Abstract
The forkhead box protein P1 (FOXP1) expression resulted from chromosome translocation was found in MALT lymphoma, and its nuclear expression in diffuse large B cell lymphoma has been believed to be a poor prognostic factor. In our study, FOXP1 expression was investigated in its relationship to the occurrence of large tumor cells, clinical features, and prognosis in a series of 115 MALT lymphomas divided into two groups with or without the large tumor cells. All cases were morphologically reviewed, and FOXP1 expression was detected both in mRNA and protein levels by real-time PCR, immunochemical staining, and Western blot hybridization. All available clinical data were collected. In the MALT lymphoma with large cells, FOXP1 expression was higher at both mRNA (P = 0.008) and protein (P = 0.000) levels than that in group without large cells, and most large tumor cells showed FOXP1 positivity. It was also found that cases beyond Ann Arbor stage I have a higher FOXP1 expression rate than cases in stage I (P = 0.01), moreover, FOXP1-positive group has more plasmacytic differentiation (P = 0.025), deeper filtrating depth in digestive tract (P = 0.039), and a higher Ki67 proliferation index (P = 0.022). However, no statistical significance was identified in the involved anatomic sites and prognosis. Our data demonstrated the close relationship between FOXP1 nuclear expression and the occurrence of large tumor cells in MALT lymphoma, which suggested the possibility of large cell transformation of FOXP1-positive cases. And FOXP1 positivity was associated with enhanced invasion and proliferation ability of tumor cells. In the thyroid cases, the FOXP1 positivity showed a poorer prognosis (P = 0.043), but the significance was not found in the overall survival analysis (P = 0.1123).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The chromosome translocations are demonstrated to be related closely with the oncogenesis and progression of mucosa-associated lymphoid tissue lymphoma (MALT lymphoma), among which the translocations t(11;18)(q21;q21), t(1;14)(p22;q32), and t(14;18)(q32;q21) seem to be more close to this type of B cell non-Hodgkin lymphoma, and the products of the three translocations, API2-MALT1 fusion protein, over-expressed BCL10 and MALT1 proteins, play a role in the signaling pathway leading to the activation of nuclear factor-kappa B [1–6]. In 2005, a novel translocation t(3;14)(p14.1;q32) was found in MALT lymphoma [7]. This translocation puts forkhead box protein P1 (FOXP1), a member of the FOX family, under transcriptional control of promoter of immunoglobin heavy chain (IgH) gene. FOX family includes more than 100 protein members taking part in many important biologic functions such as development of embryo, regulation of cell cycle, metabolism of glucide, aging, and immune regulation. And mutation or abnormal expression of these members was also detected in many human diseases [8, 9].
The expression of FOXP1 is common in many human normal and neoplastic tissues; however, its biological function and mechanism in tumors are still not very clear [10–13]. The recent research on FOXP1 concerned with human diseases focused on breast carcinoma [14] and lymphoma. Although the research results were diverse, the high-level expression of FOXP1 protein did exist in activated B cell lymphocyte and the mantle zone B cells, and some germinal center B cells also showed such a strong positive staining by immunohistochemistry (IHC) [11]. Interestingly, even in diffuse large B cell lymphoma, not otherwise specified (DLBCL-NOS), the significance of FOXP1 over-expression still remained controversial. It is reported that there was no relationship between the expression of FOXP1 and the prognosis; though, the germinal center (GC) DLBCL group had a lower expression (48 %) than non-GC DLBCL group (71 %) [15], but more studies regarded it as a valuable factor predicting for a worse prognosis of DLBCL [16, 17]. Sagaert [18] found that FOXP1 also expressed in some MALT lymphoma and predicted a poorer prognosis.
To estimate the possible significance of expression of FOXP1 in MALT lymphoma, this study investigated a series of MALT lymphoma divided into two groups (with or without large tumor cells). The relationships between strong expression of FOXP1 and the clinical manifestation, histology, and prognosis were studied.
Materials and methods
Patients selection and groups based on large tumor cell existence or not
One hundred and fifteen cases of MALT lymphoma by their first diagnosis were collected from the archival documents of the Department of Pathology, West China Hospital of Sichuan University between 1995 and 2007, all of which were diagnosed by histopathology and IHC according to the WHO classification (2008) [19]. Paraffin-embedded blocks of all 115 cases and frozen tissues of 6 cases were available. Histological examination was performed on routine HE slides. Each sample was studied by two Chinese hematopathologists separately. Immunostaining of CD5, CD10, CD23, and cyclin D1 was used to differentiate selected cases from other low-grade lymphomas, the differentiation criteria were the lack of CD5 and CD23 expression could help to distinguish cases from small B cell lymphoma, the lack of CD10 expression could help to distinguish from follicular lymphoma, and the lack of CD5 and cyclin D1 expression could help to distinguish from mantle cell lymphoma. It was noted when plasmacytic differentiation and monocytoid B-like cells of tumor cell occurred by histology.
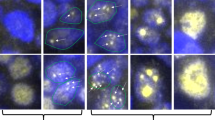
According to the cellular composition of tumor, the 115 cases of MALT lymphoma were divided into two subsets: MALT lymphoma without large tumor cell (n = 77, 70 %) and with large tumor cell (n = 38, 30 %). The former subset, we called group without large cells (MALT lymphoma without LC), histologically presented with predominant small neoplastic centrocyte-like cells and lymphocyte-like cells (Fig. 1a), while the latter subset, called as group with large cells (MALT lymphoma with LC), was a mixed morphological view composed of lymphocyte-like cells, centrocyte-like cells, and variable number of scattered large tumor cells (Fig. 1b) mimic centroblast cells or immuoblast cells (but without proliferation in sheet-like pattern, since such case should be classified as DLBCL-NOS in WHO classification 2008 [19]). The histological study and grouping were also performed by the same two hematopathologists, and the grouping criteria we chose were the large tumor cell presence or not, because in the WHO classification, there is no detailed demanding on the number or percentage of large tumor cell in the MALT lymphoma with large cell transformation.
a MALT lymphoma without LC showed centrocyte-like small tumor cells (×400). b MALT lymphoma with LC showed centroblast-like or immunoblast-like large tumor cells (×400). c Negative expression of FOXP1in MALT lymphoma without LC, note the dimly stained small tumor cells (×400). d Positive expression of FOXP1 in MALT lymphoma with LC, note the strongly stained large tumor cells (×400)
Clinical data
The patients’ records were retrospectively studied, and the collected data including gender, age, clinical symptoms, involved site, radiographic and endoscopic examinations, lymph node involvement, clinical stage, treatment, and follow-up were taken into consideration.
FOXP1 expression
Real-time quantitative PCR for FOXP1 mRNA
cDNA of 101 MALT lymphoma cases (65 cases without LC and 36 with LC) were available for relative expression quantitative detection of FOXP1 mRNA. The target fragment was amplified by PCR in a-20μL reaction containing 1 μL of a 1:10 dilution of cDNA, oligonucleotide primers (Invitrogen, Shanghai, China)at 0.5 μmol/L, and detection was guided by protocol of SYBR® Green Realtime PCR Master Mix Kit (TOYOBO, Osaka, Japan) on Applied Biosystems 7300 Real-Time PCR System(Foster city, CA, USA). The reaction mix was heated to 95 °C for 3 min followed by a two-step thermal cycling protocol: 95 °C for 30 s, 60 °C 1 min for 40 cycles. The melting curve was done after the amplification as following: 95 °C for 15 s, 60 °C for 30 s, then heated to 95 °C at 0.1 °C/0.2 s. Primers are listed in Table 1 (housekeeping gene GAPDH was used as an internal control).
Tissue microarray and immunohistochemical staining for FOXP1 protein
A tissue microarray (TMA) containing 53 cases of MALT lymphoma and 2 cases of reactive hyperplasia of lymph node was prepared for immunostaining.
Four micrometre-formalin-fixed, paraffin-embedded sections of 115 cases (including the TMA and other 62 cases) were immunostained for FOXP1 with the mouse monoclonal antibody JC12 at a dilution 1:80 (kindly donated by Prof. A.H. Banham, University of Oxford, Oxford, UK) using the EliVision™ Plus Kit (MAIXIN.BIO Corp, Fuzhou, China). The scoring of FOXP1 immunostaining was done as mentioned by Banham [17]: 0, <10 % nuclear staining of tumor cells; 1, 10–30 % nuclear expression; 2, 31–50 % nuclear expression; 3, >50 % nuclear expression of tumor cells, and scores of 0 and 1(<30 % of the cell positive) were considered negative and scores of 2 and 3(>30 % of the cell positive) were considered positive for FOXP1 expression.
Western blot for FOXP1 protein
Expression level of FOXP1 protein was detected in 8 cases with enough frozen tissue available including 4 cases of MALT lymphoma without LC, 2 cases of MALT lymphoma with LC, and 2 cases of Hashimoto thyroiditis as control. The total proteins from such cases were extracted by using extraction agents (Applygen Technologies Inc, Beijing, China) as described in the supplier’s protocol. Proteins were solubilized in 1 × SDS loading buffer [50 mM Tris (pH6.8), 2 %(w/v)SDS, 0.1 % (w/v)bromphenol blue, 5 %(v/v)glycerol, and 100 mM DTT] and resolved in 10 % acrylamide gels in 1 × SDS running buffer [25 mM Tris, 250 mM glycine, and 0.1 % (w/v)SDS]. The target proteins were transferred to polyvinylidene fluoride membrane (Millipore, Boston, USA) in transfer buffer solution (10 % methanol, 1 × SDS running buffer). Membranes were incubated in blocking buffer for 2 h at room temperature and then incubated in blocking buffer with primary antibody (dilution, JC12 1:30 and GAPDH 1:5,000) at 4 °C overnight. Membranes were washed for 30 min in three changes of wash buffer [1 × TBS, 0.05 %(v/v)Tween20] and then incubated with horseradish peroxidase–conjugated goat antimouse secondary antibody(DAKO) in blocking buffer for 2 h at room temperature. Membranes were washed as before, and protein detected using the Super ECL Plus Detection Reagent (Applygen Technologies Inc, Beijing, China).
Statistical analysis
Analysis of variance was used to analyze the mRNA expression level of FOXP1 and the χ2 or Fisher’s exact test for differences between categorical variables when appropriate. The relationship between the various variables was assessed by using the Spearman rank correlation test, and Kaplan–Meier method was used to estimate survival distributions and log-rank test for the differences of survival distributions. For all tests, significance was accepted when P < 0.05. SPSS 10.0 software (SPSS Inc, Chicago, USA) was used for the data analysis.
Results
Clinical features
In the group without LC, the patients’ age ranged from 25 to 89 years, and the median age was 56 years, with a male-to-female ratio of 1.30:1. Involved sites included stomach (31 cases), thyroid (13), intestine (13), lung (12), and others (8). Fifty-two patients (68 %) presented with Ann Arbor stage I, 22 cases (29 %) with stage II, and only 3 cases (3 %) with Stage III. Lymph node involvement was found in 24 cases. In the group with LC, the patients’ age ranged from 37 to 54 years, and the median age was 58 years, with a male-to-female ratio of 0.65:1. Involved sites included stomach (19 cases), thyroid (13), intestine (5), and lung (1). Twenty-four patients (63 %) presented with Ann Arbor stage I, 14 cases (37 %) with stage II but none with Stage III, and lymph node involvement was found in 11 cases (Table 2). The tumor morphology was showed in Fig. 1.
Ninety-two patients (80 %) had the follow-up data with an overall 5-year survival rate of 74.9 %, of which 24 patients died and 18 of these deaths resulted from disease progression or recurrence. All 92 patients were treated with local lesion resection, and 40 of them accepted additional therapy including chemotherapy, local radiotherapy, or Chinese traditional medicine, and 11 of the 18 deaths accepted additional chemotherapy and radiotherapy, 5 accepted chemotherapy and Chinese traditional medicine, and only 2 cases refused any therapy after surgery. The 5-year survival rate of MALT lymphoma with LC (61.8 %) is lower than that of MALT lymphoma without LC (80.5 %); however, the two survival curves showed no statistical significance (P = 0.0514) (Fig. 2).
Real-time PCR
The mean FOXP1 relative expression level in 65 cases without LC was 0.07 ± 0.045, while the mean expression level in 36 cases with LC was 0.127 ± 0.096.
IHC and Western blot
In group without LC, 14/77(18.2 %) cases had FOXP1-positive staining (Fig. 1c), while in group with LC FOXP1 positivity was found in 32/38(84.2 %) cases, and interestingly, the large tumor cells mostly showed positive staining (Fig. 1d).
Eighty-five kilodalton-target fragment of FOXP1 protein was detected in 6 cases of MALT lymphoma with frozen tissue available, two of them with LC (Fig. 3). Standardized by their respective GAPDH expression, the relative expression amount of FOXP1 protein was listed in Table 3.
Statistical analysis
The statistical significance was found between MALT lymphoma with and without LC both in the expression of FOXP1 mRNA by real-time PCR (P = 0.008) and in the expression of FOXP1 protein by IHC (P = 0.000). Cases beyond stage I has a higher FOXP1 expression (16/36, 44.4 %) than cases in Ann Arbor stage I (30/76, 39.5 %) (P = 0.01).
The specific relationships between FOXP1 immunostaining and clinical features, morphology and prognosis were summarized in Tables 4, 5, 6, 7. The analysis indicated the significant impact of FOXP1 protein expression on large cell occurrence in tumor, plasmacytic differentiation, tumor filtrating depth in digestion tract cases and Ki67 proliferation index.
In our study, no significance was found in the overall survival curves between FOXP1 positive and negative groups (P = 0.1123), though the positive group seemed a poorer outcome (5-year survival rate of 70.62 %) than the negative group (5-year survival rate of 78.43 %) (Fig. 4). But the significance of survival curves between FOXP1 positive and negative groups was found in the MALT lymphoma cases of thyroid (P = 0.043) (Fig. 5), while such statistical difference was not found in the cases of stomach (P = 0.571), lung (P = 0.724), and intestine (P = 0.539).
Discussion
MALT lymphoma is a common disease often occurred in digestive tract, thyroid, lung, and other different anatomic sites. Many cellular components can be seen in this disease and various number of scattered large tumor cells can be found in some cases. In our previous work, we demonstrated these centroblast-like or immunoblast-like large tumor cells and the centrocyte-like small tumor cells derived from the same clone by laser microdissection and sequence analysis on IgH gene rearrangement [20] in MALT lymphoma, however, the mechanism that drives small tumor cell to large tumor cell still remains unclear.
In this study, we chose FOXP1 gene as target and investigated its expression in MALT lymphoma at both mRNA and protein levels. Given the known literatures, research on FOXP1 has been focused on DLBCL, it seems to be a potential indicator for poor prognosis in DLBCL-NOS [16, 17, 21], and some authors think it is an important marker in the molecular subtype of DLBCL-NOS [22]. In the WHO classification (2008) [19], DLBCL-NOS with MALT lymphoma component should be diagnosed when large tumor cells proliferating in solid or sheet-like pattern. So, it’s very interesting that the MALT lymphoma with LC and the DLBCL-NOS with MALT lymphoma component are two different diseases because of their different prognosis, but both of them are composed of the same two tumor components, and the only difference in morphology is the proliferation pattern of the large tumor cells. It is reasonable that the MALT lymphoma with LC and DLBCL-NOS accompanied with MALT lymphoma component belong to the same entity to some extent, but at different stages.
Although the occurrence of scattered large tumor cells won’t change the prognosis apparently in current opinion, we detected the FOXP1 expression in the whole MALT lymphoma cases in order to try to find the possible mechanism about the large cell transformation. In our study, we found a higher FOXP1 expression in MALT lymphoma with LC than that in MALT lymphoma without LC, and more importantly, most of the large tumor cells showed FOXP1 positivity, which suggest strongly FOXP1 may be in association with the large cells of polymorphic cellular components in MALT lymphoma. Based on this, we might suggest a hypothesis that FOXP1 play a role in the transformation process from small tumor cells to large tumor cells, at least, may be a potential molecular marker in the process.
Interestingly, we found that besides the large tumor cells, some centrocyte-like small tumor cells of the MALT lymphoma with LC also showed FOXP1 positivity, and the same positive expression was found even in the tumor cells of some cases of MALT lymphoma without LC, which can be explained if some kind of relationship does exist between the small and large tumor cells of MALT lymphoma. As to our knowledge, the morphologic change usually follows the molecular alteration, so the FOXP1-positive small tumor cells may be the precursor of the large tumor cells, which has some molecular alterations already but without enlargement of cellular size yet. However, we cannot be sure for now whether the FOXP1-positive small tumor cells transform into large cells finally, and experiment on cell lines in vitro can help us to understand the process in our next step study, since our cases accepted treatment such as surgery, chemotherapy, or radiotherapy soon after their first diagnosis that makes difficulty in observing the natural outcome of this disease.
Additionally, we found a higher FOXP1 expression rate in high clinical stage, meanwhile, considering FOXP1 as a transcript regulator, the correlation between FOXP1 expression and proliferation of tumor cell was also analyzed, and the result was as expected, the positive group had a higher cell proliferation index. Interestingly, when the tumor located in digestive tract, the filtrating depth we measured was deeper in FOXP1-positive group than in negative group. All of these findings indicate that FOXP1 expression may enhance the invasion and proliferation ability of tumor cells leading to a worse clinical process. As to the more plasmacytic differentiation in FOXP1-positive group, we think the biologic function of FOXP1 as a regulator may play a role in promoting the differentiation of tumor cells in some way we haven’t known yet.
In our study, no significance of FOXP1 expression was showed in different affected anatomic sites, which means FOXP1 over-expression was not limited only in the cases with MALT lymphoma in thyroid, orbit and skin where the t(3;14)(p14.1;q32) most occurred, but this result may support Streubel’s hypothesis [7] that there must be, besides chromosome translocation, some other unknown pathway promoting over-expression of FOXP1 because translocation was responsible for only part of over-expression of FOXP1 in MALT lymphoma, and even in some cases without the translocation, the FOXP1 expression was also amazingly high. But the mechanism in detail needs more research to be revealed.
Interestingly, we only found the difference of prognosis between FOXP1-positive and FOXP1-negative groups in cases whose primary site was thyroid, but the overall survival curves showed no statistical significance, although the 5-year survival rate of positive group (70.6 %) seemed lower than that of negative group (78.4 %). This result is somewhat different with Sagaert’s research [18], which suggests FOXP1 can predict a poorer prognosis of MALT lymphoma. This difference may be due to the short follow-up period of some patients, and the effect of therapy can also change the natural progression of this disease, so in order to obtain a more precise survival analysis result, a prolonged follow-up period, a widened observation range are thought to be helpful and necessary. In our current results, we do not provide much opinion on the oncogenic mechanism of FOXP1, but we demonstrate the close relationship between FOXP1 over-expression and the occurrence of large tumor cells in MALT lymphoma, and we think it may be a useful marker in MALT lymphoma as in DLBCL-NOS, which may remind us of the possibility of large cell transformation resulting in a worse clinical process when we are encountered with a FOXP1-positive MALT lymphoma case.
References
Ott G, Katzenberger T, Greiner A, et al. The t(11;18)(q21;q21) chromosome translocation is a frequent and specific aberration in low-grade but not high-grade malignant non-Hodgkin’s lymphomas of the mucosa-associated lymphoid tissue(MALT-) type. Cancer Res. 1997;57:3944–8.
Wotherspoon AC, Pan LX, Diss TC, et al. Cytogenetic study of B-cell Lymphoma of mucosa-associated lymphoid tissue. Cancer Genet Cytogenet. 1992;58:35–8.
Streubel B, Lamprecht A, Dierlamm J, et al. T(11;14)(p22;q32) involving IGH and MALT1 is a frequent chromosome aberration in MALT lymphoma. Blood. 2003;101:2334–9.
Liu H, Hamoudi RA, Ye H, et al. t(11;18)(q21;q21) of mucosa-associated lymphoid tissue lymphoma results from illegitimate non-homologous end joining following double strand breaks. Br J Haematol. 2004;125:318–29.
Achuthan R, Bell SM, Leek JP, et al. Novel translocation of the bcl-10 gene in a case of mucosa associated lymphoid tissue lymphoma. Genes Chromosomes Cancer. 2000;29:347–9.
Du MQ, Peng H, Liu H. bcl-10 gene mutation in lymphoma. Blood. 2000;95:3885–90.
Streubel B, Vinatzer U, Lamprecht A, et al. T(3;14)(p14.1;q32) involving IGH and FOXP1 is a novel recurrent chromosomal aberration in MALT lymphoma. Leukemia. 2005;19:652–8.
Weigel D, Jürgens G, Küttner F, et al. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989;57:645–58.
Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250:1–23.
Wang B, Lin D, Li C, et al. Multiple domains define the expression and regulatory properties of Foxp1 forkhead transcriptional repressors. J Biol Chem. 2003;278:24259–68.
Banham AH, Beasley N, Campo E, et al. The FOXP1 winged helix transcription factor is a novel candidate tumor suppressor gene on chromosome 3p. Cancer Res. 2001;61:8820–9.
Hu H, Wang B, Borde M, et al. Foxp1 is an essential transcriptional regulator of B cell development. Nat Immunol. 2006;7:819–25.
Wang B, Weidenfeld J, Lu MM, et al. Foxp1 regulates cardiac outflow tract, endocardial cushion morphogenesis and myocyte proliferation and maturation. Development. 2004;131:4477–87.
Fox SB, Brown P, Han C, et al. Expression of the forkhead transcription factor FOXP1 is associated with estrogen α and improved survival in primary human breast carcinomas. Clin Cancer Res. 2004;10:3521–7.
Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–82.
Barrans SL, Fenton JAL, Banham AH, et al. Strong expression of FOXP1 identifies a distinct subset of diffuse large B-cell lymphoma(DLBCL) patients with poor outcome. Blood. 2004;104:2933–5.
Banham AH, Connors JM, Brown PJ, et al. Expression of the FOXP1 transcription factor is strongly associated with inferior survival in patients with diffuse large B-cell lymphoma. Clin Cancer Res. 2005;11:1065–72.
Sagaert X, Paepe PD, Libbrecht L, et al. Forkhead box protein P1 expression in mucosa-associated lymphoid tissue lymphomas predicts poor prognosis and transformation to diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:2490–7.
Isaacson PG, Chott A, Nakamura S, et al. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2008. p. 214–9.
Jiang W, Li GD, Li L, et al. Clonal relationship between transformed and non-transformed components in mucosa-associated lymphoid tissue lymphoma. [article in Chinese]. Zhonghua Bing Li Xue Za Zhi. 2007;36:736–41.
Hoeller S, Schneider A, Haralambieva E, et al. FOXP1 protein overexpression is associated with inferior outcome in nodal diffuse large B-cell lymphomas with non-germinal centre phenotype, independent of gains and structural aberrations at 3p14.1. Histopathology. 2010;57:73–80.
Choi W, Weisenburger D, Greiner T, et al. A new immunostain algorithm classifies diffuse large B-Cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009;15:5494–502.
Acknowledgments
We wish to give the special thanks to Professor Alison H. Banham (Leukemia Research Fund Immunodiagnostics Unit, Nuffield Department of Clinical Laboratory Sciences, John Radcliffe Hospital, University of Oxford, Headington, Oxford, United Kingdom) for kindly providing us with JC12 antibody against FOXP1. This work was supported by National Natural Science Foundation of China (30370600).
Conflict of interest
We declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, W., Li, L., Tang, Y. et al. Expression of FOXP1 in mucosa-associated lymphoid tissue lymphoma suggests a large tumor cell transformation and predicts a poorer prognosis in the positive thyroid patients. Med Oncol 29, 3352–3359 (2012). https://doi.org/10.1007/s12032-012-0288-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-012-0288-7