Abstract
Correlation between clinicopathogenetic features and the expression of specific miRNAs is unclear in papillary thyroid carcinoma (PTC). We therefore sought to assess whether miR-221 was associated with aggressive clinicopathologic characteristics and the BRAF mutation. We studied the expression levels of miR-221 using northern blot quantitated by scion image in 51 cases of PTCs. The status of BRAF of PTCs was analyzed through direct DNA sequencing. Mann–Whitney U test was used to analyze different expression of miR-221 in PTCs with distinct clinicopathogenetic characteristics including gender, age, tumor size, multifocality, extrathyroidal invasion, disease stages, node metastasis, and BRAF status. Compared with the normal thyroid tissues, the relative expression of miR-221 in tumor tissues was significantly upregulated (p < 0.001). Overexpression of miR-221 was significantly associated with extrathyroidal invasion (p = 0.001), lymph node metastasis (p = 0.046), advanced disease stages III–IV (p = 0.001), and the BRAF mutation (p = 0.014). While among BRAF wild tumors, miR-221 was only associated with extarthyroidal invasion, it showed strong association with all above aggressive features among BRAF mutation tumors. MiR-221 may be of potential importance in determining the aggressive properties of PTCs including the BRAF mutation, and it may further refine the risk stratification by BRAF mutation in PTCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy, whose biological features and clinical outcomes vary considerably. Certain clinical and pathological features such as advanced tumor, node, metastasis system (TNM) stages, multifocality, extrathyroidal extension, and lymph node metastasis have been associated with a poor prognosis of this disease [1]. The T1799A BRAF mutation is the most common genetic mutation in PTCs (50 % on average), which is reported associated with more aggressive properties such as cervical lymph node metastases, extrathyroidal invasion, advanced stage at diagnosis, and tumor recurrence [2].
Micro-RNAs (miRNAs) are small noncoding RNA molecules that function as negative regulators of gene expression by binding to the 3′-untranslated region of target mRNAs and by blocking the translation or degradation of the mRNAs that mediate various pathophysiological processes including the pathogenesis of diverse human cancers [3]. Although recent studies on miRNA deregulation have demonstrated an increased aberrant miRNA expression (particularly, miR-221 which was also reported in our lab) in PTCs compared with normal thyroid tissues [4–7], little has been reported on the association of the clinicopathogenetic features of PTC with specific miRNA expression [8, 9].
In this study, we investigated the differences of the expression patterns of miR-221 in PTCs with distinct clinicopathogenetic features using northern blot, attempting to elucidate whether deregulated expression of miR221 has any association with aggressive clinicopathologic characteristics and the BRAF mutation.
Materials and methods
Patients and clinicopathological features
Our study was performed at the First Affiliated Hospital of Wenzhou Medical College from January 2009 to December 2010 with the approval of our institutional review board and patient consent. The study population included 51 PTC patients (mean age, 43 years; range, 16–71 years), who underwent total thyroidectomy and modified neck dissection. All patients had no history of neck irradiation and underwent preoperative US and US-guided fine-needle aspiration biology (FNAB) whose diagnosis was PTC. The PTC patients included 31 women (mean age, 44 years; range, 16–69 years) and 20 men (mean age, 41 years; range, 22–71 years). The mean ± standard deviation tumor size was 1.9 ± 1.1 cm (range, 0.5–5.0 cm). Final histological classifications and findings were made by two pathologists according to the 2002 edition AJCC pathological classification of thyroid tumors. Clinicopathologic data were available for each of the 51 patients, including tumor size, extrathyroid invasion, node metastasis, multifocality and disease stage (III–IV defined as advanced stage, while I–II as low stage). Details of the clinicopathologic features of the PTCs in this study are presented in Table 1.
Tissue samples
Fresh thyroid samples including tumor tissues and paired normal thyroid tissues (which were more than 1 cm away from cancer and confirmed by pathology) from 51 PTC patients were collected. The samples were snap-frozen in liquid nitrogen at the time of thyroidectomy and subsequently stored at −80 °C. The corresponding formalin-fixed paraffin-embedded (FFPE) tissues samples were obtained at the Department of Pathology, First Affiliated Hospital of Wenzhou Medical College after Institutional Review Board approval.
BRAF mutation analysis
DNA was extracted from the Paraffin-embedded tissues with a QIAamp DNA FFPE Tissue Kit (QIAGEN), according to the manufacturer’s protocol. We amplified the BRAF exon 15 by polymerase chain reaction (PCR) with the following primers designed by Gu et al. [10]: forward, 5′-TCATAATGCTTGCTCTGATAGGA-3, reverse, 5′-GGCCAAAAATTTAATCAGTGG-3′. The amplicon size was 215 bp. The PCR conditions were: initial denaturation at 94 °C for 2 min, followed by 35 cycles of denaturation at 94 °C for 15 s, annealing at 60 °C for 30 s, and elongation at 68 °C for 20 s. The specificity and integrity of the PCR were confirmed by visualization of a single-band PCR product with the expected molecular weight on a 1.5 % agarose gel. The samples were analyzed on an ABI PRISM 3700 DNA Analyzer (Applied Biosystems) to identify the mutation.
RNA extraction
Total RNA was extracted from surgical specimens using Trizol reagent (Invitrogen). In brief, 0.5 mL Trizol reagent was added to 20 mg of each surgical specimen which was crushed to powder in liquid nitrogen to extract RNA without any DNA and protein contamination. The samples were thoroughly vortexed and 0.2 mL chloroform was added for phase separation. The samples were then centrifuged at 12,000 g for 10 min and the upper aqueous phase was transferred to fresh diethyl phosphorocyanidate-treated Eppendorf tubes to which an equal volume of isopropanol was added for RNA precipitation at −20 °C for 1 h. RNA was harvested by centrifugation at 12,000 g for 10 min at 48 °C, followed by 75 % ethanol precipitation. 20ul DEPC-treated distilled water was used to dissolve RNA. RNA concentration and purity were calculated according to spectrophotometric (Thermo) detection of RNA solution A260/A280 absorbance. A260/A280 ratio >1.8 was suitable for further experiment and the integrity of RNA was detected by the l % agarose denaturing gel electrophoresis.
Northern blot
RNA samples were cooled on ice immediately after thermal denaturation at 95 °C for 3 min. Following pre-electrophoresis of 15 % PAGE gel at 180 V for 30 min, 5 mg of total RNA was loaded per gel lane and electrophoresis was performed at 180 V for 60 min. Then, a “sandwich” structure which was formed by PAGE gel, filter paper, and nylon membrane was transmembraned at 100 mA for 60 min. Further, nylon membrane was cross-linked at UV for 2 min. The Digoxigenin (Dig)-marked miR-221 probe (5′-GAAACCCAGCAGACAAUGTAGCU-3′) and the internal reference U6 probe (5′-GCAGGGGCCAUGCUAAUCUUCUCUGUAUCG-3′) were chemically synthesized by Shanghai Invitrogen Biotechnology Co. Ltd and added into hybridization solution at 42 °C over night after prehybridization at 68 °C for 30 min. After hybridization was completed, 2× SSC (including 0.1 % SDS) and washing buffer were used to wash the membrane at 42 °C for three times and at room temperature for 10 min, respectively. Then, blocking buffer was used to block for 30 min. Further, Dig-AP antibody was bound at room temperature for 1 h and washing buffer was used to wash the membrane for 3 times. Finally, hybridization signals were visualized by radiography and quantitated using densitometry (scion image, Scion, Frederick, MD) and a Kodak gray color scale as a standard [11, 12]. MiR-221 levels were normalized to U6 to correct loading variation. The band intensity of U6 was set as 1 and the relative band intensity of miR-221/U6 was used to reveal the miR-221 expression in samples.
Statistical analysis
Age, size, and other data have been indicated as the mean and standard deviation and analyzed using Student’s t test. Data of miR-221 expression have been indicated as the median and interquartile range. The difference in miR-221 expression levels between PTC samples and matched normal tissue samples or subgroups classified according to the different clinicopathogenetic features was analyzed using Mann–Whitney U test. The BRAF classification data were analyzed by chi-square test. All statistical analyses were performed using the Statistical Package for Social Science program (SPSS for Windows, version 13.0). Differences of p < 0.05 were considered statistically significant.
Results
miR-221 is up-regulated in PTCs compared with that in corresponding paraneoplastic normal tissues
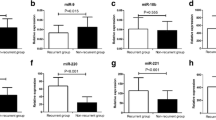
Northern blot results detected that the expression of miR-221 in PTC tissues was higher than that in paired adjacent normal tissues (Fig. 1). There were significant differences of miR-221 relative expression between the two groups (p < 0.001) (Fig. 2).
Different miR-221 expression levels in normal tissues and PTC tissues. Mann–Whitney U test (Z = −6.606, p < 0.001) revealed that the miR-221 expression in PTCs (median = 1.522, interquartile range 1.248–1.899) was significantly higher than that in adjacent normal tissues (median = 0.612, interquartile range 0.401–1.040)
BRAF mutation is associated with aggressive clinicopathologic characteristics in PTC
The BRAF mutation was found in 51.0 % (26/51) of all cases. Patients with wild BRAF status were significantly younger than that with BRAF mutation (p < 0.001). BRAF mutation was associated with advanced TNM stage (p = 0.015) and node metastasis (p = 0.040) and had no correlation with the sex, tumor size, multfocality and extrathyroid invasion (Table 1).
Over-expression of miR-221 is associated with aggressive clinicopathogenetic characteristics in PTC
Analysis of association of miR-221 expression levels in 51 PTCs with different clinicopathogenetical features was shown in Table 2. The expression levels of miR-221 were significantly associated with extrathyroidal invasion (p = 0.001), node metastasis (p = 0.046), and advanced stage (p = 0.001). However, it had no correlation with age, sex, tumor size and multifocality. The miR-221 expression levels in PTCs with BRAF mutation were significantly higher than those without this mutation (p = 0.014) (Fig. 3).
Different miR-221 expression levels in PTCs with BRAF mutation and PTCs without BRAF mutation. Mann–Whitney U test (Z = −2.459, p = 0.014) revealed that the miR-221 expression in PTCs with BRAF mutation (median = 1.650, interquartile range 1.370–1.984) was significantly higher than that in PTCs without BRAF mutation (median = 1.424, interquartile range 1.071–1.659)
Overexpression of miR-221 further refines the risk stratification by BRAF mutation in PTC
We further investigated the correlation between miR-221 expression and clinicopathologic features in BRAF mutation group and BRAF wild group, respectively. In BRAF mutation group, miR-221 was significantly upregulated in PTCs with advanced disease stage III–IV compared with in PTCs with low stage I–II (p = 0.010) (Fig. 4), while overexpression of miR-221 was associated with aggressive clinicopathologic features including tumor size (p = 0.007), extrathyroid invasion (p = 0.022), and node metastasis (p = 0.011). However, in BRAF wild group, extrathyroid invasion was the only aggressive clinicopathologic characters associated with miR-221 expression (p = 0.016) (Table 3).
Different miR-221 expression levels in different stages in PTCs with BRAF mutation. Mann–Whitney U test (p = 0.010) revealed that the miR-221 expression in advanced stage (median = 1.945, interquartile range 1.875–2.476) was significantly higher than that in low stage (median = 1.389, interquartile range 1.317–1.650) in PTCs with BRAF mutation
Discussion
PTC represents the most common thyroid cancer, with incidence increasing each year worldwide. Although PTC generally has excellent clinical outcomes, a subset of patients will have aggressive disease characterized by local recurrence and/or distant metastasis. Improved risk stratification at the time of diagnosis can help guide clinical management by optimizing surgery, adjuvant therapy, and long-term follow-up. The AJCC staging system is commonly used for stratifying recurrence risk and BRAF mutation (incidence of 28–69 % [13, 14]) has been proposed as a candidate for improved tumor risk stratification, which is reported associated with more aggressive properties such as cervical lymph node metastases, extrathyroidal invasion, advanced stage at diagnosis, and tumor recurrence [2]. Recently, specific miRNAs have been also proposed as distinguishing markers for PTC [4–7], but the association of the clinicopathogenetic features of PTC with these miRNAs is less revealed [8, 9]. In this study, we have shown that overexpression of miR-221 which particularly reported in our lab is not only associated with PTC carcinogenesis, but also related to aggressive clinicopathogenetic features of PTCs using northern blot. The potential importance of miR-221 for further refining the risk stratification by BRAF mutation in PTC was highlighted.
We confirmed that miR-221 was upregulated in PTCs compared to the matched normal thyroid tissue using northern blot which was consistent with other reports [4–7]. Further study obtained the similar results reported by Chou et al. [8] that the expression level of miR-221 was significantly higher in PTCs with aggressive clinicopathologic features including extrathyroidal invasion and advanced disease stage. In addition, we firstly prompted that overexpression of miR-221 was associated with node metastasis, which was not shown in the previous study. [8] The inconsistence may be due to our different methods for detecting miRNAs expression. All these findings support the hypothesis that alterations in miRNA expression may provide cancer cell with a selective growth advantage by altering the expression profile of a series of genes, allowing them to develop aggressive properties. Therefore, identification of target genes is crucial for understanding the function of miRNAs in PTC. Visone et al. [15] demonstrated that miR-221 inhibited the expression of cyclin-dependent kinase inhibitor protein p27kip1 and promoted cancer cell proliferation. Garofalo et al. [16] reported that miR-221 finally lead to cancer invasion and metastasis by inhibiting the expression of PTEN and TIMP3. Further studies were needed to clarify miR-221 function in PTC.
We also evaluated the role of BRAF mutation for risk stratification in PTC. Early in 2007, Xing et al. [2] pointed out that though reports on the relationship between BRAF mutation and clinicopathologic factors is not very consistent, most PTCs with BRAF mutation have one or more aggressive clinicopathologic features including node metastasis, extrathyroidal invasion, advanced disease stage and so on. In our study, BRAF mutation (our incidence of 51.0 %) was more common in PTCs with old age (≥45 years), advanced disease stage and node metastasis, but was not interrelated with extrathyroidal invasion, multifocality, tumor size, and gender. Interestingly, not all PTCs with BRAF mutation demonstrate aggressive biologic behavior, suggesting that BRAF status is not the only predictor of aggressive biologic behavior. In this study, we observed miR-221 was significantly upregulated in PTCs harboring BRAF mutation compared with those without the mutation. This result may be inconsistent with the result that only miR-146b was associated with BRAF mutation reported by Chou et al. [8], but agreed with Nikiforova et al. [5]. Furthermore, miR-221 was significantly overexpressed in the BRAF-positive PTCs that also had aggressive tumor behaviors like greater tumor size, advanced disease stage, extrathyroid invasion, and node metastasis. The positive result also showed in PTCs with extrathyroid invasion in our cohort of BRAF wild group. These findings suggest that miR-221 could be an additional molecular marker due to the potential importance for further refining the risk stratification by BRAF mutation in PTC.
Although we demonstrated the correlation of BRAF mutation with miR-221 expression, the related molecular mechanism is far from understood. Given there is not found pairing sequence in 3′-untranslated region of BRAF mRNAs binding to miR-221, BRAF may not be a target gene of miR-221. The significant association between overexpression of miR-221 and PTCs harboring BRAF mutation or with extrathyroidal invasion or with advanced tumor stage or with node metastasis is compatible with the hypothesis that miR-221 might be a downstream gene of BRAF mutation. It has been shown that BRAF V600E activates not only MAPK but also the nuclear factor-kappaB (NF-kB) signaling pathway resulting in apoptotic resistance and promotes cancer invasion [17, 18]. In fact constitutively deregulated NF-kappaB has been seen in anaplastic thyroid cancers [17]. Another report demonstrated that P65 subunit in NF-kB pathway promoted the expression of miR-221 by binding to the distal enhancer regions of miR-221 gene [19]. Therefore, we speculate in the PTC, the BRAF mutation may be regulation of miR-221 expression through NF-kB pathway. Further studies are required to address this issue.
In recent years, growing studies on the assessment of microRNA expression levels in plasma or serum have made progress [20, 21]. Fine-needle aspirate (FNA) specimens were also widely used for detecting the expression of miRNAs [4, 7, 22, 23]. According to our findings, we believe that miR-221 might potentially be an adjunct marker for diagnosis and prognosis in both plasma or serum and FNA specimens. Although our analysis is limited because of the lack of long-term follow-up results, we did highlight the importance of miR-221 in determining the aggressive properties of PTCs and the potential importance of miR-221 for further refining the risk stratification by BRAF mutation in PTC.
In conclusion, overexpression of miR-221 is associated with aggressive clinicopathologic characteristics including advanced disease stage, extrathyroidal invasion, and node metastasis in PTCs. BRAF mutation has also correlation with miR-221, which might regulate miR-221 expression through NF-kB pathway. MiR-221 might potentially be an adjunct marker for diagnosis and prognosis in both plasma or serum and FNA specimens and its use thus may provide further refinement of the risk stratification by BRAF mutation in PTCs.
References
Ito Y, Miyauchi A. Prognostic factors and therapeutic strategies for differentiated carcinomas of the thyroid. Endocr J. 2009;56(2):177–92.
Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28(7):742–62.
Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–69.
Pallante P, Visone R, Ferracin M, Ferraro A, Berlingieri MT, Troncone G, et al. MicroRNA deregulation in human thyroid papillary carcinomas. Endocr Relat Cancer. 2006;13(2):497–508.
Nikiforova MN, Tseng GC, Steward D, Diorio D, Nikiforov YE. MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab. 2008;93(5):1600–8.
He H, Jazdzewski K, Li W, Liyanarachchi S, Nagy R, Volinia S, et al. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci USA. 2005;102(52):19075–80.
Chen YT, Kitabayashi N, Zhou XK, Fahey TJ III, Scognamiglio T. MicroRNA analysis as a potential diagnostic tool for papillary thyroid carcinoma. Mod Pathol. 2008;21(9):1139–46.
Chou CK, Chen RF, Chou FF, Chang HW, Chen YJ, Lee YF, et al. miR-146b is highly expressed in adult papillary thyroid carcinomas with high risk features including extrathyroidal invasion and the BRAF(V600E) mutation. Thyroid. 2010;20(5):489–94.
Yip L, Kelly L, Shuai Y, Armstrong MJ, Nikiforov YE, Carty SE, et al. MicroRNA signature distinguishes the degree of aggressiveness of papillary thyroid carcinoma. Ann Surg Oncol. 2011;18:2035–41.
Gu LQ, Li FY, Zhao L, Liu Y, Zang XX, Wang TX, et al. BRAFV600E mutation and X-linked inhibitor of apoptosis expression in papillary thyroid carcinoma. Thyroid. 2009;19(4):347–54.
Felli N, Fontana L, Pelosi E, Botta R, Bonci D, Facchiano F, et al. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proc Natl Acad Sci USA. 2005;102(50):18081–6.
Mezentsev A, Seta F, Dunn MW, Ono N, Falck JR, Laniado-Schwartzman M. Eicosanoid regulation of vascular endothelial growth factor expression and angiogenesis in microvessel endothelial cells. J Biol Chem. 2002;277(21):18670–6.
Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12(2):245–62.
Ugolini C, Giannini R, Lupi C, Salvatore G, Miccoli P, Proietti A, et al. Presence of BRAF V600E in very early stages of papillary thyroid carcinoma. Thyroid. 2007;17(5):381–8.
Visone R, Russo L, Pallante P, De Martino I, Ferraro A, Leone V, et al. MicroRNAs (miR)-221 and miR-222, both overexpressed in human thyroid papillary carcinomas, regulate p27Kip1 protein levels and cell cycle. Endocr Relat Cancer. 2007;14(3):791–8.
Garofalo M, Di Leva G, Romano G, Nuovo G, Suh SS, Ngankeu A, et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. 2009;16(6):498–509.
Pacifico F, Leonardi A. Role of NF-kappaB in thyroid cancer. Mol Cell Endocrinol. 2010;321(1):29–35.
Bommarito A, Richiusa P, Carissimi E, Pizzolanti G, Rodolico V, Zito G, et al. BRAFV600E mutation, TIMP-1 upregulation, and NF-kappaB activation: closing the loop on the papillary thyroid cancer trilogy. Endocr Relat Cancer. 2011;18(6):669–85.
Galardi S, Mercatelli N, Farace MG, Ciafre SA. NF-kB and c-Jun induce the expression of the oncogenic miR-221 and miR-222 in prostate carcinoma and glioblastoma cells. Nucleic Acids Res. 2011;39(9):3892–902.
Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu Y, et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol. 2010;28(10):1721–6.
Asaga S, Kuo C, Nguyen T, Terpenning M, Giuliano AE, Hoon DS. Direct serum assay for microRNA-21 concentrations in early and advanced breast cancer. Clin Chem. 2011;57(1):84–91.
Mazeh H, Mizrahi I, Halle D, Ilyayev N, Stojadinovic A, Trink B, et al. Development of a microRNA-based molecular assay for the detection of papillary thyroid carcinoma in aspiration biopsy samples. Thyroid. 2011;21(2):111–8.
Kitano M, Rahbari R, Patterson EE, Xiong Y, Prasad NB, Wang Y, et al. Expression profiling of difficult-to-diagnose thyroid histologic subtypes shows distinct expression profiles and identify candidate diagnostic microRNAs. Ann Surg Oncol. 2011;18(12):3443–52.
Acknowledgments
This work was supported by the National High Technology Research and Development plan 863 major projects of China (No. 2012AA020315) and Zhejiang Provincial Natural Science Foundation (Y207526) in China.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhou, YL., Liu, C., Dai, Xx. et al. Overexpression of miR-221 is associated with aggressive clinicopathologic characteristics and the BRAF mutation in papillary thyroid carcinomas. Med Oncol 29, 3360–3366 (2012). https://doi.org/10.1007/s12032-012-0315-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-012-0315-8