Abstract
Adrenomedullin (ADM) is a potent, long-lasting angiogenic peptide that was originally isolated from human pheochromocytoma. ADM signaling is of particular significance in endothelial cell biology because the peptide protects cells from apoptosis, and ADM has been shown to be pro-tumorigenic in that it stimulates tumor cell growth and angiogenesis. ADM may be involved in micro-vessel proliferation and partially in the release of hypoxia in solid tumors, contributing to the proliferation of tumor cells as well as local tumor invasion and metastasis. However, the effect of hypoxia-induced ADM expression in bladder cancer remains unclear. Here, we found that the levels of ADM protein in tumor tissue from patients with bladder urothelial cell carcinoma were significantly increased compared to the adjacent non-tumor bladder tissues (p < 0.01). Under hypoxic conditions, the expression of ADM was significantly elevated in a time-dependent manner in human bladder cancer cell lines. Furthermore, the knockdown of ADM by shRNA in T24 cells showed obvious apoptosis compared to untransfected controls (p < 0.0001). In addition, the combination of cisplatin and ADM-shRNA significantly reduces the tumor growth in vivo compared to treatment with cisplatin (p = 0.0046) or ADM-shRNA alone (p < 0.0001). These data suggest that ADM plays an important role in promoting bladder cancer cell growth under hypoxia and that the inhibition of ADM may provide a target for bladder cancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer is a common cancer affecting men worldwide and is associated with high levels of morbidity and mortality. In the United States, it is estimated that there are 73,510 new cases of bladder cancer, and there were 14,880 deaths in 2012 [1]. The most frequent histologic type is urothelial cell carcinoma (UCC), accounting for approximately 90 % of all bladder cancers [2]. Without treatment, locally advanced or metastatic bladder cancers are fatal. Before the development of effective chemotherapy, median survival rarely exceeded 3–6 months. Most combination chemotherapy used to treat bladder cancer includes cisplatin. Unfortunately, disease recurrence is observed in 30–56 % of patients despite improvements in surgery and chemotherapy [3]. The prognosis for patients with bladder cancer recurrence following cystectomy is poor. Thus, it is necessary to identify the risk biomarkers to improve diagnosis and to explore the novel therapeutic targets for treatment.
Tumor growth and progression rely on the process of angiogenesis because tumor cells eventually need an additional nutrient supply. Angiogenesis and the production of angiogenic factors are critical for tumor growth, invasion and metastasis [4]. Tumor angiogenesis is mainly triggered by the release of endothelial cell-specific growth factors from neoplastic cells that stimulate the growth of blood vessels in the host. The switch depends on the increased production of one or more positive regulators of angiogenesis, such as hypoxia-inducible factor-1 (HIF-1), which activates the expression of genes involved in the glycolytic system, angiogenesis and cell survival [5, 6]. Previous studies have demonstrated the importance of angiogenic factors, such as vascular endothelial growth factor (VEGF), measured in the urine or in the tumor, in mediating bladder tumor angiogenesis [7, 8]. This process clearly involves more than the simple up-regulation of angiogenic activity and has thus been regarded as the result of a net balance of positive and negative regulators.
Adrenomedullin (ADM) is a vascular-derived peptide originally extracted from pheochromocytoma tissue [9]. ADM was later found to be expressed by many body tissues and to play a key role as a mediator of inflammation via paracrine, autocrine and endocrine mechanisms [10]. In addition, recent studies have revealed that ADM has a significant effect in the promotion angiogenesis [11] and tumor growth [12]. Moreover, ADM mRNA has been found to be overexpressed in multiple tumor tissues, such as ovarian cancer [13], prostate cancer [13], breast cancer [14], pancreatic cancer [15] and hepatocellular carcinoma [16]. ADM is also implicated in bladder cancer given that its expression in bladder cancer cell lines is up-regulated 17-fold by hypoxia [17]. However, ADM signaling has not been extensively explored during bladder cancer progression, particularly with respect to the induction of hypoxia and cellular proliferation.
This study therefore aimed to evaluate whether ADM is overexpressed in bladder cancer and whether down-regulating its expression by RNA interference (RNAi) affects the growth of bladder cancer cells and tissues.
Materials and methods
Patients and surgical specimens
The study protocol was approved by the Institutional Review Board of the Cancer Institute of Xinxiang Medical University, and all of the participants provided their written informed consent before participation. Bladder tumor samples from resection specimens were collected from patients with primary bladder UCC who underwent a transurethral resection or a total cystectomy at the Department of Urology, First Affiliated Hospital of Xinxiang Medical University, between September 2011 and August 2012. The patients were selected based on distinctive histopathologic diagnosis and availability of detailed clinicopathologic data. The patients who received anticancer treatment prior to surgery or who had evidence of other malignancies were excluded from the study. We studied a total of 55 patients (34 males and 21 females; median age 53.2 years, range 35–72 years) from whom fresh tumor samples coupled with adjacent normal bladder tissues were obtained for the analysis. The tumors were staged according to the 6th edition of the pTNM classification of the International Union Against Cancer (UICC, 2002).The patients were divided into four groups as follows: 21 cases were stage Ta or T1, 16 were stage T2, 11 were stage T3, and 7 were stage T4. The fresh-harvested tumor tissues were immediately frozen in liquid nitrogen and stored at −80 °C until use.
Cell culture and reagents
The human bladder cancer cell lines T24, UMUC-3, 253J and 5637 were obtained from the American Type Culture Collection (Rockville, MD, USA). The cells were cultured in DMEM supplemented with 10 % fetal bovine serum, 100 μg/mL penicillin and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere containing 5 % CO2. The anti-ADM antibody was obtained from Abcam Technology (Cambridge, MA). The anti-VEGF and anti-HIF-1 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-GAPDH antibody was obtained from Cell Signaling Technology (Beverly, MA).
Induction of hypoxia in bladder cancer cells
T24, UMUC-3, 253J and 5637 cells were seeded in 6-well plates at a density of 5 × 103 cells/well to allow for an exponential growth rate. Four groups were included in each cell line: the 6 h hypoxia group, the 12 h hypoxia group, the 24 h hypoxia group and a control, non-hypoxia group. Each group had three wells with identical treatments. Initially, the 24 h hypoxia group was placed in the hypoxia condition (1 % O2, 5 % CO2 and 94 % N2, at 37 °C); 12 and 18 h later, the 12 h hypoxia group and 6 h hypoxia groups, respectively, were placed into the same hypoxia condition. The normal control group was placed into a normal culture condition in parallel. Twenty-four hours later, the cells were digested with trypsin, and total RNA and protein were extracted for further analysis.
Gene silencing in the T24 cell line using ADM-shRNA
Sixth passage T24 cells were seeded in 6-well plates (1 × 105 cells/well) and allowed to adhere for 24 h before transfection. Using Lipofectamine 2000 (Invitrogen, Groningen, Netherlands), the cells were transfected with plasmids containing shRNA directed against human ADM (ADM-shRNA) (Santa Cruz, CA, USA) or the non-targeting vector control shRNA (NC-shRNA) (Santa Cruz, CA, USA). In addition, the copGFP control plasmid (Santa Cruz, CA, USA) was used to monitor and optimize the transfection efficiency. At 72-h post-transfection, puromycin (7.0 μg/mL) was added to the culture medium for selection and further characterization. The transfection efficiency was assessed using flow cytometry.
Immunoblot analysis
Cells or tissue samples were lysed for 20 min on ice with lysis buffer (50 mM Tris–HCl, pH 7.5; 10 % glycerol; 1 % NP-40; 0.25 % sodium deoxycholate; 150 mM NaCl; 1 mM EDTA; 1 mM EGTA; 1 mM PMSF; 1 μg/mL aprotinin, leupeptin, and pepstatin; 1 mM Na3VO4; 1 mM NaF) and centrifuged at 14,000 g for 10 min at 4 °C. The supernatant was collected and the protein concentration was determined using a Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA). The proteins were separated on a 12 % SDS–polyacrylamide gel and transferred to a PVDF membrane (Bio-Rad, Hercules, CA). The membranes were then blocked with 5 % skim milk for 1 h and incubated overnight with the following primary antibodies (all diluted 1:1,000): anti-ADM (Abcam, ab69117), anti-VEGF (Santa Cruz, sc-152), anti-HIF-1α (Santa Cruz, sc-10790) and anti-GAPDH (Cell Signaling, #2118). Next, the membranes were incubated with a secondary antibody for 1.5 h at room temperature. Bound antibodies were visualized using a chemiluminescent substrate (ECL, Amersham, Arlington Heights, IL) and exposed to Kodak X-OMAT film. Immunoreactive protein bands were quantified using densitometry with Quantity One (Bio-Rad, Hercules, CA).
RNA extraction and real-time quantitative reverse transcription-PCR
Total RNA was extracted from cells and tissues using the RNA iso Plus Kit (Takara, Dalian, China). The quantity of total RNA was assessed using a Nanodrop-2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) One microgram total RNA was used for first-strand DNA synthesis using PrimeScript RT reagents Kit (Takara, Dalian, China), and qPCR was performed using human-specific primers and a SYBR Green Premix Kit (Takara, Dalian, China). The following primers were used for amplification: ADM, forward primer (5′-ACTTGGCAGATCACTCTCTTAGCA-3′) and reverse primer (5′-ATCAGGGCGACGGAAACC-3′); VEGF, forward primer (5′-GCACCCATGGCAGAAGG-3′) and reverse primer (5′-GGGGTACCCCTCACCGCCTCGGCTTGTC-3′); HIF-1α, forward primer (5′-GAAAGCGCAAGTCCTCAAAG-3′) and reverse primer (5′-TGGGTAGGAGATGGAGATGC-3′); and GAPDH, forward primer (5′-CAAATTCCATGGCACCGTC-3′) and reverse primer (5′-CCCATCTGATTTTGGAGGGA-3′). For each primer pair, the annealing temperature was optimized by gradient PCR. The expression of each target mRNA relative to GAPDH mRNA was calculated based on Ct as 2−Δ(ΔCt).
Flow cytometry
The extent of apoptosis in T24 cells was assayed by Annexin V/PI staining. After 72 h of cell growth, T24 cells stably expressing ADM-shRNA were stained with Annexin V-FITC and PI using an Apoptosis Detection Kit (Sigma-Aldrich, St. Louis, MO), according to the manufacturer’s instructions. T24 cells transfected with nonspecific vector shRNA were used as the vehicle control. The cells were counted with a FACSCalibur flow cytometer.
Animal studies
All of the animal experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) .A total of 48 male BALB/c mice (5–6 weeks old) were randomly divided into six groups (cisplatin + ADM-shRNA, cisplatin + vector, cisplatin + PBS, saline + ADM-shRNA, saline + vector, saline + PBS; n = 8 for each group) to receive subcutaneous injections of 0.1 mL of T24 cells (at a density of 1 × 107 cell/mL) into their right flanks. Intratumoral administration of 10 μg/kg of body weight, ADM-shRNA, NC-shRNA or the same volume of PBS were followed by intraperitoneal injections of cisplatin (5 mg/kg of body weight) for three of the six groups. The other three groups were treated with saline as controls. shRNA, cisplatin, saline or PBS were injected every three days for 28 days. The mean tumor volume was measured in three dimensions (a, b, c) every 3 days with a caliper and calculated according to the following formula: a × b × c × 0.52. At the end of the experiment, the mice were killed, the tumors were harvested, and tumor proteins were analyzed by western blot.
Statistical analysis
All in vitro experimental data presented represent at least three independent experiments and are expressed as the mean ± SEM. The significant differences were evaluated using one-way analysis of variance (ANOVA) followed by LSD multiple comparisons tests through using a commercially available software package, SPSS (SPSS Inc., USA). The results were considered to be statistically significant when the p value was less than 0.05.
Results
Expression of ADM mRNA and protein in UCC tissues
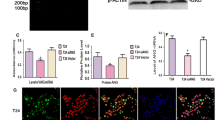
We first examined ADM mRNA expression levels in bladder cancer tissues and paired adjacent normal bladder tissues by quantitative RT-PCR. GAPDH was used as an internal control, and the ratio of ADM/GAPDH was used to reflect the relative mRNA expression level of ADM. As shown in Fig. 1a, the relative level of ADM mRNA in UCC tissues was significantly higher than that in adjacent normal bladder tissues (F 4,105 = 12.72, p < 0.0001). The ADM levels in stage T3 and T4 cancer tissues were significantly higher than those in normal controls, suggesting that the expression of ADM is up-regulated in human UCC tissues, facilitating their growth.
Quantitative RT-PCR detection of ADM protein and mRNA levels in 55 cases of bladder UCC tissues and adjacent normal bladder tissues. Twenty-one cases were stage Ta or T1, 16 were stage T2, 11 were stage T3, and 7 were stage T4. GAPDH was used as an internal control. ADM levels in adjacent normal bladder tissues were set at 100 %. The data are presented as the mean ± SEM. *p < 0.05; **p < 0.01; and ***p < 0.0001 compared with normal controls
To investigate whether ADM was also elevated at the protein level, western blot analysis was performed on those tissues. We found that the relative level of ADM protein in UCC tissues was significantly higher than that in adjacent normal bladder tissues (F 4,105 = 4.332, p < 0.01, Fig. 1b), which is consistent with the results of the RT-PCR.
Hypoxia-induced ADM, VEGF and HIF-1 expressions in bladder cancer cells
To investigate whether the hypoxia activates ADM expression in bladder cancer cells, we measured the mRNA levels of ADM and certain important cancer-promoting genes, such as VEGF and HIF-1. Quantitative RT-PCR showed that the mRNA levels for ADM, VEGF and HIF-1 increased significantly in four bladder UCC cell lines upon exposure to hypoxic conditions (Fig. 2). Among the three types of human UCC cell lines, T24 cells appear more sensitive to the stress of hypoxia (up to 4.11-fold at 24 h of hypoxia compared to normal controls). See Supplemental Table 1 for the detailed statistical results.
Relative mRNA levels of ADM, VEGF and HIF-1 after hypoxia treatment in a T24, b UMUC-3, c 253J and d 5637 cells. The normal control group was treated with a normal culture condition in parallel. The data are presented as the mean ± SEM. *p < 0.05; **p < 0.01 and ***p < 0.0001 compared with normal controls
Generation of T24 cells that stably express ADM-shRNA
To examine the effect of tumor-derived ADM on bladder cancer development, we constructed a stable UCC cell line with ADM knockdown. As T24 cells are sensitive to the stress of hypoxia, lentiviral ADM-shRNA was transfected into T24 cells. After puromycin selection, the transfection efficiency was calculated as 87.3 % using flow cytometry (Fig. 3a). According to real-time quantitative PCR and immunoblotting, both the ADM protein (F 2,15 = 10.95, p = 0.0012) and mRNA (F 2,15 = 11.32, p = 0.0022) levels in T24 cells decreased significantly after the transfection of ADM-shRNA (Fig. 3b). The relative ADM mRNA expression in cells transfected with ADM-shRNA was 0.32 ± 0.07-fold lower than that in normal T24 cells (p < 0.01). The expression of ADM was not different between the vector control cells and untransfected normal cells. These results reveal that the ADM-shRNA used in the present study successfully knocks down ADM in T24 cells.
T24 cells were transfected with plasmids containing ADM-shRNA. a After selection with puromycin, the transfection efficiency in the untransfected cells was 7.1 % (left) compared to 87.3 % in the stably expressing cells (right) using flow cytometry. b Gene silencing of ADM by ADM-shRNA causes down-regulation of ADM protein (left) and mRNA (right) levels. The data are presented as the mean ± SEM. ***p < 0.0001 compared with normal controls
ADM knockdown promotes apoptosis in T24 cells
After 72 h of cell growth, T24 cells stably expressing ADM-shRNA (mean: 40.11 ± 0.47 % cell death) had significantly increased cell death rates (F 2,14 = 21.09, p < 0.0001; post hoc, p < 0.0001) relative to the untransfected normal cells (mean: 12.67 ± 0.29) (Fig. 4). The apoptosis in the vector control group (mean: 10.02 ± 0.61 % cell death) showed a minimum elevation over the normal T24 cells, but this effect failed to reach statistical significance.
Therapeutic efficacy of the combination of cisplatin and ADM inhibition on tumor growth in vivo
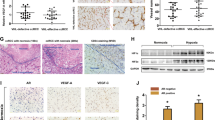
To investigate whether ADM-shRNA inhibits tumor growth in vivo, a nude mouse xenograft model of bladder cancer was established and intratumoral injection with the ADM-shRNA or control shRNA complex in the tumor mice was performed. Cisplatin was also administered as a positive control. As shown in Fig. 5a, the tumor volume of the ADM-shRNA group from days 25 to the end of the study was significantly smaller than in the vector control groups (saline + shADM vs. saline + vector, shADM effect: F 1,112 = 51.6, p < 0.0001). Tumor growth was significantly inhibited in mice injected with cisplatin (cisplatin + vector vs. saline + vector, cisplatin effect: F 1,112 = 85.7, p < 0.0001). When cisplatin treatment was combined with ADM silencing, tumor growth was inhibited significantly more compared to treatments with cisplatin treatment alone (cisplatin + shADM vs. cisplatin + vector, main effect: F 1,112 = 8.35, p = 0.0046). Furthermore, treatment with ADM-shRNA resulted in a significant decrease in the tumor volume and weight as measured at the end of the experiment (day 34) compared with the vector control, and this tumor-inhibiting effect was more prominent for the combination with cisplatin treatment (shADM:F 1,28 = 1112, p < 0.0001; cisplatin: F 1,28 = 1272, p < 0.0001; interaction: F 1,28 = 213, p < 0.0001; Fig. 5b).
Therapeutic efficacy of cisplatin when used in combination with ADM-shRNA to treat tumor growth in vivo. a Four representative subcutaneous tumors from the various intratumoral injection therapies. Each group contained 8 animals. The inhibited tumor volume in each treatment group is shown in the lower panel. b The mean ADM expression levels from randomly selected tumors in each treatment group exhibited a remarkable decrease in both the cisplatin + ADM-shRNA and saline + ADM-shRNA groups. **p < 0.05 and ***p < 0.0001 relative to the control group treated with vector shRNA
Western blot analysis of tumors showed a remarkable decrease in ADM (Fig. 5c) for tumors treated with ADM-shRNA. Two-way ANOVA analysis revealed significant effects for both cisplatin (F 1,20 = 6.77, p = 0.017) and ADM-shRNA (F 1,20 = 19.1, p = 0.0003) on tumor ADM expression, with a significant interaction between those two factors (F 1,20 = 2.66, p = 0.1183). Consistent with the results of tumor growth, injections of the ADM-shRNA plasmid significantly suppressed ADM expression in the tumor tissue (saline + shADM vs. saline + vector, p < 0.0001).Cisplatin combined with ADM-shRNA significantly inhibited tumor ADM expression when compared to treatment with cisplatin alone (cisplatin + shADM vs. cisplatin + vector, p < 0.05). Our findings support the hypothesis that the combination of cisplatin and ADM-shRNA can significantly suppress bladder cancer tumor growth in vivo.
Discussion
In this study, we have found that ADM is overexpressed in human bladder cancer tissues and that hypoxia induces ADM expression, which accelerates cell proliferation. ADM-shRNA has been successfully constructed and transfected into T24 cells, which reduced ADM expression. In addition, the T24 cells have an increased cell death rate after ADM expression is knocked down by RNAi. Furthermore, the inhibition effect of cisplatin on the tumor growth in vivo was significantly enhanced by combined treatment with ADM-shRNA. Therefore, ADM protein may be a new target for obstructing the growth of tumor neovascularization and arresting the proliferation of bladder cancer cells.
ADM expression has been demonstrated in several cancer cell lines, including those for brain tumor [18], neuroblastoma [19] and lung cancer [20]. In addition, the expression of ADM and its receptor have been demonstrated in many human epithelial cancer cell lines of diverse origins (e.g., lung, colon, ovary, breast, bone marrow, prostate and cartilage cell lines) [21]. The present study demonstrates that ADM mRNA expression is higher in tissue from human bladder cancer than in adjacent normal bladder tissue. It has also been observed that hypoxia can increase the expression of both ADM mRNA and secreted protein in a variety of tissues [12, 22, 23]. The increases are at least partly mediated by the activation of HIF-1 and VEGF [24–26]. The increased expression of ADM mRNA may result in increased angiogenic activity in tumor tissues [11, 27]. There is increasing evidence that angiogenesis is important for bladder cancer invasion and metastasis [28–31].
VEGF is well documented as a key angiogenic factor that plays an important role in mediating bladder tumor angiogenesis [7, 8] and micro-vessel density. Angiogenesis is significantly associated with poor outcome in invasive bladder cancer [28]. In addition, hypoxia is a key microenvironmental regulatory factor that induces up-regulation of the expression of both VEGF and its receptors via HIF [32]. These findings suggest that genes induced by hypoxia may be related to the aggressive phenotype of bladder cancer. Consistent with previous results from Ord and colleagues [17], our data showed that hypoxia-induced HIF-1, VEGF and ADM expressions in four types of human bladder cancer cell lines and that T24 cells were more sensitive to the hypoxia condition. There is a complex interrelationship between tumor hypoxia and tumor angiogenesis. Hypoxia is an important stimulus of angiogenesis, and angiogenesis is closely related to tumor prognosis. In clinical practice, cases with a high density of blood vessels have a poor prognosis. Low oxygen levels activate hypoxia-inducible transcription factors (HIF-1α, -2α, -3α) that bind as heterodimers with HIF-1α onto hypoxia response elements in the promoter region of various genes encoding angiogenesis factors [33]. Tumors are often hypoxic, and HIFs are generally believed to promote tumor growth by driving angiogenesis [34].
ADM’s inhibition of tumor growth may result from the interaction of ADM with tumor micro-vessels. Tumor growth and progress rely on angiogenesis; tumor cells eventually need an additional nutrient supply for survival. ADM is up-regulated under hypoxic conditions in tumor cells and could promote tumor vessel growth by enhancing vascular smooth muscle cell maturation [10, 35] via the cAMP-dependent pathway and/or mitogen-activated protein kinase (MAPK) or PI3 K/Akt signaling [10, 35]. Future studies should clarify the mechanisms that underlie ADM regulation of hypoxic cell death in bladder cancer. Because ADM levels have been found to be correlated with N-cadherin expression, which is an adhesion molecule involved in the cell migration, invasion and metastasis of cancer cells [36], the overexpression of ADM may be a prognostic indicator in bladder cancer.
Given that hypoxia-inducible ADM accelerates bladder cancer cell growth, we used shRNA against ADM to investigate whether the inhibition of ADM expression in bladder cancer cells decreases hypoxia-induced tumor growth. The suppression of ADM significantly reduced cell proliferation and increased cell death under hypoxic conditions in the T24 cell line. Similarly, work by other groups has shown the effectiveness of an exogenous ADM antagonist or RNAi specific for ADM expression in the reduction of ovarian cancer cell migration and osteosarcoma cell proliferation [37, 38]. Furthermore, we found that the combination of cisplatin and ADM inhibition treatment significantly decreased tumor growth compared to treatment with cisplatin or ADM-shRNA alone in mice bearing subcutaneous tumors, suggesting that ADM inhibition and chemotherapy exert a synergistic effect on tumor growth.
In summary, this study demonstrates that ADM signaling is hypoxia inducible and functionally active in bladder cancer. Furthermore, our findings suggest that the down-regulation of ADM using RNAi significantly inhibits bladder cancer cell proliferation and promotes apoptosis. Combined therapy with ADM-shRNA and cisplatin further inhibited tumor growth compared to treatment with cisplatin alone in vivo. Thus, selectively interfering with ADM signaling may be therapeutically efficacious in patients with bladder cancer.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29.
Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374(9685):239–49.
Shariat SF, Karakiewicz PI, Palapattu GS, Lotan Y, Rogers CG, Amiel GE et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol 2006; 176(6 Pt 1):2414-22; discussion 22.
Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64(2):327–36.
Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88(4):1474–80.
Maxwell PH, Pugh CW, Ratcliffe PJ. Activation of the HIF pathway in cancer. Curr Opin Genet Dev. 2001;11(3):293–9.
Chow NH, Liu HS, Chan SH, Cheng HL, Tzai TS. Expression of vascular endothelial growth factor in primary superficial bladder cancer. Anticancer Res. 1999;19(5C):4593–7.
Crew JP, O’Brien T, Bicknell R, Fuggle S, Cranston D, Harris AL. Urinary vascular endothelial growth factor and its correlation with bladder cancer recurrence rates. J Urol. 1999;161(3):799–804.
Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192(2):553–60.
Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21(2):138–67.
Nikitenko LL, Fox SB, Kehoe S, Rees MC, Bicknell R. Adrenomedullin and tumour angiogenesis. Br J Cancer. 2006;94(1):1–7.
Zudaire E, Martinez A, Garayoa M, Pio R, Kaur G, Woolhiser MR, et al. Adrenomedullin is a cross-talk molecule that regulates tumor and mast cell function during human carcinogenesis. Am J Pathol. 2006;168(1):280–91.
Rocchi P, Boudouresque F, Zamora AJ, Muracciole X, Lechevallier E, Martin PM, et al. Expression of adrenomedullin and peptide amidation activity in human prostate cancer and in human prostate cancer cell lines. Cancer Res. 2001;61(3):1196–206.
Fox SB, Generali DG, Harris AL. Breast tumour angiogenesis. Breast Cancer Res. 2007;9(6):216.
Ramachandran V, Arumugam T, Langley R, Hwang RF, Vivas-Mejia P, Sood AK, et al. The ADMR receptor mediates the effects of adrenomedullin on pancreatic cancer cells and on cells of the tumor microenvironment. PLoS ONE. 2009;4(10):e7502.
Park SC, Yoon JH, Lee JH, Yu SJ, Myung SJ, Kim W, et al. Hypoxia-inducible adrenomedullin accelerates hepatocellular carcinoma cell growth. Cancer Lett. 2008;271(2):314–22.
Ord JJ, Streeter EH, Roberts IS, Cranston D, Harris AL. Comparison of hypoxia transcriptome in vitro with in vivo gene expression in human bladder cancer. Br J Cancer. 2005;93(3):346–54.
Satoh F, Takahashi K, Murakami O, Totsune K, Sone M, Ohneda M, et al. Adrenomedullin in human brain, adrenal glands and tumor tissues of pheochromocytoma, ganglioneuroblastoma and neuroblastoma. J Clin Endocrinol Metab. 1995;80(5):1750–2.
Zimmermann U, Fischer JA, Frei K, Fischer AH, Reinscheid RK, Muff R. Identification of adrenomedullin receptors in cultured rat astrocytes and in neuroblastboma x glioma hybrid cells (NG108-15). Brain Res. 1996;724(2):238–45.
Martinez A, Miller MJ, Unsworth EJ, Siegfried JM, Cuttitta F. Expression of adrenomedullin in normal human lung and in pulmonary tumors. Endocrinology. 1995;136(9):4099–105.
Miller MJ, Martinez A, Unsworth EJ, Thiele CJ, Moody TW, Elsasser T, et al. Adrenomedullin expression in human tumor cell lines. Its potential role as an autocrine growth factor. J Biol Chem. 1996;271(38):23345–51.
Oehler MK, Norbury C, Hague S, Rees MC, Bicknell R. Adrenomedullin inhibits hypoxic cell death by upregulation of Bcl-2 in endometrial cancer cells: a possible promotion mechanism for tumour growth. Oncogene. 2001;20(23):2937–45.
Ogita T, Hashimoto E, Yamasaki M, Nakaoka T, Matsuoka R, Kira Y, et al. Hypoxic induction of adrenomedullin in cultured human umbilical vein endothelial cells. J Hypertens. 2001;19(3 Pt 2):603–8.
Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol. 2005;7(2):134–53.
Frede S, Freitag P, Otto T, Heilmaier C, Fandrey J. The proinflammatory cytokine interleukin 1beta and hypoxia cooperatively induce the expression of adrenomedullin in ovarian carcinoma cells through hypoxia inducible factor 1 activation. Cancer Res. 2005;65(11):4690–7.
Oladipupo S, Hu S, Kovalski J, Yao J, Santeford A, Sohn RE, et al. VEGF is essential for hypoxia-inducible factor-mediated neovascularization but dispensable for endothelial sprouting. Proc Natl Acad Sci USA. 2011;108(32):13264–9.
Ribatti D, Nico B, Spinazzi R, Vacca A, Nussdorfer GG. The role of adrenomedullin in angiogenesis. Peptides. 2005;26(9):1670–5.
Bochner BH, Cote RJ, Weidner N, Groshen S, Chen SC, Skinner DG, et al. Angiogenesis in bladder cancer: relationship between microvessel density and tumor prognosis. J Natl Cancer Inst. 1995;87(21):1603–12.
Streeter EH, Harris AL. Angiogenesis in bladder cancer–prognostic marker and target for future therapy. Surg Oncol. 2002;11(1–2):85–100.
Theodoropoulos VE, Lazaris A, Sofras F, Gerzelis I, Tsoukala V, Ghikonti I, et al. Hypoxia-inducible factor 1 alpha expression correlates with angiogenesis and unfavorable prognosis in bladder cancer. Eur Urol. 2004;46(2):200–8.
Shariat SF, Youssef RF, Gupta A, Chade DC, Karakiewicz PI, Isbarn H, et al. Association of angiogenesis related markers with bladder cancer outcomes and other molecular markers. J Urol. 2010;183(5):1744–50.
Baek JH, Jang JE, Kang CM, Chung HY, Kim ND, Kim KW. Hypoxia-induced VEGF enhances tumor survivability via suppression of serum deprivation-induced apoptosis. Oncogene. 2000;19(40):4621–31.
Yamakawa M, Liu LX, Date T, Belanger AJ, Vincent KA, Akita GY, et al. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res. 2003;93(7):664–73.
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–32.
Iwase T, Nagaya N, Fujii T, Itoh T, Ishibashi-Ueda H, Yamagishi M, et al. Adrenomedullin enhances angiogenic potency of bone marrow transplantation in a rat model of hind limb ischemia. Circulation. 2005;111(3):356–62.
Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000;148(4):779–90.
Pang X, Shang H, Deng B, Wen F, Zhang Y. The interaction of adrenomedullin and macrophages induces ovarian cancer Cell migration via activation of RhoA signaling pathway. Int J Mol Sci. 2013;14(2):2774–87.
Dai X, Ma W, He XJ, Jha RK. Elevated expression of adrenomedullin is correlated with prognosis and disease severity in osteosarcoma. Med Oncol. 2013;30(1):347.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Ag., Zhang, Xz., Li, Fb. et al. RNA interference targeting adrenomedullin induces apoptosis and reduces the growth of human bladder urothelial cell carcinoma. Med Oncol 30, 616 (2013). https://doi.org/10.1007/s12032-013-0616-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0616-6