Abstract
Discoidin domain receptors (DDRs) are a novel class of receptor tyrosine kinases that respond to several collagens and facilitate cell adhesion. DDR1 is highly expressed in a variety of human cancers, and it is clear that DDR1 is primarily expressed in epithelial cells including lung, colon and brain. Moreover, DDR1 expression can be stimulated by collagen types I, II, III, IV, V, VIII and XI, and aberrant signaling induced by DDR1 dysregulated expression is involved in various steps of tumorigenesis. However, the molecular mechanism underlying the role of DDR1 in cancer development is not well documented. In this study, we found that the expression of DDR1 is upregulated in non-small cell lung cancer (NSCLC) tissues and cells when compared with counterpart normal tissues and cells. Furthermore, collagen I could induce DDR1 expression, and activated DDR1 promoted NSCLC cell migration and invasion, while knockdown of DDR1 by transfection with siRNA resulted in a significant decrease in cell migrativeness and invasiveness. Enhanced DDR1 expression mediated by collagen I could activate MMP-2, N-cadherin and vimentin expression, but reduce E-cadherin expression; however, inhibition of DDR1 expression could suppress MMP-2, N-cadherin and vimentin expression and induce E-cadherin activation. In conclusion, our findings indicated that upregulation of DDR1 induced by collagen I may contribute to the development and progression of NSCLC and this effect may be associated with increased invasiveness, at least in part, via promoting epithelial-to-mesenchymal transition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer death worldwide, and non-small cell lung cancer (NSCLC), which includes adenocarcinoma and squamous cell carcinoma, is the predominant form of lung cancer [1]. Because of the limited palliation provided by surgery, chemotherapy and radiation, the improvement in prognosis and survival of patients with lung cancer in the past 20 years is still unfavorable [2]. A continuing problem in the management of NSCLC is tumor cell metastasis; thus, gaining a detailed understanding of the molecular pathways and mechanisms that are activated in metastatic cells is essential for improving diagnosis, prevention and treatment of this disease.
Discoidin domain receptors (DDRs), a unique subfamily of non-integrin collagen receptors, are receptor tyrosine kinases containing a discoidin homology region in their N-terminal domain [3]. DDR and tyrosine kinases have been reported to be involved in cellular morphogenesis, differentiation, migration and invasion [4, 5]. There are two types of DDRs: DDR1 and DDR2. DDR1 is primarily stimulated by collagens I–IV and VIII, and DDR1 signaling is essential for cerebellar granule differentiation, mammary gland development and arterial wound repair [6–9]. Recently, many studies have reported altered expression of DDR1 in multiple human cancers, including lung, breast, ovary and prostate cancers, suggesting a definite role of DDR1 in tumor progression [10–13]. Moreover, the enhanced expression of DDR1 in a lot of fast-growing invasive tumors indicates that this matrix-activated receptor tyrosine kinase may act as a critical regulator of cell adhesion, migration, invasion and subsequent tumor metastasis [14]. However, DDR1 downstream signaling pathways or functions in different cell types and its transcriptional targets have not yet been described. Similarly, there is as yet relatively little information concerning the regulation of DDR1 expression, and so far, only a few inflammatory mediators including TNF-α, IL-1β and LPS have been shown to induce DDR1 expression, but the regulatory mechanisms governing the expression of DDR1 remain to be documented [15].
Given the important role of DDR1 in cancer development and progression, we in an effort to explore the expression in NSCLC tissues and cells identify downstream signaling targets of DDR1, in particular those that may contribute to the DDR1-induced NSCLC cell migration and invasion pathways. In this study, we found that DDR1 was significantly overexpressed in NSCLC tissues and cells when compared with their compact normal tissues and cells through qRT-PCR and Western blot. Furthermore, collagen I could stimulate DDR1 expression, and activated DDR1 promotes NSCLC cell migration and invasion, while knockdown of DDR1 by transfection with siRNA resulted in a significant decrease in cell migrativeness and invasiveness. Enhanced DDR1 expression mediated by collagen I could activate MMP-9, N-cadherin and vimentin expression, but reduced E-cadherin expression; however, inhibition of DDR1 expression could suppress MMP-9, N-cadherin and vimentin expression and induce E-cadherin activation. These data indicated that upregulation of DDR1 induced by collagen I may contribute to the development and progression of NSCLC and this effect may be associated with increased invasiveness, at least in part, via promoting epithelial-to-mesenchymal transition (EMT).
Materials and methods
Tissue samples
Non-small cell lung cancer and normal lung tissues were obtained from patients who underwent primary surgical resection of NSCLC with informed consent at Nanjing Drum Tower Hospital Affiliated to Medical School of Nanjing University between 2006 and 2007. No local or systemic treatment had been conducted in these patients before the operation. All these tissue samples were immediately snap-frozen in liquid nitrogen and stored at −80 °C until total RNA was extracted. The study was approved by the Research Ethics Committee of Nanjing University (Nanjing, Jiangsu, PR China). Informed consent was obtained from all patients.
Cell lines and culture conditions
Non-small cell lung cancer adenocarcinoma cell lines (A549, H322, H358, H460 and NCI-H1299), a NSCLC squamous carcinoma cell line (SK-MES-1) and a normal human bronchial epithelial cell line (BEAS-2B) were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). All cells were cultured in RPMI 1640 (GIBCO-BRL) medium supplemented with 10 % fetal bovine serum (10 % FBS), 100 U/ml penicillin and 100 mg/ml streptomycin (Invitrogen) in humidified air at 37 °C with 5 % CO2. Cells were grown on 250 ng/ml type I collagen (BD Biosciences, San Diego, CA, USA) for all relative experiments.
RNA extraction and qRT-PCR analyses
Total RNA was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. For analysis of DDR1, E-cadherin, N-cadherin, vimentin, MMP-2 and MMP-9 mRNA expression, 100 ng total RNA was reverse transcribed in a final volume of 10 μl using random primers under standard conditions using the PrimeScript RT reagent Kit and SYBR Premix Ex Taq (TaKaRa, Dalian, China) according to the manufacturer’s instructions. GAPDH gene was used as an internal control. The primers were designed as follows: DDR1, forward primer: 5′-GCGTCTGTCTGCGGGTAGAG-3′, reverse primer: 5′-ACGGCCTCAGATAAATACATTGTCT-3′; MMP-2, forward primer: 5′-CGTCTGTCCCAGGATGACATC-3′, reverse primer: 5′-TGTCAGGAGAGGCCCCATAG-3′; MMP-9, forward primer: 5′-TGGGCAGATTCCAAACCTTT-3′, reverse primer: 5′-TCTTCCGAGTAGTTTTGGATCCA-3′; E-cadherin, forward primer: 5′-TCCCATCAGCTGCCCAGAAA-3′, reverse primer: 5′-TGACTCCTGTGTTCCTGTTA-3′; N-cadherin, forward primer: 5′-CCCTGC TTCAGGCGTCTGTA-3′, reverse primer: 5′-TGCTTGCATAATGCGATTTCACC-3′; vimentin, forward: 5′-TCTACGAGGAGGAGATGCGG-3′, reverse: 5′-GGTCAAGACGTGCCAGAGAC-3′; GAPDH, forward primer: 5′-GGGAGCCAAAAGGGTCAT-3′, reverse primer: 5′-GAGTCCTTCCACG ATACCAA-3′. The relative levels of mRNA expression were calculated based on the difference between amplification of target genes and GAPDH mRNA using the 2−Δct method. All experiments were performed three times with three technical replicates.
Transfection of NCSCL cells
DDR1-siRNA named si-DDR1 and non-specific control siRNA (si-NC) were purchased from GeneChem, Shanghai, China. The si-DDR1 was transfected into cultured A549 cells, respectively. A549 cells were grown on six-well plate to confluence and transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Forty-eight hours after transfection, cells were harvested for qRT-PCR analyses or Western blot.
Wound-healing assay
For the wound-healing assay, 5 × 105 cells were seeded in six-well plates, cultured overnight and treated with type I collagen or respective controls. When the culture had reached nearly 90 % confluency, the cell layer was scratched with a sterile plastic tip and then washed with culture medium and cultured again for 48 h with serum-reduced medium containing 1 % FBS. At different time points, photographic images of the plates were acquired under a microscope.
Cell migration and invasion assays
For the migration assays, 24 h after transfection, 3 × 104 cells in serum-free media were placed into the upper chamber of an insert (8 μm pore size, millipore). For the invasion assays, 6 × 105 cells in serum-free media were placed into the upper chamber of an insert coated with Matrigel (BD, San Diego, CA, USA). Media containing 10 % FBS were added to the lower chamber. After 24 h of incubation, the cells remaining on the upper membrane were removed with cotton wool, whereas the cells that had migrated or invaded through the membrane were stained with methanol and 0.1 % crystal violet, imaged and counted using an IX71 inverted microscope (Olympus, Tokyo, Japan). Experiments were independently repeated three times.
Western blotting assay
Cells were lysed using mammalian protein extraction reagent RIPA (Beyotime) supplemented with protease inhibitors cocktail (Roche) and PMSF (Roche). Protein concentration was measured with the Bio-Rad protein assay kit; 40 μg of protein extractions was separated by 10 % sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), then transferred to 0.22-μm NC membranes (Sigma) and incubated with specific antibodies. Autoradiograms were quantified by densitometry (Quantity One software; Bio-Rad). GAPDH was used as control. GAPDH antibody was purchased from sigma; E-cadherin, N-cadherin, vimentin and vimentin antibody were purchased from BD (San Diego, CA, USA); MMP-2 and MMP-9 antibody were purchased from CST.
Statistical analysis
Student’s t test (two-tailed), one-way ANOVA and Mann–Whitney test were performed to analyze the data using SPSS 16.0 software. P values <0.05 were considered statistically significant.
Results
Expression of DDR1 is upregulated and correlated with lymph node metastasis of non-small cell lung cancer
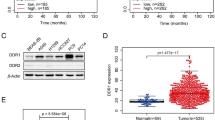
The expression of DDR1 was detected in 82 NSCLC samples and normal tissues by qRT-PCR and normalized to GAPDH. The level of DDR1 mRNA was significantly upregulated in cancerous tissues (median ratio of 2.8-fold, P < 0.01) compared with corresponding normal tissues (Fig. 1a). Furthermore, correlation analysis of DDR1 expression with clinical pathological features of NSCLC patients revealed a significant association between DDR1 upregulation and lymph node metastasis and NSCLC cell distance metastasis (Fig. 1b, c). However, DDR1 expression was not correlated with patient age, gender, etc. (Table 1).
DDR1 expression in NSCLC tissues and its clinical significance. a Determining DDR1 expression in NSCLC tissues. DDR1 was detected in 82 pairs of NSCLC tissues by qRT-PCR. Data are presented as fold changes in tumor tissues relative to normal tissues. b, c Data are presented as fold changes in tumor tissues relative to normal tissues. DDR1 expression was significantly higher in patients with lymph node metastasis and NSCLC cell distance metastasis. d Patients with high levels of DDR1 expression showed reduced survival times compared to patients with high levels of DDR1 expression. e Analysis of DDR1 protein level in tumor tissues relative to normal tissues by Western blot. All experiments were performed in biological triplicate with three technical replicates. **P < 0.01
Kaplan–Meier survival analysis was performed to further evaluate the correlation between DDR1 expression and NSCLC patient prognosis. According to the mean ratio of relative DDR1 expression (2.8) in tumor tissues, 82 NSCLC patients were classified into two groups: high-DDR1 group (n = 50, DDR1 expression ratio ≥ mean ratio) and low-DDR1 group (n = 32, DDR1 expression ratio ≤ mean ratio). The Kaplan–Meier survival curve showed that patients with increased DDR1 expression levels had shorter survival times than those with low DDR1 expression levels (Fig. 1d). Moreover, the results of Western blot assays revealed that DDR1 protein was also increased in NSCLC tissues and cells compared with counterpart normal tissues and cells (Fig. 1e). These results indicated that increased DDR1 expression may play an important role in NSCLC progression and development.
Modulation of DDR1 expression by collagen I stimulation and siRNA transfection
We next performed qRT-PCR and Western blot analysis to examine the expression of DDR1 in six human NSCLC cell lines, including both adenocarcinoma and squamous carcinoma subtypes. The results of qRT-PCR and Western blot analysis showed that DDR1 expression was also upregulated in NSCLC cells compared with the normal bronchial epithelial cell BEAS-2B (Fig. 2a, b).
Modulation of DDR1 expression by collagen I simulation and siRNA transfection. a Analysis of DDR1 expression in NSCLC cell lines (A549, H322, H358, H460, NCI-H1299 and SK-MES-1) compared with the normal bronchial epithelial cell line (BEAS-2B) by qRT-PCR and Western blot. b Analysis of DDR expression following treatment of collagen I in A549 cells by qRT-PCR and Western blot. c Analysis of DDR expression following treatment of siRNA transfection in A549 cells by qRT-PCR and Western blot. All experiments were performed in biological triplicate with three technical replicates. *P < 0.05, **P < 0.01
To determine whether collagen can induce the expression of DDR1 in NSCLC cells, we treated A549 cells with collagen I for 16 h. The results of qRT-PCR showed that DDR1 mRNA and protein level were significantly upregulated in A549 cells treated with collagen I when compared with control (Fig. 2c). To dissect the possible contribution of DDR1 in lung cancer development and progression, we knocked down DDR1 levels by transfection of three different siRNAs in A549 cells. The expression levels of DDR1 mRNA and protein in A549/si-DDR1 cells were significantly decreased compared with si-NC-transfected cells (Fig. 2d).
Effects of collagen I stimulation and DDR1 knockdown on cell migration and invasion
As collagen I has been shown to induce the expression of DDR1, we elucidated whether the induction of DDR1 by collagen I had a functional effect in cell migration and invasion. The results of wound-healing assays showed that A549 cells with collagen I stimulation presented a faster closing of scratch wound, compared with the control cells (Fig. 3a). Moreover, the cell migration and invasion assays showed that collagen I stimulation resulted in increased migration and invasion rate of A549 cells compared with the control (Fig. 3c).
Effect of collagen I simulation and DDR1 on cell migration and invasion in vitro. a, b Wound-healing assay was performed to investigate the migratory ability of A549 cells treated with collagen I or DDR1 siRNA transfection. c, d Transwell assays were performed to investigate the migratory and invasive ability of A549 cells treated with collagen I or DDR1 siRNA transfection. All experiments were performed in biological triplicate with three technical replicates. *P < 0.05 and **P < 0.01
To investigate whether DDR1 had a direct functional effect in facilitating NSCLC cell migration and invasion, we evaluated cancer cell invasion through Matrigel and migration through wound-healing and transwell assays. As shown in Fig. 3b, d, knockdown of DDR1 expression impeded the migration of A549 cells compared with control. Similarly, invasion of A549 cells was also reduced following inhibition of DDR1. These data indicated that DDR1 can promote the migratory and invasive phenotype of NSCLC cells.
Collagen I-induced EMT is partly via DDR1 signaling
Epithelial-to-mesenchymal transition, a fundamental biological process in embryonic development, has been found to be involved in tissue homeostasis, wound healing, tumor invasion and metastasis. Recent studies show that interactions with extracellular matrix molecules, such as collagen I, can induce EMT in some cell types. Therefore, we investigated the mechanism whereby interaction of A549 cells with collagen I might induce EMT via DDR1 signaling. The results of qRT-PCR showed that collagen I stimulation could induce N-cadherin, vimentin and MMP-9 mRNA expression and decrease E-cadherin mRNA expression, while inhibition of DDR1 could downregulate N-cadherin, vimentin and MMP-9 mRNA expression and increase E-cadherin mRNA expression reversely (Fig. 4a). Moreover, Western blot analysis also showed the same results (Fig. 4b). These data indicated that collagen I may influence NSCLC cell migration and invasion through promoting the epithelial-to-mesenchymal transition via partly inducing DDR1 expression.
Collagen I-induced EMT is partly via DDR1 signaling. a Analysis of E-cadherin, N-cadherin, vimentin, MMP-2 and MMP-9 mRNA expression in A549 treated with collagen I or DDR1 siRNA transfection cells by qRT-PCR. b Analysis of E-cadherin, N-cadherin, vimentin, MMP-2 and MMP-9 protein levels in A549 treated with collagen I or DDR1 siRNA transfection cells by Western blot. All experiments were performed in biological triplicate with three technical replicates. *P < 0.05, **P < 0.01 and NS not significant
Discussion
DDR1 is a receptor tyrosine kinase that is identified during the search for tyrosine kinase proteins expressed in human malignancies [16]. DDR1 kinase contains a homology domain to discoidin, which is distinct from other members of the large receptor tyrosine kinase and could be activated by various types of collagens and is found to be involved in cell attachment, migration and invasion [17]. Accumulating evidence indicates that DDR1 is overexpressed in invasive tumors including breast, prostate, lung and cancer cells overexpressing DDR1 display increased migration and invasion [7, 18]. For instance, upregulated DDR1 expression promotes cancer development by enhancing cancer cell survival and invasion, and high DDR1 expression is associated with short hormone resistance interval in prostate carcinoma [13].
In the present study, we found that DDR1 expression is upregulated in NSCLC tissues and cells compared to normal tissues and cells, which indicated that enhanced DDR1 expression may contribute to NSCLC development and progression. It has been reported that DDR1 can be primarily activated by collagens I–IV and VIII, and Ruiz et al. found that collagen I induces the expression of DDR1 in a dose- and time-dependent manner in primary human lung fibroblasts [19]. Moreover, our data also showed that collagen I stimulation could significantly induce DDR1 expression in NSCLC cells. Several reports support that collagen I can induce activation of tyrosine kinase FAK and recruitment to the E-cadherin/catenin complex; meanwhile, collagen I activates JNK, which upregulates N-cadherin expression, initiates EMT and promotes invasion and metastasis of pancreatic cancer cells [20, 21]. These data point out the highly significant influence of collagen I on the outcome of pancreatic cancer.
Here, we showed that collagen I stimulation could promote NSCLC cell migration and invasion, while inhibition of DDR1 expression resulted in a decreased number of migration and invasion of NSCLC cells when compared with control cells. In addition, we found that collagen I could induce expression of N-cadherin, vimentin and MMP-9 and inhibit E-cadherin expression; however, knockdown of DDR1 expression leads to decreased N-cadherin, vimentin and MMP-9 expression and increased E-cadherin expression. Loss of E-cadherin expression or function and increased expression of other cadherins such as E-cadherin and vimentin is necessary for the increased cell motility that accompanies EMT [22, 23].
Epithelial-to-mesenchymal transition is first recognized as a central differentiation process allowing the remodeling of tissues during early embryogenic and is implicated in the promotion of tumor invasion and metastasis [24, 25]. EMT can be initiated by external signals originating from outside the cell, such as transforming growth factor (TGF)-b, hepatocyte growth factor (HGF), epidermal growth factor (EGF) and fibroblast growth factor (FGF) [26, 27]. Furthermore, it has been proposed and supported by numerous publications that EMT process would be a potent mechanism that enhances the detachment of cancer cells from primary tumors. One characteristic of cells that underwent EMT is the loss of E-cadherin expression, and decreased E-cadherin expression has been reported to be associated with poor clinical outcome in NSCLC [28, 29]. Therefore, EMT-inducing pathways may be good candidates for intervention in the treatment for cancer, and it is important to understand the molecular mechanisms that drive EMT for the prevention of metastasis. In this study, we showed that collagen I activates DDR1 signaling is an effective pathway promoting EMT, while inhibiting the DDR1 pathway prevented EMT in NSCLC cells.
In conclusion, this study provides evidence that DDR1 is significantly upregulated in NSCLC tissues and cells. Collagen I triggers the expression of DDR1 and promotes NSCLC cells migration and invasion, and inhibition of DDR1 in NSCLC cells resulted in significantly increased cell motility and invasiveness. In addition, our results demonstrate that the collagen I activated DDR1 signaling, which may be an effective pathway promoting EMT and contribute to NSCLC cell invasion. These findings indicate that upregulation of DDR1 may be involved in the progression of NSCLC and associated with increased invasiveness. Although this study provides a possible molecular mechanism for DDR1 affecting NSCLC cell migration and invasion, further studies are needed to elucidate the details of the regulation of DDR1 expression.
References
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60(5):277–300. doi:10.3322/caac.20073.
Carney DN. Lung cancer–time to move on from chemotherapy. N Engl J Med. 2002;346(2):126–8. doi:10.1056/NEJM200201103460211.
Alves F, Vogel W, Mossie K, Millauer B, Hofler H, Ullrich A. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 1995;10(3):609–18.
Medici D, Nawshad A. Type I collagen promotes epithelial-mesenchymal transition through ILK-dependent activation of NF-kappaB and LEF-1. Matrix Biol. 2010;29(3):161–5. doi:10.1016/j.matbio.2009.12.003.
Ongusaha PP, Kim JI, Fang L, Wong TW, Yancopoulos GD, Aaronson SA, et al. p53 induction and activation of DDR1 kinase counteract p53-mediated apoptosis and influence p53 regulation through a positive feedback loop. EMBO J. 2003;22(6):1289–301. doi:10.1093/emboj/cdg129.
Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1(1):13–23.
Ram R, Lorente G, Nikolich K, Urfer R, Foehr E, Nagavarapu U. Discoidin domain receptor-1a (DDR1a) promotes glioma cell invasion and adhesion in association with matrix metalloproteinase-2. J Neurooncol. 2006;76(3):239–48. doi:10.1007/s11060-005-6874-1.
Hou G, Vogel W, Bendeck MP. The discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. J Clin Invest. 2001;107(6):727–35. doi:10.1172/JCI10720.
Vogel WF, Aszodi A, Alves F, Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21(8):2906–17. doi:10.1128/MCB.21.8.2906-2917.2001.
Johnson JD, Edman JC, Rutter WJ. A receptor tyrosine kinase found in breast carcinoma cells has an extracellular discoidin I-like domain. Proc Natl Acad Sci USA. 1993;90(22):10891.
Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, Scurry J, Scolyer RA, Davies MJ, et al. Overexpression of the cell adhesion molecules DDR1, Claudin 3, and Ep-CAM in metaplastic ovarian epithelium and ovarian cancer. Clin Cancer Res. 2004;10(13):4427–36. doi:10.1158/1078-0432.CCR-04-0073.
Yang SH, Baek HA, Lee HJ, Park HS, Jang KY, Kang MJ, et al. Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung carcinomas. Oncol Rep. 2010;24(2):311–9.
Shimada K, Nakamura M, Ishida E, Higuchi T, Yamamoto H, Tsujikawa K, et al. Prostate cancer antigen-1 contributes to cell survival and invasion though discoidin receptor 1 in human prostate cancer. Cancer Sci. 2008;99(1):39–45. doi:10.1111/j.1349-7006.2007.00655.x.
Ford CE, Lau SK, Zhu CQ, Andersson T, Tsao MS, Vogel WF. Expression and mutation analysis of the discoidin domain receptors 1 and 2 in non-small cell lung carcinoma. Br J Cancer. 2007;96(5):808–14. doi:10.1038/sj.bjc.6603614.
Kamohara H, Yamashiro S, Galligan C, Yoshimura T. Discoidin domain receptor 1 isoform-a (DDR1alpha) promotes migration of leukocytes in three-dimensional collagen lattices. FASEB J. 2001;15(14):2724–6. doi:10.1096/fj.01-0359fje.
Zerlin M, Julius MA, Goldfarb M. NEP: a novel receptor-like tyrosine kinase expressed in proliferating neuroepithelia. Oncogene. 1993;8(10):2731–9.
L’Hote CG, Thomas PH, Ganesan TS. Functional analysis of discoidin domain receptor 1: effect of adhesion on DDR1 phosphorylation. FASEB J. 2002;16(2):234–6. doi:10.1096/fj.01-0414fje.
Park HS, Kim KR, Lee HJ, Choi HN, Kim DK, Kim BT, et al. Overexpression of discoidin domain receptor 1 increases the migration and invasion of hepatocellular carcinoma cells in association with matrix metalloproteinase. Oncol Rep. 2007;18(6):1435–41.
Ruiz PA, Jarai G. Collagen I induces discoidin domain receptor (DDR) 1 expression through DDR2 and a JAK2-ERK1/2-mediated mechanism in primary human lung fibroblasts. J Biol Chem. 2011;286(15):12912–23. doi:10.1074/jbc.M110.143693.
Koenig A, Mueller C, Hasel C, Adler G, Menke A. Collagen type I induces disruption of E-cadherin-mediated cell–cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res. 2006;66(9):4662–71. doi:10.1158/0008-5472.CAN-05-2804.
Shintani Y, Hollingsworth MA, Wheelock MJ, Johnson KR. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006;66(24):11745–53. doi:10.1158/0008-5472.CAN-06-2322.
Maeda M, Johnson KR, Wheelock MJ. Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci. 2005;118(Pt 5):873–87. doi:10.1242/jcs.01634.
Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118(3):277–9. doi:10.1016/j.cell.2004.07.011.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. doi:10.1016/j.cell.2009.11.007.
Acloque H, Thiery JP, Nieto MA. The physiology and pathology of the EMT. Meeting on the epithelial-mesenchymal transition. EMBO Rep. 2008;9(4):322–6. doi:10.1038/embor.2008.30.
Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172(7):973–81. doi:10.1083/jcb.200601018.
Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24(37):5764–74. doi:10.1038/sj.onc.1208927.
Bremnes RM, Veve R, Gabrielson E, Hirsch FR, Baron A, Bemis L, et al. High-throughput tissue microarray analysis used to evaluate biology and prognostic significance of the E-cadherin pathway in non-small-cell lung cancer. J Clin Oncol. 2002;20(10):2417–28.
Liu D, Huang C, Kameyama K, Hayashi E, Yamauchi A, Kobayashi S et al. E-cadherin expression associated with differentiation and prognosis in patients with non-small cell lung cancer. Ann Thorac Surg. 2001;71(3):949–54; discussion 54–5.
Acknowledgments
This research was supported by the Natural Science Fund of Jiangsu Province (BK2011658) to Song Yong.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miao, L., Zhu, S., Wang, Y. et al. Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung cancer and promotes cell invasion via epithelial-to-mesenchymal transition. Med Oncol 30, 626 (2013). https://doi.org/10.1007/s12032-013-0626-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0626-4