotherapy plus leuprolide acetate group (P > 0.05). As per our definition of effective treatment, ovarian suppression with leuprolide acetate effectively preserved the ovarian function after chemotherapy (P < 0.01). Time to resumption of menses was shown in Fig. 1. The median time to resume menstruation was 9.2 months in the patients treated with chemotherapy plus leuprolide acetate, whereas the median time to resume menstruation was not reached in chemotherapy-only group. FSH and E2 levels were measured at 3, 6, 9 and 12 months after the end of chemotherapy (Fig. 2a, b). The mean values of E 2 level were significantly decreased in both groups relative to the values at study entry. However, there was no statically significant difference in the mean values of E 2 level between chemotherapy-only group and chemotherapy plus leuprolide acetate group at 12 months after the end of chemotherapy. In contrast, the mean values of FSH level were significantly elevated in both groups relative to the values at study entry, whereas the mean values of FSH level in patients of chemotherapy-only group were significantly higher than that of chemotherapy plus leuprolide acetate group at 12 months after the end of chemotherapy (P < 0.05).
Leuprolide-acetate-related toxicities
No treatment-related death occurred during this study. The majority of adverse effects were considered to be related to chemotherapy, including hematological adverse events (leucopenia, neutropenia and anemia), nausea, alopecia and fatigue. All patients reported leuprolide-acetate-related adverse effects, including hot flush, mood swings and urogenital symptoms, were Grade I and II cases.
Discussion
Long-term survival of breast cancer has been improved by adjuvant chemotherapy. However, the cytotoxic chemotherapeutics may cause profound and lasting consequence on gonadal function including ovarian function damage [6, 25, 26]. Apart from the essential role of ovarian function in preserving fertility, premature ovarian failure is associated with vasomotor symptoms, osteoporosis, urogenital symptoms and heart disease [27–29]. Therefore, a few approaches have been applied to preserve ovarian function or fertility for patients undergoing chemotherapy, including oophoropexy, ovarian suppression and cryopreservation of ovarian tissue, oocytes and embryos. However, only the efficacy of embryo cryopreservation has been proved, which is also not practical for patients without a partner due to the requirement of a current sperm donor [30].
Gonadotropin-releasing hormone analogue treatment has been proposed to be a less invasive and easier way to prevent the cytotoxic effects of chemotherapeutics on ovaries. A few mechanisms have been proposed on how GnRH analogues may protect ovaries. Blumenfeld et al. [31] suggested that GnRH analogues worked by suppressing gonadotropin levels to stimulate prepubertal hormonal milieu and subsequently preventing primordial follicles from maturation and therefore decreasing the number of follicles that are more vulnerable to chemotherapy. A later study of the same group suggested that the protective effect of GnRH analogues was associated with decrease in utero-ovarian perfusion, which leads to decreased exposure of the ovaries to the chemotherapeutic agents, upregulation of intragonadal anti-apoptotic molecules such as sphingosine-1-phosphate and protection of the undifferentiated germline stem cells [32]. A study by Imai et al. [33] showed that GnRH analogues protected ovarian function by directly activating GnRH receptors on ovaries. Although these studies formed the theoretical basis for the role of GnRH analogue treatment in ovarian protection, clinical studies have provided inconclusive evidences for its effectiveness [19, 20].
Recently, the results of several randomized clinical trials on the efficacy of ovarian function preservation with GnRH analogue in patients with breast cancer were published. The results of GBG37 ZORO study, which was conducted in 60 patients with hormonal-insensitive breast cancer, demonstrated that premenopausal patients with breast cancer receiving goserelin simultaneously with modern neoadjuvant chemotherapy did not experience statistically significantly less amenorrhea 6 months after the end of chemotherapy compared with those receiving chemotherapy alone [23]. Khan et al. [34] randomized 49 patients and found that triptorelin did not make significant difference in menses resumption between patients treated with chemotherapy alone and combined with triptorelin. In contrast, a phase III trial conducted in 78 patients with early-stage breast cancer showed an absolute reduction of 56 % in the occurrence of premature ovarian failure when goserelin was given simultaneously with chemotherapy compared to chemotherapy only [22]. Furthermore, the results of PROMISE-GIM6 study, which was so far the largest phase III trial, revealed that the use of triptorelin during chemotherapy in premenopausal patients with early-stage breast cancer reduced the occurrence of chemotherapy-induced early menopause [35]. In consistent with the results of PROMISE-GIM6 study, our present study suggested that leuprolide acetate, before and continuing every 4 weeks during cyclophosphamide–doxorubicin-based chemotherapy, resulted in decreased occurrence of premature menopause in patients with breast cancer. Early menopause was observed in 28.7 % of patients treated with chemotherapeutics only and 16.9 % of patients treated with chemotherapeutics simultaneously with leuprolide acetate.
The previous study suggested that younger patients are less susceptible to chemotherapy-induced premature ovarian failure [9]. However, our study showed that patient age did not significantly affect the risk of developing premature ovarian failure. We also examined whether higher cumulative dose of chemotherapy would increase the occurrence of premature ovarian failure. No significant difference was observed between patients receiving more than four cycles of chemotherapy and those receiving four cycles or less than four cycles, which agree with the observation from the previous study with patients receiving CEF regimen. Interestingly, we found that patients receiving CA + Taxol regimen were associated with lower occurrence of premature menopause relative to those receiving CA regimen (0.01 < P < 0.05). We further analyzed the effect of paclitaxel in each group and found that CA + taxol regimen was associated with lower risk only in chemotherapy plus leuprolide acetate group. Although the gonadal toxicity of paclitaxel has not been extensively investigated, several studies have demonstrated that paclitaxel might cause ovarian damage [36–39]. In addition, a couple of studies with rat model have demonstrated that the GnRH agonists could act as ovarian protection against paclitaxel-induced gonadotoxicity [36, 40]. Therefore, the less premature menopause in patients received chemotherapy plus leuprolide acetate group that received CA + Taxol regimen might be explained by the ovarian protective effect of leuprolide acetate. However, since CA + Taxol regimen compromised only four cycles of chemotherapy whereas CA regimen comprised six cycles of chemotherapy, the influence of low cumulative chemotherapy dose could not be ruled out. Further studies are needed to determine whether paclitaxel acts as an independent factor affecting the efficacy of leuprolide acetate.
During 12-month follow-up, 39 patients in chemotherapy-only group and 53 patients in chemotherapy plus leuprolide acetate group resume menses (0.01 < P < 0.05). In addition, a total of 21 patients have restored premenopausal level of FSH and E 2 (7 in chemotherapy-only group and 14 in chemotherapy plus leuprolide acetate group). However, since tamoxifen treatment given to patients with hormonal-sensitive breast cancer might affect the FSH and E 2 levels [16, 35, 41], FSH and E 2 levels are of less value to assess the decrease in ovarian reserve and predict future menstrual activity resumption.
In our study, leuprolide acetate did not cause any severe toxicity. In addition, previously randomized studies showed that concurrent ovarian suppression during chemotherapy had no significant effect on patients’ outcomes [42, 43]. However, the chance of negative interaction between leuprolide acetate and chemotherapy cannot be ruled out, especially in patients with hormonal-sensitive tumors, because the occult tumor cells in these patients might be stimulated by ovarian function resumption [23, 44]. In conclusion, this study provided clinical evidence that leuprolide acetate treatment before and during adjuvant chemotherapy reduced the risk of developing premature ovarian failure in premenopausal patients with breast cancer. Further clinical trials are warranted to assess the appropriate patient group that will benefit most from the ovarian suppression treatment.
References
Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53(1):5–26.
Bokser L, Szende B, Schally AV. Protective effects of D-Trp6-luteinising hormone-releasing hormone microcapsules against cyclophosphamide-induced gonadotoxicity in female rats. Br J Cancer. 1990;61(6):861–5.
Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26(20):3324–30. doi:10.1200/JCO.2007.14.2471.
Kreuser ED, Hetzel WD, Billia DO, Thiel E. Gonadal toxicity following cancer therapy in adults: significance, diagnosis, prevention and treatment. Cancer Treat Rev. 1990;17(2–3):169–75. doi:0305-7372(90)90043-F.
Petrek JA, Naughton MJ, Case LD, Paskett ED, Naftalis EZ, Singletary SE, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol. 2006;24(7):1045–51. doi:10.1200/JCO.2005.03.3969.
Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14(5):1718–29.
Del Mastro L, Venturini M, Sertoli M. Phase III adjuvant trial comparing standard versus accelerated FEC regimen in early breast cancer patients. Results from GONO-MIG1 study. Breast Cancer Res Treat. 2003;82(Suppl 1):S9 (Abstr).
Levine MN, Bramwell VH, Pritchard KI, Norris BD, Shepherd LE, Abu-Zahra H, et al. Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1998;16(8):2651–8.
Del Mastro L, Venturini M, Sertoli MR, Rosso R. Amenorrhea induced by adjuvant chemotherapy in early breast cancer patients: prognostic role and clinical implications. Breast Cancer Res Treat. 1997;43(2):183–90.
Schover LR. Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility. J Clin Oncol. 2008;26(5):753–8. doi:10.1200/JCO.2007.14.1655.
Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–31. doi:10.1200/JCO.2006.06.5888.
Lamar CA, DeCherney AH. Fertility preservation: state of the science and future research directions. Fertil Steril. 2009;91(2):316–9. doi:10.1016/j.fertnstert.2008.08.133.
Porcu E, Venturoli S, Damiano G, Ciotti PM, Notarangelo L, Paradisi R, et al. Healthy twins delivered after oocyte cryopreservation and bilateral ovariectomy for ovarian cancer. Reprod Biomed Online. 2008;17(2):265–7.
Meirow D. Fertility preservation in cancer patients using stored ovarian tissue: clinical aspects. Curr Opin Endocrinol Diabetes Obes. 2008;15(6):536–47. doi:10.1097/MED.0b013e32831a44a8.
Ataya K, Rao LV, Lawrence E, Kimmel R. Luteinizing hormone-releasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod. 1995;52(2):365–72.
Del Mastro L, Catzeddu T, Boni L, Bell C, Sertoli MR, Bighin C, et al. Prevention of chemotherapy-induced menopause by temporary ovarian suppression with goserelin in young, early breast cancer patients. Ann Oncol. 2006;17(1):74–8. doi:10.1093/annonc/mdj029.
Urruticoechea A, Arnedos M, Walsh G, Dowsett M, Smith IE. Ovarian protection with goserelin during adjuvant chemotherapy for pre-menopausal women with early breast cancer (EBC). Breast Cancer Res Treat. 2008;110(3):411–6. doi:10.1007/s10549-007-9745-y.
Recchia F, Saggio G, Amiconi G, Di Blasio A, Cesta A, Candeloro G, et al. Gonadotropin-releasing hormone analogues added to adjuvant chemotherapy protect ovarian function and improve clinical outcomes in young women with early breast carcinoma. Cancer. 2006;106(3):514–23. doi:10.1002/cncr.21646.
Oktay K, Sonmezer M, Oktem O, Fox K, Emons G, Bang H. Absence of conclusive evidence for the safety and efficacy of gonadotropin-releasing hormone analogue treatment in protecting against chemotherapy-induced gonadal injury. Oncologist. 2007;12(9):1055–66. doi:10.1634/theoncologist.12-9-1055.
Ben-Aharon I, Gafter-Gvili A, Leibovici L, Stemmer SM. Pharmacological interventions for fertility preservation during chemotherapy: a systematic review and meta-analysis. Breast Cancer Res Treat. 2010;122(3):803–11. doi:10.1007/s10549-010-0996-7.
Zhang J, Gao W, Wang P, Wu Z. Relationships among hope, coping style and social support for breast cancer patients. Chin Med J (Engl). 2010;123:2331–5.
Badawy A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: prospective randomized study. Fertil Steril. 2009;91(3):694–7. doi:10.1016/j.fertnstert.2007.12.044.
Gerber B, von Minckwitz G, Stehle H, Reimer T, Felberbaum R, Maass N, et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study. J Clin Oncol. 2011;29(17):2334–41. doi:10.1200/JCO.2010.32.5704.
Brookmeyer R, Crowley J. Confidence interval for the median survival time. Biometrics. 1982;38:29–41.
Chapman RM, Sutcliffe SB, Malpas JS. Cytotoxic-induced ovarian failure in women with Hodgkin’s disease I. Hormone function. JAMA. 1979;242(17):1877–81.
Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17(8):2365–70.
Blumenfeld Z, Avivi I, Linn S, Epelbaum R, Ben-Shahar M, Haim N. Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel to chemotherapy. Hum Reprod. 1996;11(8):1620–6.
Santoro A, Bonadonna G, Valagussa P, Zucali R, Viviani S, Villani F, et al. Long-term results of combined chemotherapy-radiotherapy approach in Hodgkin’s disease: superiority of ABVD plus radiotherapy versus MOPP plus radiotherapy. J Clin Oncol. 1987;5(1):27–37.
Bokemeyer C, Schmoll HJ, van Rhee J, Kuczyk M, Schuppert F, Poliwoda H. Long-term gonadal toxicity after therapy for Hodgkin’s and non-Hodgkin’s lymphoma. Ann Hematol. 1994;68(3):105–10.
Park HJ, Koo YA, Im YH, Yoon BK, Choi D. GnRH agonist therapy to protect ovarian function in young Korean breast cancer patients. J Korean Med Sci. 2010;25(1):110–6. doi:10.3346/jkms.2010.25.1.110.
Blumenfeld Z, Avivi I, Ritter M, Rowe JM. Preservation of fertility and ovarian function and minimizing chemotherapy-induced gonadotoxicity in young women. J Soc Gynecol Investig. 1999;6(5):229–39.
Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist co-treatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist. 2007;12(9):1044–54. doi:10.1634/theoncologist.12-9-1044.
Imai A, Sugiyama M, Furui T, Tamaya T, Ohno T. Direct protection by a gonadotropin-releasing hormone analog from doxorubicin-induced granulosa cell damage. Gynecol Obstet Invest. 2007;63(2):102–6. doi:10.1159/000096062.
Ismail-Khan R, Minton S, Cox C, Sims I, Laceivic M, Gross-king M, et al. Preservation of ovarian function in young women treated with neoadjuvant chemotherapy for breast cancer: a randomized trial using the GnRH agonist (triptorelin) during chemotherapy [abstract]. J Clin Oncol. 2008;26(155):524.
Del Mastro L, Boni L, Michelotti A, Gamucci T, Olmeo N, Gori S, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA. 2011;306(3):269–76. doi:10.1001/jama.2011.991.
Ozcelik B, Turkyilmaz C, Ozgun MT, Serin IS, Batukan C, Ozdamar S, et al. Prevention of paclitaxel and cisplatin induced ovarian damage in rats by a gonadotropin-releasing hormone agonist. Fertil Steril. 2010;93(5):1609–14. doi:10.1016/j.fertnstert.2009.02.054.
Gucer F, Balkanli-Kaplan P, Doganay L, Yuce MA, Demiralay E, Sayin NC, et al. Effect of paclitaxel on primordial follicular reserve in mice. Fertil Steril. 2001;76(3):628–9.
Yucebilgin MS, Terek MC, Ozsaran A, Akercan F, Zekioglu O, Isik E, et al. Effect of chemotherapy on primordial follicular reserve of rat: an animal model of premature ovarian failure and infertility. Aust N Z J Obstet Gynaecol. 2004;44(1):6–9. doi:10.1111/j.1479-828X.2004.00143.x.
Mailhes JB, Carabatsos MJ, Young D, London SN, Bell M, Albertini DF. Taxol-induced meiotic maturation delay, spindle defects, and aneuploidy in mouse oocytes and zygotes. Mutat Res. 1999;423(1–2):79–90.
Matsuo G, Ushijima K, Shinagawa A, Takahashi S, Fujiyoshi N, Takemoto S, et al. GnRH agonist acts as ovarian protection in chemotherapy induced gonadotoxicity: an experiment using a rat model. Kurume Med J. 2007;54(1–2):25–9.
Klijn JG, Beex LV, Mauriac L, van Zijl JA, Veyret C, Wildiers J, et al. Combined treatment with buserelin and tamoxifen in premenopausal metastatic breast cancer: a randomized study. J Natl Cancer Inst. 2000;92(11):903–11.
Arriagada R, Le MG, Spielmann M, Mauriac L, Bonneterre J, Namer M, et al. Randomized trial of adjuvant ovarian suppression in 926 premenopausal patients with early breast cancer treated with adjuvant chemotherapy. Ann Oncol. 2005;16(3):389–96. doi:10.1093/annonc/mdi085.
Rivkin SE, Green S, O’Sullivan J, Cruz AB, Abeloff MD, Jewell WR, et al. Adjuvant CMFVP versus adjuvant CMFVP plus ovariectomy for premenopausal, node-positive, and estrogen receptor-positive breast cancer patients: a Southwest Oncology Group study. J Clin Oncol. 1996;14(1):46–51.
Gerber B, Dieterich M, Muller H, Reimer T. Controversies in preservation of ovary function and fertility in patients with breast cancer. Breast Cancer Res Treat. 2008;108(1):1–7. doi:10.1007/s10549-007-9572-1.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guiping Song and Hui Gao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Song, G., Gao, H. & Yuan, Z. Effect of leuprolide acetate on ovarian function after cyclophosphamide–doxorubicin-based chemotherapy in premenopausal patients with breast cancer: results from a phase II randomized trial. Med Oncol 30, 667 (2013). https://doi.org/10.1007/s12032-013-0667-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0667-8