Abstract
Combined chemoradiation (CRT) is the standard therapy in locally advanced non-small cell lung cancer (NSCLC). Nevertheless, the best approach in the elderly population is still poorly defined. We retrospectively reviewed the charts of elderly (≥65 years) patients with unresectable, locally advanced NSCLC, diagnosed at the Brazilian National Cancer Institute between 2003 and 2007. The primary outcome was overall survival (OS), measured from diagnosis until death. Palliative therapy (PT) included best supportive care radiation therapy (RT; ≤40 Gy) and palliative chemotherapy. Among patients treated with radical RT, OS was measured from date of treatment beginning until death (OST). One hundred seventy-one patients were included, with median age of 71 years (range 65–90). Thirty-nine percent received PT, 32 % exclusive RT (>40 Gy), and 29 % CRT (concomitant or sequential). Patients treated with RT and CRT had better OS (median 13.7 months [95 % CI 10.9–16.4] and 15.5 months [95 % CI 13.0–17.9]) than PT (median 4.1 months [95 % CI 3.6–4.6]; p < 0.0001). In the multivariate analysis, RT (HR 0.28 [95 % CI 0.18–0.42]; p < 0.0001) and CRT (HR 0.17 [95 % CI 0.1–0.27]; p < 0.0001) were independently correlated to better survival in comparison with PT. Among patients receiving radical RT, the addition of chemotherapy was correlated to longer OST (median 13.8 [95 % CI 10.6–17.0] vs. 10.8 months [95 % CI 8.6–13.1]; p = 0.018). This benefit was confirmed in the multivariate analysis (HR 0.59 [95 % CI 0.36–0.97]; p = 0.039). Elderly patients with locally advanced NSCLC derived significant survival benefit from radical RT and CRT, suggesting that age should not be a contraindication for these aggressive therapeutic strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer death in both genders worldwide [1], and approximately one-third of non-small cell lung cancer (NSCLC) patients are diagnosed with unresectable, locally advanced disease. In this setting, the combination of chemotherapy and radiotherapy (CRT) is considered the gold standard for fit patients, based on randomized trials. In a meta-analysis [2], the absolute benefits of adding chemotherapy to radiation (RT) were 4 % at 2 years and 2.2 % at 5 years. Furthermore, concomitant CRT was demonstrated to be superior to sequential schedules, but at the cost of a higher acute toxicity [3].
It should be noted, however, that the best approach in the elderly population remains a matter of debate, since this subgroup was mostly underrepresented in clinical trials. Advanced age has been associated with worse prognosis, and older patients tend to have more comorbidities, reduced tolerance to cancer treatment, and receive less intense therapies [4]. Notwithstanding, a survival benefit was suggested for CRT in a subgroup analysis involving 164 patients older than 70 years in a meta-analysis (HR 0.67; 95 % CI 0.48–0.94) [5]. Moreover, the combination of a daily low-dose carboplatin schedule to thoracic RT was superior to exclusive RT in a Japanese phase III trial involving 200 elderly patients [6].
Since elderly patients are frequently excluded from clinical trials, and approximately two-thirds of NSCLC cases occur in people aged 65 years or older [7], we speculated that a real-world study should be more adequately representative. In order to accomplish it, we performed a comprehensive retrospective review of aged patients with unresectable, stage III NSCLC, diagnosed at the Brazilian National Cancer Institute (INCA) over a 5-year period. The main questions were whether there was a benefit in treating elderly patients with locally advanced NSCLC overall, and whether CRT was superior to RT alone.
Patients and methods
Patients
The total cohort included incident cases of unresectable, stage III NSCLC (American Joint Commission on Cancer, 6th edition), diagnosed at INCA between 2003 and 2007, aged 65 years or more. Patients with malignant pleural effusion (former “wet IIIB”) were excluded, as well as patients treated with primary surgery or at other institutions. Both treated and untreated patients were included in this cohort. In a second analysis, we evaluated exclusively patients who received radical schedules of RT, as defined below. The local Hospital Data System provided the list of patients, and data were collected from medical records and exams database by two investigators (P.M.D. and R.Z.). The study was approved by the local Institutional Ethics Committee.
Outcomes
The primary outcome was overall survival (OS), calculated as the interval between diagnosis and death. In order to assess the impact of different therapeutic strategies, we defined palliative therapy (PT) as including best supportive care, RT at doses of less than 40 Grays (Gy), and palliative chemotherapy. On the other hand, patients treated with RT at doses of 40 Gy or higher were classified in the RT group. When a combination of chemotherapy and RT was used, patients were classified in the CRT group, independently of receiving concomitant or sequential schedules. In a second analysis, only patients treated with radical RT (≥40 Gy) or CRT were included, and OS was measured from the date of treatment beginning until death (OST).
Statistical analysis
Baseline characteristics were compared between the cohort of patient receiving PT and patients treated with radical schedules of RT (exclusive RT or CRT) using the chi square, Fisher exact, and Mann–Whitney tests. OS and OST were estimated using the Kaplan–Meier method [8]. p values of ≤0.05 were considered to indicate statistical significance, and the 95 % confidence interval (CI) was calculated. Survival curves were compared according to putative prognostic factors using logrank [9], and then, multivariate analyses of all matched variables were carried out using stepwise Cox model [10]. Statistical analyses were performed using the SPSS 18.0 software (SPSS Inc., CA, USA).
Results
Total cohort
One hundred seventy-one patients were included (Table 1). Median age was 71 years (range 65–90), 75 % were male, and 77 % were white. Most patients (53 %) had squamous cell carcinoma, and 82 % were diagnosed with stage IIIB. Seventy-three percent of patients had at least 5 % weight loss documented at the diagnosis, and most had performance status (PS) 0–1 (42 %), while PS 2 and 3 were documented in 31 and 27 %, respectively. Ninety-five percent were current/former smokers, and Charlson Index was 0 in 66 % and 1–2 in 34 %. Sixty-two patients (37 %) received either no therapy overall or only palliative RT (<40 Gy), while 4 patients (2 %) received palliative chemotherapy, comprising 66 (39 %) in the PT group. Fifty-four patients (32 %) were treated with exclusive radical RT, and 49 (29 %) with CRT, consisting of concomitant and sequential schedules in 30 and 19 patients, respectively. All chemotherapy regimes were based in platinum contents.
Patients receiving RT at radical doses (N = 103) presented higher proportions of PS 0–1, stage IIIA, and no weight loss than patients in the PT group (Table 1). PS 0–1, 2, and 3 were documented in 61, 34, and 5 % in the radical treatment cohort and in 12, 27, and 61 % in the PT group, respectively (p = 0.001). The frequencies of patients without weight loss were 33 and 18 % in the radical RT and in the PT groups, respectively (p = 0.031), while stage IIIA was documented in 23 and 11 % (p = 0.045). Conversely, most other baseline characteristics were in line with the cohort of patients receiving PT (Table 1).
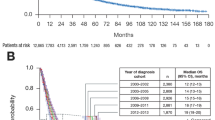
After a median follow-up of 8.8 months, 160 deaths (94 %) were documented, and the median OS was 9.7 months (95 % CI 8.0–11.5). Survival was significantly longer among patients with PS 0–1 (median 15.2 months [95 % CI 13.4–17.0]) in contrast to PS 2 and 3 (medians 9.8 [95 % CI 8.1–11.6] and 3.9 months [95 % CI 3.5–4.2], respectively; p < 0.0001), and among patients without weight loss (median 14.2 months [95 % CI 11.1–17.2] vs. 7.7 months [95 % CI 5.7–9.6]; p = 0.015). On the other hand, histology (p = 0.22), tumor stage (p = 0.32), Charlson Index (p = 0.30), and age (p = 0.58) were not considered prognostic factors. Patients treated with either exclusive RT or CRT had better survival (medians 13.7 months [95 % CI 10.9–16.4] and 15.5 months [95 % CI 13.0–17.9], respectively) than patients receiving PT (median 4.1 months [95 % CI 3.6–4.6]; p < 0.0001) (Fig. 1). The data from univariate analyses are summarized in Table 2. In the multivariate analyses, RT (HR 0.28 [95 % CI 0.18–0.42]; p < 0.0001) and CRT (HR 0.17 [95 % CI 0.11–0.27]; p < 0.0001) were independently correlated to better survival in comparison with PT.
Patients receiving radical RT
One hundred and three patients received radical doses of RT, with or without chemotherapy (Table 3). More patients in the CRT group presented with better PS (p < 0.001), adenocarcinoma histology (p = 0.02), and less weight loss (p = 0.02) than patients receiving exclusive RT. Moreover, patients treated with CRT were slightly younger (medians 70 and 72 years; p = 0.03). There was no significant difference in salvage chemotherapy exposure in the RT and CRT groups (6 vs. 16 %, respectively; p = 0.11). All other factors were well balanced between the two groups.
Ninety-six deaths (93 %) were documented in this cohort, with a median OST of 11.4 months (95 % CI 10.5–12.3). The addition of chemotherapy was significantly correlated to a longer OST (medians 13.8 ms [95 % CI 10.6–17.0] vs. 10.8 ms [95 % CI 8.6–13.1] for CRT and exclusive RT, respectively; p = 0.018) (Fig. 2). In the CRT group, patients receiving concomitant schedules had a numerically superior OST than sequential, but this difference was not statistically significant (medians 14.1 months [95 % CI 9.7–18.4] vs. 11.2 months [95 % CI 7.9–14.5]; p = 0.59). In addition, patients with better PS also presented longer OST (medians 14.1 months [95 % CI 11.0–17.1], 10.4 months [95 % CI 8.2–12.7], and 5.9 months [0.0–15.3] for PS 0–1, 2, and 3, respectively; p = 0.004). In contrast, other characteristics were not correlated to OST, including stage (p = 0.84), histology (p = 0.09), weight loss (p = 0.11), Charlson Index (p = 0.73), and salvage chemotherapy (p = 0.10) (Table 4). In the multivariate analysis, PS 0–1 (HR 0.24 [95 % CI 0.09–0.63]; p = 0.004), PS 2 (HR 0.25 [95 % CI 0.09–0.67]; p = 0.006), and CRT (HR 0.59 [95 % CI 0.36–0.97]; p = 0.039) were independently correlated to better OST.
Discussion
The best approach to locally advanced NSCLC in the elderly population has been poorly defined in the literature. In the current study, our group compiled the data from elderly patients presenting with unresectable, stage III disease, in a real-world scenario. Notably, we demonstrated that selected patients derived survival benefit from RT at radical doses, with or without chemotherapy, corroborating that advanced age should not be seen as a stigma to preclude aggressive therapies in this setting. Furthermore, the addition of chemotherapy was significantly correlated to longer survival in comparison to exclusive RT among treated patients. These statements were accordingly confirmed in multivariate analyses.
We are aware of two comprehensive studies evaluating the outcomes of elderly patients with locally advanced NSCLC in real-world scenarios. Davidoff et al. [11] retrospectively reviewed data from 6,325 patients in the North American SEER database. These patients were older than 65 years and presented with stages IIIA or IIIB, diagnostic between 1997 and 2002. In their cohort, 26.5 % did not receive specific oncologic therapy and had the worst survival (median, 6.9 months). On the other hand, 41.3 and 45.2 % received exclusive RT and CRT, respectively, and their median OS were 7.6 months and 12.0 months. In a Canadian study [12], Coate et al. retrospectively showed that older patients were more likely to receive palliative therapy. However, aged patients treated with curative intent—including surgery—had similar survival in comparison with the younger. It is important to mention that these studies used a 65-years cutoff for selecting older patients, even though a criterion of 70 years is being currently used, at least for metastatic lung cancer [13]. For this reason, we also decided to select patients based on the 65-years cutoff. In our cohort, the described survival is comparable to those found in the aforementioned large-scale studies. For instance, we also had nearly one-third of patients receiving PT, with a median survival of 4.1 months. On the other hand, median OS among patients receiving RT and CRT were 13.7 and 15.5 months, respectively.
In the present study, all medical charts were directly reviewed by the investigators, which tend to minimize the risk of systematic errors in contrast to larger database studies like Davidoff et al.s’ [11]. In their analysis, treatment classification was based on Medicare claims files, which may be flawed in defining the treatments or sequences that were actually used. In our study, for instance, the local Hospital Data System provided a list with 319 patients, from which only 171 were really eligible. Most other patients were misclassified, since they in fact had metastatic disease. Moreover, we limited the evaluation to a 5-year period in order to avoid bias related to the implementation of novel technologies, which could impact on patient selection over time through stage migration. In fact, all patients in our cohort were staged in an era prior to positron emission tomography (PET) availability. Nonetheless, we believe that this cohort is representative of a real-world population. Of note, around 60 % had very poor PS (2 or 3), and approximately 40 % were only approached with palliative measures. In this regard, the high frequency of PS 2–3 documented here likely reflects the delayed diagnosis and the lower access to medical care in low- and middle-income countries around the globe, where poorer health systems are routine.
This study was not intended to evaluate neither the toxicity profile of each treatment schedule nor the best CRT combination or sequence. Cavalcanti et al. [14] recently evaluated these aspects in a retrospective study at a North American single institution between 1997 and 2010. Their cohort comprised 64 patients aged 70 years or older, treated with concurrent CRT in 43 cases and with sequential schedules in 21. The most common chemotherapy regimens were carboplatin and paclitaxel (44 %) and carboplatin and etoposide (15 %), and there was a trend toward superior survival in the concurrent versus sequential groups (median survival of 19 and 11 months, respectively), but this difference was not statistically significant (p = 0.67). They also demonstrated that such approaches were feasible, with esophagitis (42 %), anemia (39 %) and pneumonia (24 %) being the most frequent side effects. In our cohort, 30 patients received sequential and 19 concurrent CRT. In line with the above study, we also found a numerically but not statistically significant superior survival for the concurrent versus sequential schedules (median OST of 14.1 and 11.2 months, respectively; p = 0.59). As older patients commonly present with a narrower therapeutic window, combined chemotherapy should comprise agents with a favorable toxicity profile. In this regard, the substitution of carboplatin for cisplatin might be a first reasonable concept.
Deteriorated PS and weight loss were correlated to inferior survival in our cohort, although these factors were no longer significant after adjusting to treatment approaches. These findings are in line with other large-scale studies [11, 12], where age, comorbidities, and PS were considered factors predictive of worse prognosis in the elderly. As expected, poor PS and weight loss were more frequently documented in the PT cohort, which might be an important confounding bias. These and other unmeasurable factors—including patients’ preference—might have impacted on the selection characteristics for choosing a more or less aggressive treatment. These limitations were predictable and are inherent to a retrospective study. It is noteworthy that Charlson comorbidity index was not a prognostic factor in the present study. This might be explained by the relatively lower number of patients included, and to some degree of recall bias, which is inherent to the retrospective data collection. We also did not notice a survival difference according to distinct age categories. In this regard, it should be emphasized that 78 % of the studied patients were in the stratum aged between 65 and 75 years, and hence, our data might not be accurately extrapolated to an older population. In this cohort, salvage chemotherapy did not significantly impact on survival after aggressive RT protocols, probably due to the low proportion of patients receiving such approach.
In summary, our study indicates that selected elderly patients with locally advanced NSCLC derive survival benefit with more aggressive approaches including radiation and chemotherapy combinations, suggesting that age per se should not be a contraindication. The best sequence and schedule should be accordingly evaluated in prospective trials especially designed for patients at advanced ages, ideally involving novel agents with optimized efficacy and toxicity profile.
References
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Aupérin A, Le Péchoux C, Pignon JP, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol. 2006;17:473–83.
Auperin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–90.
Schild SE, Stella PJ, Geyer SM, et al. The outcome of combined-modality therapy for stage III non-small-cell lung cancer in the elderly. J Clin Oncol. 2003;21:3201–6.
Langer CJ, Hsu C, Curran WJ, et al. Elderly patients (pts) with local advanced non-small cell lung cancer (LA-NSCLC) benefit from combined modality therapy: Secondary analysis of Radiation Therapy Oncology Group (RTOG) 94-10. Proc Am Soc Clin Oncol. 2002;21:75s (abstr 1193).
Okamoto H, Atagi S, Kawahara M, et al. Updated results of a phase III trial comparing standard thoracic radiotherapy (RT) with or without concurrent daily low-dose carboplatin in elderly patients (pts) with locally advanced non-small cell lung cancer (NSCLC): JCOG0301. J Clin Oncol. 2012;30 (suppl; abstr 7017).
Ries LAG, Melbert D, Krapcho M, et al. SEER cancer statistics review, 1975–2005. Bethesda: National Cancer Institute; 2007. Available at: http://seer.cancer.gov/csr/1975_2005/. Accessed 15 April 2012.
Kaplan EL, Meier P. Nonparametric estimation for incomplete observation. J Am Stat Assoc. 1958;53:457–81.
Lawless JS. Statistical models and methods for life-time data. New York: Wiley; 1982.
Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220.
Davidoff AJ, Gardner JF, Seal B, et al. Population-based estimates of survival benefit associated with combined modality therapy in elderly patients with locally advanced non-small cell lung cancer. J Thorac Oncol. 2011;6(5):934–41.
Coate LE, Massey C, Hope A, et al. Treatment of the elderly when cure is the goal: the influence of age on treatment selection and efficacy for stage III non-small cell lung cancer. J Thorac Oncol. 2011;6(3):537–44.
Quoix E, Zalcman G, Oster JP, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet. 2011;378(9796):1079–88.
Cavalcanti L, Kutwak F, Ruiz AL, et al. Outcomes of combined modality treatment in elderly patients with stage III non-small cell lung cancer. J Clin Oncol. 2012;30 (suppl; abstr 7049).
Conflict of interest
The authors declare no conflicts of interest related to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Domingues, P.M., Zylberberg, R., da Matta de Castro, T. et al. Survival data in elderly patients with locally advanced non-small cell lung cancer. Med Oncol 30, 449 (2013). https://doi.org/10.1007/s12032-012-0449-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-012-0449-8