Abstract
This phase II trial assessed temsirolimus, an inhibitor of mammalian target of rapamycin (mTOR), as second-line therapy in patients with metastatic transitional carcinoma of the urothelium (TCCU) after failure of platinum containing therapy. From June/2009 to June/2011, we enrolled 15 patients in this trial. Primary endpoint was overall survival, as secondary endpoints we defined time to disease progression, safety and QoL along treatment. Patients with progressive TCCU after prior platinum-based chemotherapy received weekly 25 mg of temsirolimus for 8 weeks. Evaluation for response was accomplished every 8 weeks according to the RECIST criteria, QoL assessment was done every 4 weeks using the QLQ-C30 questionnaire, adverse events (AEs) were recorded and graded using NCI-CTC criteria. Fifteen patients were enrolled in this study, of whom 14 (93 %) were available for activity, safety and QoL assessment. We treated 10 (71 %) male and 4 female (29 %) patients. Median age was 64,7 years (45–76). Patients received on average 13 (3–15) infusions of temsirolimus. As per protocol, no sufficient benefit on overall survival was observed, we early stopped the study after 14 patients. Median time to progression was 2.5 months (77 days), median overall survival was 3.5 months (107 days). Four patients with stable disease were observed. QoL assessment along treatment revealed a reduction of EORTC-QLQ-C30, Global Health Status subscale, from initial 7.86 to 5.00. Temsirolimus was well tolerated. As Grade 3–4 adverse events, we observed fatigue (n = 2), leukopenia (n = 2) and thrombopenia (n = 2). All other adverse events were graded 1–2 in nature. Temsirolimus seems to have poor activity in patients with progressive metastasized TCCU after failure of platinum containing first-line therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transitional cell carcinoma of the urothelium is known to be sensitive for chemotherapy. The two first-line chemotherapy regimens for metastatic urothelial carcinoma that have been widely adopted combine either cisplatin and gemcitabine (GC) or methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) [1]. In metastatic disease, chemotherapy is rarely curative, and most of the clinically localized cancers relapse after first-line therapy. To date, various drugs have been investigated in the second-line setting for metastatic or advanced TCCU, including many of the newer targeted agents that were initially approved for mRCC. So far, only two randomized phase III trials investigating second-line chemotherapy in advanced TCCU have been reported [2, 3]. After years without standard second-line therapy for patients with advanced urothelial cancer, vinflunine was approved in 2009 by the EMA because of a survival advantage of 2.4 months over best supportive care (BSC). Since then, several new drugs have been tested in this configuration. However, due to the lack of convincing results, none passed the phase II setting of clinical investigation.
Although mammalian target of rapamycin (mTOR) inhibition seems to be an attractive therapeutic strategy for TCCU, the development of clinical trials with mTOR inhibitors in this setting has been quite slow. It is known that on the molecular level, cell lines representing advanced tumors differ from those representing superficial cancer cells. Thus in preclinical studies, predominantly advanced cell lines have been investigated. Wu and colleagues investigated 4 different TCCU cell lines, T24, J82 and UMUC-3 as widely used and representative of advanced bladder cancer and RT4 being more representative for superficial tumor cells. They found out that phosphatase and tensin homolog mutations are present in about 30 % of patients with TCCU, and the phosphatidylinositol 3-kinase pathway regulates TCC cell invasion [4]. Animal studies for one mTOR inhibitor, everolimus, suggest remarkable antitumor activity in TCCU, confirming the effects seen in vitro when analyzing transitional carcinoma cell lines representing invasive or metastatic manifestations as UM-UC lines and the 253-JP and 253 J-BV cell lines [5]. A new mTOR inhibitor, temsirolimus, significantly reduced cell viability and induced apoptosis and cell cycle arrest in bladder cancer cell lines [6]. In conclusion, mTOR inhibition was considered to be an attractive therapeutic strategy for this disease.
Herein, we present the results of a phase II clinical trial, which evaluated the clinical activity and tolerability of temsirolimus as second-line treatment after platin failure for patients with locally advanced or metastatic TCCU.
Materials and methods
Patient population
From June/2009 through June/2011, we enrolled 15 patients in this phase II trial.
All patients provided written informed consent prior to enrollment. Eligible patients had to be 18 years or older with a minimum karnofsky performance status (KPS) of 60 %. Pathologically documented and measurable locally advanced or metastatic TCCU of the urinary bladder or upper urinary tract with documented disease progression after first-line platinum-based therapy was required for entering the study. Prior surgical cure was not a requirement for being enrolled. Patients had to be disease-free from other malignancies within the 2 years preceding study entry except basalioma of the skin. Patients who had received chemo- or radiation therapy within the preceding 30 days or who had participated in a clinical trial within the preceding 60 days were not eligible for enrollment. Included patients were required to have acceptable organ function as indicated by a (1) serum creatinine <3.0 mg/dl (2) leukocyte count ≥1,500/mm3 and platelets ≥100,000/mm3; hemoglobin >7 g/dl and (3) SGOT (AST) ≤ three times upper limit of normal.
Study design and treatment plan
The study protocol of this investigator-initiated trial was approved by the local ethics committee and regulatory agencies. We performed this clinical trial in compliance with good clinical practice and the guiding principles of the declaration of Helsinki. This trial was designed as a monocentric, non-randomized, open-label phase II study with the primary endpoint being overall survival. Secondary endpoints included time to disease progression, safety and QoL along treatment using the QLQ-C30 questionnaire [7]. The pre-treatment evaluation included a complete medical history as well as a complete physical examination. Baseline radiographic studies included abdominal and pelvic computerized tomography (CT) scans and chest radiograph. When clinically indicated, additional bone scans, chest CT and head CT were performed. Radiographic studies and definition of target lesions were required to be performed within 21 days of enrollment. Additional baseline studies included electrocardiogram (ECG), complete blood count with differential (CBC), serum chemistries including creatinine, liver functional parameters, T3, T4, TSH blood sugar. Physical examination, ECG and bloodwork were repeated prior to each administration of therapy. Temsirolimus was given i.v. at a dose of 25 mg in a weekly 30-min intravenous infusion for 8 consecutive weeks. Premedication with 50 mg of intravenous dimetindenmaleat or a similar H1 blocker was given approximately 30 min prior to each weekly temsirolimus infusion as prophylaxis against allergic reactions [8]. The patients were evaluated for response every 8 weeks according to the RECIST 1.1 criteria [9]. QoL assessment was done every 4 weeks using the “Global Health Status” section of the QLQ-C30 questionnaire. All adverse events (AEs) were recorded and graded using NCI-CTC criteria. Standard serum chemistries and hematology parameters were monitored weekly. Treatment was continued until disease progression, withdrawal from the study because of unacceptable toxicity, or decrease in QoL score more than 20 scoring points on QLQ-C30 compared to the previous assessment result. Patients with objective remission or stable disease at the end of 8 applications were continued on therapy for at least 8 additional applications or until disease progression. When progression was clinically suspected, radiologic assessment could be performed earlier than every 8 weeks. Therapy with temsirolimus was scheduled to continue indefinitely in patients with either stable or responding disease until disease progression, patient intolerance or at the discretion of the investigator. Following RECIST 1.1, we defined complete response (CR) as the complete disappearance of all clinically detectable target lesions, any pathological lymph node was defined to have reduction to <10 mm. A partial response (PR) was determined by at least 30 % decrease in the sum of diameters of target lesions. Progressive disease (PD) was defined as at least 20 % increase in the sum of diameters of target lesions, taking as reference the smallest sum on study. Additionally, the sum was determined to show an absolute increase of at least 5 mm as well as the appearance of one or more new lesions was defined as progression. Stable disease (SD) was defined as neither sufficient tumor shrinkage to qualify for PR nor sufficient increase to qualify for PD. For statistical considerations, we used a two-stage design as suggested by Gehan et al. [10] to define the total number of patients [10]. We set an OS of 6 months as the target benefit level and choose 40 % as lowest overall response rate of interest. For a total of 25 calculated patients, 14 were planned to be accrued during the first stage and at least 11 during the possible second stage. If none of the first 14 treated patients would reach the target benefit level, the study was predicted to be finished. If 5 or fewer patients with an OS of 6 months were observed by the end of the second stage, then no further investigation of the drug would be considered warranted in this indication.
Time to progression and survival were measured from the date of trial registration to the date of confirmed disease progression and to the date last known alive or of death, respectively. Estimated time to progression and survival were calculated by the Kaplan–Meier method [11].
Results
Patient characteristics
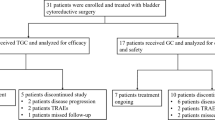
Between June 2009 and June 2011, fifteen patients were accrued to this study. One patient died prior to receiving the study medication. Hence, this patient was therefore deemed ineligible, leaving 14 eligible patients available for activity and safety assessment. The majority of the patients were male, with 21 % of the patients aged >75 years. Mean age of the patients was 66 years (range 45–76). 78.6 % (11/14) of patients had the bladder as their primary site of disease, whereas 22.4 % (3/14) had renal pelvis or ureter as primary tumor localization. Previous ablative surgery was performed in 12/14 (85.7 %) of the treated patients. All patients had previously received systemic platinum-based chemotherapy combinations for metastatic disease. The most common first-line platinum-based chemotherapy regimen was GC (100 %). Additionally, two patients had received chemotherapy combinations involving paclitaxel and carboplatin along the treatment course. Two patients had received radiation therapy. At average, patients received 3.6 (2–10) chemotherapy cycles prior to study inclusion. Disease duration from initial diagnosis to study enrollment was 2.6 years. Patient characteristics are displayed in Table 1.
Response and survival
A total of 107 applications of temsirolimus were administered to the 14 patients resulting in a mean applied total dosis of 188 mg of temsirolimus. The mean number of applications of study treatment was 13 (range 3–15). Four of the 14 eligible patients responded to therapy with transient stabilization of disease. Median overall survival of the group was 3.5 months (107 days). Median time to disease progression was 2.5 months (77 days) (Fig. 1a + b).
QoL/Safety
QoL assessment using the “Global Health Status” of the QLQ-C30 questionnaire revealed a moderate reduction of the score from 7.68 to 5.00 along treatment. Table 2 and Fig. 2 display how patients evaluated their global health status along treatment duration. All treated patients (N = 14) were assessable for toxicity. There were no treatment-related deaths. Overall, therapy with temsirolimus was reasonably well tolerated. Clinically significant hematologic toxicity manifested primarily as thrombocytemia/granulocytopenia and was observed as Grade 3/4 treatment-related toxic effects in 2 patients with thrombocytopenia and 2 patients, with pancytopenia respectively. Treatment interruption because of thrombocytopenia was necessary for 5 weeks in one patient. Fatigue was the only non-hematologic Grade 3 or 4 adverse event, seen in 4 patients. The relevant adverse events are displayed in Table 3.
Discussion
The main treatment objective in the management of patients with advanced, metastasized transitional cell carcinoma remains palliation of disease-related symptoms at minimal additional drug-related toxicity. Many patients with advanced bladder cancer have multiple comorbidities and normally show a reduced performance status after several previous therapies. In this context, novel agents that may have less toxicity are worth being tested in the second-line setting. Primarily in investigator-initiated trials, oncologic activity of temsirolimus was clinically investigated in numerous solid and hematologic cancers. As temsirolimus has shown moderate toxicity profiles in phase I trials, the drug was predominantly tested in malignancies for which few or no effective treatment options exist, including advanced, relapsed or refractory disease. Among the numerous phase II trials with temsirolimus in other tumor entities, there are few comparable studies to the one presented here. A literature search revealed 5 studies that investigated temsirolimus in advanced solid tumors with prior chemotherapy failure that were comparable to the study reported here [12–16]. The drug was mainly administered in doses ranging from 25 to 250 mg; however in those trials, only little or no activity could be attributed to temsirolimus (in contrast to chemo-naive tumors) that is in concordance to our results. On the basis of those findings and the results from a phase II study in mRCC, we decided to apply temsirolimus in the 25-mg dosis in order to reduce possible dose-related additional toxicities [17]. Although response rates (considered the gold standard for testing single agents in previously treated patients) do not always correlate with improved survival, we defined overall survival (OS) as primary endpoint in this trial. Time to progression, safety and QoL were defined as secondary endpoints. As per protocol, this study was closed after enrollment of the minimum required number of 14 patients since the primary endpoint, prolongation of overall survival was not met, and further application of temsirolimus was not considered warranted. Median OS had reached 3.5 months (107 days) at this point and did not reach the overall survival previously described for BSC in a phase III trial evaluating vinflunine as standard treatment for this indication in Europe [2]. While the primary endpoint was not met, nearly one-third of patients (29 %) derived some clinical benefit in terms of transient disease stabilization. The median time to progression of 2.5 months is comparable to durations observed in previously reported studies in the same setting [18]. Thus on this base, temsirolimus had modest activity as second-line treatment in advanced TCCU. When analyzing oncologic activity in clinical trials in the palliative second-line setting of advanced TCCU, appropriate outcome criteria that mostly serve to fit the patients’ real requirements should be implemented into the study design. In our opinion, the aim of chemotherapy treatment in this setting should be a prolonged survival at lowest toxicity and at highest achievable QoL. In contrast to complete remission, the impact of response rates (RR) or stable disease on overall survival is low [19, 20]. Therefore, the data have to be interpreted carefully, and the achieved results in this trial of clinical benefit in 29 % with 4/14 disease stabilizations have to be balanced against the poor OS. Only one patient got benefit in terms of prolonged survival also receiving additional vinflunine therapy [21]. Two recent phase II studies in a similar setting with vinflunine underscore that an elevated RR does not imperatively correlate with improved survival. Culine and co-workers achieved an OS of 6.6 months at a RR of 18 %, whereas a consecutive larger phase II study with 15 % RR ended up in a prolonged OS of 8.8 months. Compared to other second-line studies in TCCU, we note a reduced OS rate in our patients, which may be caused by the poor prognostic features of the patients enrolled here. The moderate reduction in the “Global Health Status” from 7.68 to 5.00 along treatment reflects the appropriate tolerability of the drug in this setting and suggests that at least QoL has not been dramatically decreased by the treatment. This hypothesis is supported by the moderate toxicity profile with a total of only 6 Grade 3/4 AE`s.
Patient selection can significantly influence the oncologic outcome of phase II clinical trials in advanced bladder cancer. Bajorin and co-workers have characterized prognostic factors with impact on survival as KPS and involvement of visceral metastases [22]. In addition to those factors, Bellmunt and colleagues recently identified additional pretreatment prognostic factors for overall survival (OS) in patients with mTCCU after treatment failure with the first-line, platinum-based regimen when reanalyzing results of their phase III vinflunine trial [23]. On the basis of a multivariate and internal validation, they identified three main adverse prognostic factors for OS: (1) ECOG performance status (PS) more than 0, (2) hemoglobin level <10 g/dL and (3) the presence of liver metastases. On that basis, they developed a scoring system that classifies patients with platinum-refractory disease on second-line chemotherapy into four risk groups with different outcome. Although not yet implemented in our trial, this scoring system is likely to be used in the future when designing trials for the second-line setting or for single agent or combination approaches.
Moreover, in this palliative setting, additional factors are likely to influence response rates, including the time to disease progression, the drugs initially received and the setting of initial chemotherapy (adjuvant/neoadjuvant vs therapy for metastatic disease). In this study, 13/14 patients had visceral metastases as unfavorable prognostic marker, none of the patients had a KPS <80, thus according to Bajorin, 93 % of the included patients had one risk factor. It is important to note that patients treated in this study had all been diagnosed with metastatic disease prior to initial chemotherapy, thus decreasing their prognostic disposition prior to first- and second-line therapy.
As vinflunine was approved for second-line therapy while this trial was recruiting, we offered progressive patients this opportunity as third-line treatment when desired and clinically justified. In total, three patients were treated with vinflunine following temsirolimus, one patient received both drugs consecutively over 24 weeks with a disease stabilization of 3.8 months under temsirolimus [21].
Overall, as most of the targeted agents have been or are currently investigated in phase II trials in the salvage setting of mTCCU, the development seems to proceed to investigate combination therapies. The largest concluded phase II trial in the salvage setting of TCCU has been recently published by Choueiri and colleagues who investigated 142 patients in a multi-center randomized double-blind configuration combining docetaxel with vandetanib [24]. However, the combination of both drugs did not show advantage in OS nor ORR compared to Docetaxel and placebo.
Currently, there are few ongoing clinical trials evaluating mTOR-inhibitor everolimus as single agent or in combination in metastatic TCCU. A phase II clinical trial with everolimus as second-line treatment in patients with progressive urothelial cancer after previous cytotoxic chemotherapy has been recently presented. A total of 45 patients have been enrolled. The investigators report a PFS of 3.3 months with 2 partial responses, a median OS of 10.5 months. Toxicity was relevant with a total of 42 grade 3–4 adverse events. On the basis of those data, the authors concluded clinical activity of everolimus in patients with advanced TCCU [25]. In contrast, Seront et al. [26] did not see neither complete nor partial responses when applying everolimus in another second-line phase II trial. SD was found in 3 patients at 8 weeks and was maintained for 234 days in one patient. A total of 10 grade 3–4 toxicities were reported. Finally, the authors concluded modest activity for everolimus in this indication [26]. Besides those two single agent trials, the multi-center phase II clinical trial NCT00933374 evaluates Paclitaxel in combination with everolimus in mTCCU after failure of prior platin-based chemotherapy [27]. The response rate has been claimed primary outcome measure. As secondary goals, the investigators defined progression free survival (PFS), overall survival (OS) and duration of response.
Conclusion
In this first trial evaluating mTOR-inhibitor temsirolimus in the salvage setting of advanced TCCU, the drug has shown little oncologic activity. Temsirolimus did not reach the primary study goal of prolongation of OS. However, as single agent temsirolimus was well tolerated and did not dramatically affect QoL.
Abbreviations
- TCCU:
-
Transitional cell carcinoma of the urothelium
- OS:
-
Overall survival
- TTP:
-
Time to progression
- RR:
-
Response rate
- AE:
-
Adverse event
- QoL:
-
Quality of life
- BSC:
-
Best supportive care
- mRCC:
-
Metastatic renal cell carcinoma
- TWIST:
-
Time without symptoms and
- Q-TWiST:
-
Quality adjusted time without symptoms and toxicity
- CT:
-
Computed tomography
- GC:
-
Gemcitabine/cisplatin
- EMA:
-
European medicines agency
References
Bellmunt J, Albiol S. Chemotherapy for metastatic or unresectable bladder cancer. Semin Oncol. 2007;34:135–44.
Bellmunt J, Theodore C, Demkov T, Komyakov B, Sengelov L, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27:4454–61.
Albers P, Park SI, Niegisch G, Fechner G, Steiner U, et al. Randomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: short-term versus prolonged treatment [German Association of Urological Oncology (AUO) trial AB 20/99]. Ann Oncol. 2011;22:288–94.
Wu X, Obata T, Khan Q, Highshaw RA, De Vere White R, et al. The phosphatidylinositol-3 kinase pathway regulates bladder cancer cell invasion. BJU Int. 2004;93:143–50.
Mansure JJ, Nassim R, Chevalier S, Rocha J, Scarlata E, et al. Inhibition of mammalian target of rapamycin as a therapeutic strategy in the management of bladder cancer. Cancer Biol Ther. 2009;8:2339–47.
Schedel F, Pries R, Thode B, Wollmann B, Wulff S, et al. mTOR inhibitors show promising in vitro activity in bladder cancer and head and neck squamous cell carcinoma. Oncol Rep. 2011;25:763–8.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.
Torisel [package insert]. Philadelphia PWPI.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Gehan EA. The determination of the number of patients required in a preliminary and a follow-up trial of a new chemotherapeutic agent. J Chronic Dis. 1961;13:346–53.
EL Kaplan MP. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Galanis E, Buckner JC, Maurer MJ, Kreisberg JI, Ballman K, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North central cancer treatment group study. J Clin Oncol. 2005;23:5294–304.
Margolin K, Longmate J, Baratta T, Synold T, Christensen S, et al. CCI-779 in metastatic melanoma: a phase II trial of the California cancer consortium. Cancer. 2005;104:1045–8.
Javle MM, Shroff RT, Xiong H, Varadhachary GA, Fogelman D, et al. Inhibition of the mammalian target of rapamycin (mTOR) in advanced pancreatic cancer: results of two phase II studies. BMC Cancer. 2010;10:368.
Pandya KJ, Dahlberg S, Hidalgo M, Cohen RB, Lee MW, et al. A randomized, phase II trial of two dose levels of temsirolimus (CCI-779) in patients with extensive-stage small-cell lung cancer who have responding or stable disease after induction chemotherapy: a trial of the eastern cooperative oncology group (E1500). J Thorac Oncol. 2007;2:1036–41.
Chang SM, Wen P, Cloughesy T, Greenberg H, Schiff D, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–61.
Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–18.
Bachner M, De Santis M. Second-line therapy in bladder cancer. Curr Opin Urol. 2009;19:533–9.
Logothetis CJ, Dexeus FH, Finn L, Sella A, Amato RJ, et al. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol. 1990;8:1050–5.
Igawa M, Ohkuchi T, Ueki T, Ueda M, Okada K, et al. Usefulness and limitations of methotrexate, vinblastine, doxorubicin and cisplatin for the treatment of advanced urothelial cancer. J Urol. 1990;144:662–5.
Gerullis H, Ecke TH, Janusch B, Arndt C, Heidari M, et al. Long-term response in advanced bladder cancer involving the use of temsirolimus and vinflunine after platin resistance. Anticancer Drugs. 2011;22:940–3.
Bajorin DF, Dodd PM, Mazumdar M, Fazzari M, McCaffrey JA, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17:3173–81.
Bellmunt J, Choueiri TK, Fougeray R, Schutz FA, Salhi Y, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28:1850–5.
Choueiri TK, Ross RW, Jacobus S, Vaishampayan U, Yu EY et al. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol. 2012;30(5):507–12.
Milowsky MI, Regazzi AM, Garcia-Grossman IR et al. Final results of a phase II study of everolimus (RAD001) in metastatic transitional cell carcinoma (TCC) of the urothelium. J Clin Oncol. 2011;29(suppl; abstr 4606).
Seront E, Rottey S, Sautois B et al. A single arm, multicenter, phase II trial of everolimus as monotherapy in the palliative treatment of patients with locally advanced or metastatic transitional cell carcinoma after failure of platinum-based chemotherapy. J Clin Oncol. 2010;28(No 15_suppl; abstr e15087).
Acknowledgment
We thank all patients and their families who participated in this study. Appreciation for assisting with data analysis is reserved for Kurt Witt and Antje Gottberg. Sarah Goretzki is appreciated for her help in data acquisition. Special thanks go to Christoph Heuck for language assistance. This trial was registered under Eudra-CT 2008-008478-30.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerullis, H., Eimer, C., Ecke, T.H. et al. A phase II trial of temsirolimus in second-line metastatic urothelial cancer. Med Oncol 29, 2870–2876 (2012). https://doi.org/10.1007/s12032-012-0216-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-012-0216-x