Abstract
Dicer11 plays an important role in generation of microRNA and is dysregulated in a variety of human cancers. The purpose of this study was to evaluate Dicer11 expression and its prognostic value in nasopharyngeal carcinoma (NPC). The protein expression of Dicer1 was examined by immunohistochemistry in 276 NPC specimens, and the mRNA levels of Dicer1 were analyzed by qRT-PCR in 56 NPC and 11 nasopharyngitis tissues. Cox regression analysis was used to identify independent prognostic factors, and a prognostic score model was constructed for survival prediction. Expression of Dicer1 was downregulated in NPC tissues at both the mRNA and the protein levels, and there was a notable positive correlation between the expression levels of Dicer1 mRNA and protein. Low Dicer1 expression was positively correlated with distant metastasis (P < 0.01) and death (P = 0.01). In addition, low expression of Dicer1 was significantly associated with poorer overall survival (HR, 2.32; 95 % CI 1.30–4.14; P < 0.01) and poorer distant metastasis-free survival (HR, 2.56; 95 % CI 1.39–4.74; P < 0.01). Furthermore, multivariate analysis showed that low expression of Dicer1 and tumor-node-metastasis (TNM) stage was independent prognostic indicators for NPC patients. A prognostic score model combining the Dicer1 expression and TNM stage had a better prognostic value than the TNM stage alone model or Dicer1 expression alone model (P < 0.05). Dicer1 was downregulated in NPC tissues at both the mRNA and the protein levels, and low expression of Dicer1 could be served as novel prognostic biomarker for NPC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial malignancy, and the global statistics have revealed its extremely unbalanced distribution across the world in the age-standardized incidence rate per 100,000 males, which ranges from 20 to 50 in South China to 0.5 in white populations [1]. According to the data from the International Agency for Research on Cancer, there were an estimated 84,400 cases and 51,600 deaths in 2008 [2].
A precise prognosis is essential for making better therapeutic choices for NPC patients. At present, however, only the tumor-node-metastasis (TNM) staging system is widely accepted as a prognostic indicator for NPC patients [3]. According to the guidelines of the National Comprehensive Cancer Network, NPC patients with early-stage disease should receive radiotherapy, whereas patients with advanced disease should be treated with chemoradiotherapy. However, large variations in treatment outcomes have been observed in NPC patients who presented with the same stage and received similar therapies, indicating that the current staging system remains inadequate for making accurate predictions. Multiple refinements have been made to the staging system [4, 5], and identification of new prognostic biomarkers has progressed [6–8]; however, the prognostic prediction for NPC patients is still dismayed. Therefore, the discovery of novel biomarkers of NPC would provide additional value to prognostic predictions and further facilitate more personalized therapies for this disease.
Dicer1, an RNase III endonuclease, is critical for the maturation of microRNAs and small interfering RNAs (siRNAs) from pre-microRNAs and dsRNA, respectively [9, 10]. Due to its central role in post-transcriptional regulation of microRNA, more focus is being directed to Dicer1 in cancer-related investigations. The expression levels and prognostic value of Dicer1 have been demonstrated in a variety of human malignancies, including ovarian cancer [11–13], lung cancer [14, 15], esophageal cancer [16], prostate cancer [17], breast cancer [18], endometrial adenocarcinoma [19], and colorectal cancer [20]. To date, however, little is known about the expression levels and prognostic value of Dicer1 in NPC.
Therefore, in the present study, we examined the expression dynamics of Dicer1 in tumor specimens from NPC patients and analyzed the association between Dicer1 expression and the clinical characteristics of NPC. In addition, we evaluated the prognostic value of Dicer1, which could provide information for developing a more personalized therapy for NPC patients.
Materials and methods
Tissue specimens
Two hundred and seventy-six non-distant-metastasis and paraffin-embedded NPC biopsy specimens and eleven nasopharyngitis tissues were retrieved from pathology archive at Department of Pathology, Sun Yat-sen University Cancer Center between January 2003 and February 2006. None of the NPC patients received radiotherapy or chemotherapy treatment before biopsy. All patients were pathologically diagnosed as World Health Organization type II or III disease. The clinical characteristics of all of the patients were recorded (Table 1). All of the MRI/CT materials and clinical records were reviewed, and all of the patients were restaged according to the criteria of the AJCC Cancer Staging Manual, 7th edition. After explaining the purpose of the study, written informed consent was obtained from all patients. This study was approved by the Institutional Ethical Review Boards of our institute.
Patient treatment and follow-up
All of the patients were treated with conventional two-dimensional radiotherapy, and details regarding the radiation therapy techniques used at Sun Yat-sen University Cancer Center have been previously reported [21]. Of the stage III–IV patients (classified as stage T3–T4 and/or stage N2–N3), 91/191 (47.6 %) also received concurrent platinum-based chemotherapy [22, 23]. In cases of documented relapse or persistent disease, salvage treatments, such as intracavitary brachytherapy, surgery, and chemotherapy, were provided.
The follow-up duration was calculated from the first day of treatment to either the day of death or the day of the last examination. Patients were examined at least every 3 months during the first 2 years and, thereafter, every 6 months until death. The data of the last follow-up were May 2011, and the median follow-up period was 63.6 months (range: 5.2–91.9 months). The following end points (time to the first defining event) were assessed: overall survival (OS) and distant metastasis-free survival (DMFS).
Immunohistochemistry
The sections of 276 paraffin-embedded NPC specimens were detected for Dicer1 protein by immunohistochemical staining, as described previously [24]. Briefly, blocks were cut into 5-μm-thick sections, followed by deparaffinization and rehydration. The sections were microwaved for antigenic retrieval in an EDTA buffer, and 3 % hydrogen peroxide was used to quench the endogenous peroxidase activity. Nonspecific binding was blocked with 1 % bovine serum albumin. The sections were then incubated overnight at 4 °C with mouse monoclonal anti-Dicer1 antibody (1:200; Abcam, Cambridge, MA). Normal goat serum was used as a negative control. After rinsing, the sections were incubated with biotinylated secondary antibody bound to a streptavidin–horseradish peroxidase complex. The bound antibody was visualized by adding 3,3-diaminobenzidine, and sections were counterstained with hematoxylin, dehydrated, and mounted.
The sections were reviewed and scored independently by 2 pathologists, and any disagreements were resolved by consensus. Both the proportion of positively stained tumor cells and the intensity of staining were assessed [24, 25]. The proportion of positive tumor cells was scored as follows: 1 (<10 %), 2 (10–35 %), 3 (35–70 %), and 4 (>70 %). The intensity of staining was graded on a scale from 0 to 3: 0 (no staining), 1 (weak staining, light yellow), 2 (moderate staining, yellow brown), and 3 (strong staining, brown). The staining index was calculated as the product of the staining intensity score and the proportion of positive tumor cells. Using this method, we evaluated Dicer1 expression in NPC tissues by the staining index with scores of 0, 1, 2, 3, 4, 6, 8, 9, or 12. The cutoff value for high and low expression levels was selected based on a measure of heterogeneity according to the log-rank test with respect to overall survival. The following optimal cutoff value was used: a staining index score of ≤4 was used to define tumors with low Dicer1 expression, and a staining index score of ≥6 was designated as high Dicer1 expression.
RNA extraction and quantitative RT-PCR
Total RNA was extracted using an acid phenol/chloroform extraction method followed by ethanol precipitation, as previously described [26]. The extracted RNA (2 μg) was reverse transcribed using random primers (Promega, Madison, WI, USA) and M-MLV reverse transcriptase (Promega) for 60 min at 37 °C and then 15 min at 70 °C, and the product was stored at −20 °C until use.
Quantitative PCR was carried out on the PRISM 7900HT sequence detection system (Applied Biosystems, Carlsbad, CA, USA) in a 15-μL reaction volume containing 1 μL of reverse transcription product, 7.5 μL of Platinum SYBR Green qPCR SuperMix-UDG reagents (Invitrogen, Carlsbad, CA, USA), 1.25 μL of each PCR forward primer and reverse primer (2.5 μM), and 4 μL of ddH2O. The reactions were pre-incubated at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 30 s and annealing/extension at 60 °C for 1 min; the reactions were then ramped from 60 to 95 °C to obtain a melting curve. The PCR primers were designed using PerlPrimer [27]. The following primers were used for Dicer1 amplification: 5′-CTGATGAACTCAACTTTAGAAGGC-3′ (forward) and 5′-GTGTGGAATCTGAGGTATGG-3′ (reverse). In addition, the following primers were used for GAPDH amplification: 5′-CTCCTCCTGTTCGACAGTCAGC-3′ (forward) and 5′-CCCAATACGACCAAATCCGTT-3′ (reverse). All of the assays were performed in triplicate, and reactions containing either no template or no reverse transcriptase were used as negative controls.
Statistical analysis
The Statistical Package for Social Sciences, version 16.0 (SPSS, Inc., Chicago, IL, USA), was used for the statistical analysis. The χ2 tests were used to analyze the relationship between protein expression of Dicer1 and clinical characteristics. The differences in Dicer1 mRNA expression between NCN and NPC tissues were analyzed using the Student’s t test. The correlation between the Dicer1 mRNA and protein expression was evaluated using the Spearman’s correlation analysis.
The Kaplan–Meier method was used to estimate the OS and DMFS, and the differences were compared using the log-rank test. HR values were calculated using univariate Cox regression analysis. Multivariate Cox regression analysis was used to test the independent significance. The Dicer1 expression level, age and sex of patients, and clinical stage were used as covariates. Further, a prognostic score model was constructed using the significant variables from the multivariate analysis [28], and the receiver operating characteristics (ROC) curves were used to compare the sensitivity and specificity for the prediction of survival. All of the tests were two-tailed, and P values of <0.05 were considered significant.
Results
Expression of Dicer1 is downregulated in NPC tissues
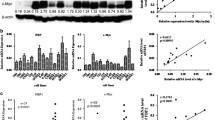
We first detected expression dynamics of Dicer1 in 276 NPC paraffin-embedded biopsy tissues using immunohistochemistry. Representative staining of Dicer1 in NPC tissues was shown in Fig. 1a–d. Positive expression of Dicer1 in epithelial tissue cells was observed in the cytoplasm. Dicer1 protein expression was detected in 257 of 276 (93.3 %) samples. Weak, moderate, or strong staining was detected in 112 (40.6 %), 118 (42.8 %), and 27 (9.8 %) of the 276 samples, respectively. As shown in Fig. 1e, Dicer1 was found to be downregulated in NPC tissues compared to the adjacent normal epithelial cells in the same section.
Expression analysis of Dicer1 protein by immunohistochemistry in nasopharyngeal carcinoma tissues. Dicer1 protein expression is mainly localized to the cytoplasm. a Negative staining (×400); b weak staining: light yellow (×400); c moderate staining: yellow brown (×400); d strong staining: brown (×400). e Dicer1 expression was downregulated in nasopharyngeal carcinoma (solid arrow) compared to the adjacent normal epithelial cells (hollow arrow)
To investigate whether Dicer1 was downregulated at the transcriptional level, we performed qRT-PCR for Dicer1 mRNA in 56 NPC tissue samples and 11 nasopharyngitis tissue samples. We found that expression levels of Dicer1 mRNA were significantly reduced in NPC compared to nasopharyngitis tissues (Fig. 2a), and the levels correlated significantly with the results of the immunohistochemical analysis (Fig. 2b).
Expression analysis of Dicer1 mRNA by qRT-PCR in nasopharyngeal carcinoma tissues and its correlation with Dicer1 protein expression. a The relative expression of Dicer1 mRNA normalized to GAPDH, as detected by qRT-PCR, is shown. The data are presented as the mean ± SEM, and the statistical significance was calculated using the Student’s t test. b Correlation analysis of Dicer1 mRNA and protein expression levels using the Spearman’s correlation analysis
Correlation between Dicer1 expression and clinical features of NPC
We analyzed the relationship between Dicer1 expression and clinical features of NPC patients. As shown in Table 1, reduced expression of Dicer1 was significantly associated with distant metastasis (P < 0.01) and death (P = 0.01). However, there was no significant association between Dicer1 expression and the other clinical features, such as age, gender, clinical stage (T/N/TNM status), and locoreginal failure (P > 0.05).
Reduced expression of Dicer1 is associated with poor prognosis
We defined 170 (61.59 %) patients as low expression of Dicer1 and 106 (38.41 %) patients as high expression of Dicer1 using the staining index of 4 as the cutoff point. Kaplan–Meier analysis showed that patients with low expression of Dicer1 were significantly associated with poorer OS (HR, 2.32; 95 % CI, 1.30–4.14; P < 0.01) and poorer DMFS (HR, 2.56; 95 % CI, 1.39–4.74; P < 0.01) than those with high expression of Dicer1 (Fig. 3a). The cumulative 5-year survival rate was only 67.06 % (95 % CI, 59.99–74.13 %) in the low Dicer1 expression group, whereas it was 81.13 % (95 % CI, 73.68–88.58 %) in the high Dicer1 expression group.
Kaplan–Meier curves of overall survival and distant metastasis-free survival of nasopharyngeal carcinoma patients stratified by Dicer1 expression level and clinical stage. Stage I–IV patients (n = 276). b Stage I–II patients (n = 85). c Stage III–IV patients (n = 191). HR hazard ratio, CI confidence interval. HR values and P values were calculated using the univariate Cox regression analysis
Furthermore, we performed stratified analysis with regard to Dicer1 expression in subsets of NPC patients with different tumor stages. The result displayed that in the advanced disease subgroups (stage III–IV, n = 191), patients with low Dicer1 expression had significantly worse OS (HR, 1.94; 95 % CI, 1.05–3.55; P = 0.03) and worse DMFS (HR, 2.23; 95 % CI, 1.17–4.24; P = 0.01) than those with high Dicer1 expression level (Fig. 3c). However, in the early-stage subgroup (stage I–II, n = 85), the differences of OS and DMFS between patients with low or high Dicer1 expression were not significant (P = 0.10 and P = 0.14, respectively; Fig. 3b).
Low expression of Dicer1 is an independent prognostic factor
We did univariate Cox regression analysis and found that Dicer1 expression, T stage, N stage, TNM stage were significantly associated with OS and DMFS (P < 0.05, Table 2). Further, we performed multivariate analyses to determine whether Dicer1 expression level is an independent prognostic factor of patient DFS and DMFS. The Dicer1 expression, age, sex, and clinical stage were used as covariates. We found that the Dicer1 expression (HR, 2.17; 95 % CI, 1.21–3.89; P = 0.01; HR, 2.42; 95 % CI, 1.31–4.48; P = 0.01, respectively) and TNM stage (HR, 3.08; 95 % CI, 1.46–6.49; P < 0.01; HR, 3.57; 95 % CI, 1.62–7.86; P < 0.01, respectively) were independent prognostic factors associated with OS and DMFS (Table 3).
Expression of Dicer1 add prognostic value to TNM clinical stage
To make more sensitive prognostic prediction, we constructed a prognostic score model combining the above-mentioned independent prognostic factors, Dicer1 expression and TNM stage. The regression coefficient of Dicer1 expression was divided by the regression coefficient of TNM stage and rounded into integer value to get risk score (Table 3). We then calculated the cumulative risk score for each patient and used the ROC curves to compare the prognostic validity of the combined model with TNM stage alone and the Dicer1 expression alone models. The model combining Dicer1 expression and TNM stage had a better prognostic value than the TNM stage alone and Dicer1 expression alone models for OS and DMFS (P < 0.05, Fig. 4).
Comparisons of the sensitivity and specificity for the prediction of survival by the combined Dicer1 expression and TNM stage model, the TNM stage alone model, and the Dicer1 expression alone model. Receiver operating characteristics (ROC) curves for the prediction of overall survival (a), and distant metastasis-free survival (b). P values showed the area under the ROC (AUROC) of the combined Dicer1 expression and TNM stage model versus AUROCs of the TNM stage alone model or the Dicer1 expression alone model
Discussion
In this study, we examined the expression of Dicer1 and its prognostic significance in patients with NPC. Our results showed that Dicer1 was downregulated in NPC tissues at both the mRNA and the protein levels. The expression of Dicer1 was found to significantly correlate with the prognosis of NPC patients, as patients with low Dicer1 expression had poor survival. Furthermore, we found that Dicer1 expression added prognostic value to the TNM clinical stage. These results suggest that expression of Dicer1 may sever as a prognostic biomarker to guide clinical personalized therapy in NPC.
Dicer1, as a member of microRNA biogenesis machinery, cleaves pre-microRNA and dsRNA into mature microRNAs and siRNAs [9, 10]. Alterations in microRNA expression have important roles in human tumorigenesis, including the development and progression of nasopharyngeal carcinoma [29–31]. Previous studies have showed that the disruption of Dicer1 results in the loss of mature microRNAs, indicating that Dicer1 is critical for microRNA maturation and may implicate as a possible cause of microRNA alterations between cancer and normal tissues [13, 32]. Dysregulated expression of Dicer1 has been previously demonstrated in a range of human cancers, and these alterations might be involved in tumorigenesis and progression [11–20]. In the present study, we examined the protein expression of Dicer1 in a large number of NPC specimens and found that the protein expression of Dicer1 was downregulated in NPC tissues, which was consistent with the results of a recent report [33]. In addition, we revealed that Dicer1 was also downregulated at mRNA level in NPC tissues compared to the control NCN tissues. These results suggested that downregulated expression of Dicer1 may have important roles in NPC tumorigenesis and progression.
As we know, the prognostic assessment is critical for making better therapeutic choice. Although multiple refinements to the TNM staging system have been made, it is still far from accurately predicting survival in NPC patients [4, 5]. Adding molecular biomarkers to the prognostic prediction system has been suggested, and various biomarkers have been analyzed in NPC [6–8]. However, an ideal marker is still lack. Recently, several studies indicate that Dicer1 has important roles in tumorigenesis and prognosis in a range of cancers [11–20]. In this study, we found that low expression of Dicer1 was significantly associated with poor OS and DMFS in NPC patients. Stratified analysis showed that low expression of Dicer1 was significantly associated with survival in advanced disease stage (i.e., stage III–IV), while not significant in early disease stage (stage I–II). Furthermore, multivariate analysis showed that low expression of Dicer1 was an independent prognostic factor. In addition, combining the Dicer1 expression and TNM stage had a better prognostic value than the TNM stage alone. These results indicated that low expression of Dicer1 was an unfavorable prognostic biomarker in NPC patients.
With the advent of intensity-modulated radiotherapy, local control of NPC has improved significantly, and distant metastasis is now the major cause of treatment failure and patient death [34]. Thirty percent of patients suffer treatment failure due to distant metastasis [35], indicating that a subgroup of patients may have an aggressive signature and that identification of these patients will improve the prognostic model and lead to a more personalized therapy. In present study, we constructed a prognostic score model using the significant variables from the multivariate analysis. ROC analysis showed that the model combining Dicer1 expression and TNM stage had a better prognostic value than the TNM stage alone and the Dicer1 expression alone models. These results suggested that the prognostic score model might be used as a more useful tool for identifying patients who are at high risk of metastasis or treatment failure at the time of diagnosis and give more aggressive therapies, such as more effective chemotherapy, targeted therapy, and biotherapy.
In addition, new intensive treatment strategies need to be designed for these patients. A recent study reported that downregulation of Dicer1 enhances the invasion of tumor cells by activating the p-AKT signal pathway [36]. Other studies demonstrated that miR-103/107 inhibits the expression of Dicer1, causing global microRNA downregulation, which fosters breast cancer cell acquisition with the mesenchymal characteristics needed for migration and metastatic dissemination [37]. Merritt et al. [11] found that cells with a low Dicer1 expression level could not effectively silence genes when synthetic shRNA constructs were transfected. These results suggested that the molecular mechanisms by which Dicer1 functioned in tumorigenesis are complicated, and further work is needed to investigate and understand the role of Dicer1 in the development and progression of NPC, which may provide additional targets and strategies for NPC treatment.
The mechanism of dysregulated expression of Dicer1 in human cancers, including NPC, appears to be complicated. Recently, Chiosea et al. [15] reported that the decreased Dicer1 expression in lung carcinoma was caused by a deletion at the Dicer1 locus, whereas Karube et al. [14] found that the methylation of CpG sites in the promoter region of Dicer1 altered its expression. The human Dicer1 gene is located at the subtelomeric region of chromosome 14 (14q32.13), which carries a cluster of genes affected by allelic deletion, rearrangement, and mutation in various tumors. Interestingly, loss of heterozygosity (LOH) was found in a high percentage of NPC on chromosome 14q, specifically at 14q32 [38, 39]. This region is the site of the Dicer1 locus, suggesting that the underlying mechanism involved in decreased Dicer1 expression in NPC maybe due to genomic instability at this region.
Conclusions
In summary, this study revealed that Dicer1 is downregulated in NPC and demonstrated that low expression of Dicer1 was significantly associated with poor prognosis of NPC patients. Furthermore, a prognostic score model combining the Dicer1 expression and TNM stage had a better prognostic value than the TNM stage alone. These results indicate that Dicer1 can sever as a novel biomarker to better predict prognosis and appropriate treatment. However, our findings need to be confirmed in a different patient population, and further studies are needed to define the mechanism of Dicer1 involving in the NPC tumorigenesis and progression.
References
Curado MP, Edwards BK. Cancer incidence in five continents. Vol IX. IARC Scientific Pub. No. 160. Lyon: IARC; 2007.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Edge SB, Byrd DR. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
Au JS, Law CK, Foo W, Lau WH. In-depth evaluation of the AJCC/UICC 1997 staging system of nasopharyngeal carcinoma: prognostic homogeneity and proposed refinements. Int J Radiat Oncol Biol Phys. 2003;56:413–26.
Mao YP, Xie FY, Liu LZ, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2009;73:1326–34.
Leung SF, Zee B, Ma BB, et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol. 2006;24:5414–8.
Turen S, Ozyar E, Altundag K, Gullu I, Atahan IL. Serum lactate dehydrogenase level is a prognostic factor in patients with locoregionally advanced nasopharyngeal carcinoma treated with chemoradiotherapy. Cancer Invest. 2007;25:315–21.
Lv X, Xiang YQ, Cao SM, et al. Prospective validation of the prognostic value of elevated serum vascular endothelial growth factor in patients with nasopharyngeal carcinoma: more distant metastases and shorter overall survival after treatment. Head Neck. 2011;33:780–5.
Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–6.
Hammond SM. Dicing and slicing: the core machinery of the RNA interference pathway. FEBS Lett. 2005;579:5822–9.
Merritt WM, Lin YG, Han LY, et al. Dicer1, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med. 2008;359:2641–50.
Flavin RJ, Smyth PC, Finn SP, et al. Altered eIF6 and Dicer1 expression is associated with clinicopathological features in ovarian serous carcinoma patients. Mod Pathol. 2008;21:676–84.
Faggad A, Budczies J, Tchernitsa O, et al. Prognostic significance of Dicer1 expression in ovarian cancer-link to global microRNA changes and oestrogen receptor expression. J Pathol. 2010;220:382–91.
Karube Y, Tanaka H, Osada H, et al. Reduced expression of Dicer1 associated with poor prognosis in lung cancer patients. Cancer Sci. 2005;96:111–5.
Chiosea S, Jelezcova E, Chandran U, et al. Overexpression of Dicer1 in precursor lesions of lung adenocarcinoma. Cancer Res. 2007;67:2345–50.
Sugito N, Ishiguro H, Kuwabara Y, et al. RNASEN regulates cell proliferation and affects survival in esophageal cancer patients. Clin Cancer Res. 2006;12:7322–8.
Chiosea S, Jelezcova E, Chandran U, et al. Up-regulation of Dicer1, a component of the MicroRNA machinery, in prostate adenocarcinoma. Am J Pathol. 2006;169:1812–20.
Grelier G, Voirin N, Ay AS, et al. Prognostic value of Dicer1 expression in human breast cancers and association with the mesenchymal phenotype. Br J Cancer. 2009;101:673–83.
Zighelboim I, Reinhart AJ, Gao F, et al. Dicer1 expression and outcomes in endometrioid endometrial adenocarcinoma. Cancer. 2011;117:1446–53.
Faber C, Horst D, Hlubek F, Kirchner T. Overexpression of Dicer1 predicts poor survival in colorectal cancer. Eur J Cancer. 2011;47:1414–9.
Ma J, Liu L, Tang L, et al. Retropharyngeal lymph node metastasis in nasopharyngeal carcinoma: prognostic value and staging categories. Clin Cancer Res. 2007;13:1445–52.
Chen Y, Liu MZ, Liang SB, et al. Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of china. Int J Radiat Oncol Biol Phys. 2008;71:1356–64.
Chan AT, Teo PM, Ngan RK, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 2002;20:2038–44.
Li W, Yu CP, Xia JT, et al. Sphingosine kinase 1 is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2009;15:1393–9.
Liao WT, Wang X, Xu LH, et al. Centromere protein H is a novel prognostic marker for human nonsmall cell lung cancer progression and overall patient survival. Cancer. 2009;115:1507–17.
Korbler T, Grskovic M, Dominis M. Antica M (2004) A simple method for RNA isolation from formalin-fixed and paraffin-embedded lymphatic tissues. Exp Mol Pathol. 2003;74:336–40.
Marshall OJ. PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics. 2004;20:2471–2.
Yang HI, Yuen MF, Chan HLY, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of the predictive score. Lancet Oncol. 2011;12:568–74.
Liu N, Chen NY, Cui RX, et al. Prognostic value of a microRNA signature in nasopharyngeal carcinoma: a microRNA expression analysis. Lancet Oncol. 2012;13:633–41.
Sengupta S, Den Boon JA, Chen IH, et al. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci USA. 2008;105:5874–8.
Chen HC, Chen GH, Chen YH, et al. MicroRNA deregulation and pathway alterations in nasopharyngeal carcinoma. Br J Cancer. 2009;100:1002–11.
Cheng C, Fu X, Alves P, Gerstein M. mRNA expression profiles show differential regulatory effects of microRNAs between estrogen receptor-positive and estrogen receptor-negative breast cancer. Genome Biol. 2009;10:R90.
Guo X, Liao Q, Chen P, et al. The microRNA-processing enzymes: Drosha and Dicer1 can predict prognosis of nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2012;138:49–56.
Lai SZ, Li WF, Chen L, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80:661–8.
Hui EP, Leung SF, Au JS, et al. Lung metastasis alone in nasopharyngeal carcinoma: a relatively favorable prognostic group. A study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Cancer. 2004;101:300–6.
Han L, Zhang A, Zhou X, et al. Downregulation of Dicer1 enhances tumor cell proliferation and invasion. Int J Oncol. 2010;37:299–305.
Martello G, Rosato A, Ferrari F, et al. A MicroRNA targeting Dicer1 for metastasis control. Cell. 2010;141:1195–207.
Shao JY, Huang XM, Yu XJ, et al. Loss of heterozygosity and its correlation with clinical outcome and Epstein-Barr virus infection in nasopharyngeal carcinoma. Anticancer Res. 2001;21:3021–9.
Cheng R, Lo K, Huang D, Tsao S. Loss of heterozygosity on chromosome 14 in primary nasopharyngeal carcinoma. Int J Oncol. 1997;10:1047–50.
Acknowledgments
This work was supported by grants from the Science Foundation of the Key Hospital Clinical Program of the Ministry of Health, P.R. China (No. 2010-178), the National Natural Science Foundation of China (No. 81071835, 31170151), the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2010), and the Key Scientific and Technological Innovation Program for Universities of Guangdong Province (No. cxzd1005).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Na Liu and Rui-Xue Cui contributed equally to this article.
Rights and permissions
About this article
Cite this article
Liu, N., Cui, RX., He, QM. et al. Reduced expression of Dicer11 is associated with poor prognosis in patients with nasopharyngeal carcinoma. Med Oncol 30, 360 (2013). https://doi.org/10.1007/s12032-012-0360-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-012-0360-3