Abstract
We enrolled 45 patients with metastatic renal cell carcinoma (RCC) at a progressive disease between March 2003 and April 2008 to assess the impact of an anti-inflammatory treatment regime in combination with metronomic low-dose chemotherapy. 42% of the patients had been systemically pre-treated. Therapy consisted of etoricoxib 60 mg daily plus pioglitazone 60 mg daily, day 1+, low-dose interferon-α 4.5 MU sc three times a week, week 1+ and low-dose capecitabine 1 g/m2 twice daily orally for 14 days, every 3 weeks, day 1+, until disease progression. Objective response was observed in 35% of the patients (PR 27, CR 9%), which was paralleled by strong CRP decline for all patients with initially elevated CRP levels (n = 32). CRP values decreased from mean 42.3 mg/L (range 9.1–236), to 11.1 mg/L, (range 1.1–35.6), P = 0.006. Median overall survival and progression-free survival for the total cohort were 26.9 and 7.2 months for patients with elevated CRP 24.4 and 11.3 months (95% CI, 22.8–31.0/5.7–16.9) and 13.8–2.6 months (95% CI, 6.5–21.1/0.4–4.8) for the non-elevated CRP group, respectively (P = 0.082/0.017). Median observation time: 26.1 months; Overall survival at 5 years: 18%. Toxicity>WHO grade 3 was reported: Hand-foot syndrome in 16 patients (36%), diarrhea in 4, and pneumonia in 2 patients. Our data allow us to conclude that the control of tumor-associated inflammation is an important therapeutic principle in patients with metastatic RCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The interaction among signaling networks of tumor and neighboring stroma cells constitutes a critical factor in solid tumor growth [1, 2]. However, the mechanisms and complexity of growth signals and its interactions with hypoxia, inflammation, stroma, and tumor microenvironment are largely unexplored. An improved understanding of only one of these pathways—disrupting angiogenesis within the tumor microenvironment—has revolutionised the treatment and management of metastatic renal cell carcinoma (RCC). Intervening in angiogenesis means to tie in multiple pathomechanisms, either tumor cell- or stroma cell-derived. Selected targets in RCC are FMS-like tyrosine kinase 3 (Flt-3), mammalian target of rapamycin (mTOR), platelet-derived growth factor receptor b (PDGFRb), phosphatidylinositol-3-kinase (PI3 K), and vascular endothelial growth factor receptor (VEGFR) [3]. For the majority of the patients, these targeted therapies are associated with a survival benefit over interferon-alpha monotherapy. However, the main benefit of such therapies is inducing stable disease.
Several ways to improve the activity of targeted agents are being explored, such as sequential treatment and combinations with immunotherapy. Bevacizumab, a monoclonal antibody against the VEGF receptor, showed efficacy in the treatment of metastatic RCC when added to interferon-α (IFN-α) [4, 5], and the combination of sorafenib with maximum tolerated gemcitabine and metronomic capecitabine resulted in a clinical benefit rate greater than previously observed with sorafenib monotherapy [6]. Future treatment strategies for advanced RCC will probably incorporate a combination of molecular approaches, using multi-drug regimens consisting of tyrosine kinase inhibitors with biologic therapies or immunomodulatory therapies, or both.
The inflammatory component of the tumor microenvironment represents another potential target for biomodulatory therapy approaches. Biomodulatory therapies are characterised by poor or no monoactivity of single combined drugs. However, concerted single drugs may finally alter the denotation of tumor-associated inflammatory processes by therapeutically focusing on the validity of systems’ features promoting tumor growth [7].
In a previous study we demonstrated that attenuation of tumor-associated inflammation in RCC, as indicated by declining C-reactive protein (CRP) levels, can be linked with objective tumor response [8]. In this historical comparison, the addition of interferon-α to low-dose capecitabine, pioglitazone, rofecoxib, or etoricoxib highlighted the impact of distinct biomodulary acting combination therapies on inflammation control for improving survival: The regimen without interferon may attenuate inflammation but did not have the capacity to induce objective tumor response. In an amendment approved by the local ethic committee, the study on capecitabine, pioglitazone, and etoricoxib plus low-dose interferon-α was extended due to the fact that long-term complete remissions had been observed in non-resectable metastatic RCCs. Here, we report on the final results of 45 patients with metastatic, non-resectable, and partially systemically pre-treated RCC.
Patients and methods
Eligibility
The local ethics committee approved the study protocol, and patients needed to provide written informed consent before enrollment. Eligible patients were required to have progressive metastatic (according to Response Evaluation Criteria in Solid Tumors—RECIST—requirements) and locally recurrent or contralateral non-resectable RCC. If nephrectomy was not indicated because of non-operability, histology was confirmed at a metastatic site. Patients with primarily metastatic disease underwent nephrectomy at least 21 days before initiation of treatment according to protocol. Patients were allowed to have received an unlimited number of previous systemic therapies including chemotherapy and immunotherapy or antiangiogenic agents such as thalidomide and IFN-α, or both (Table 1) IFN-α pretreatment was no exclusion criterion because we suggested synergistic anti-inflammatory activity of pioglitazone/COX-2 inhibitor/IFN-α. Previous treatment with pioglitazone or capecitabine presented an exclusion criterion. The remaining inclusion criteria included those of the Eastern Cooperation Oncology Group (ECOG).
Pre-treatment evaluation
Baseline evaluation included, i.e., the assessment of ECOG performance status, computed tomography scanning of the thorax and abdomen, and facultative bone scanning or CT scanning of the brain, if metastasis was clinically suspected. Patients were subsequently monitored before the start of chemotherapy and every 3 weeks thereafter (assessment of toxicity, serum chemistry assays, one of which measured CRP levels, and a physical examination). For patients continuing study medication, target lesions were assessed (via abdominal ultrasound or chest X-ray) before each 3-week therapy cycle. If these techniques suggested response to treatment or progressive disease, CT scans were taken before the routinely scheduled response evaluations by CT scans in 12-week intervals.
Treatment
Patients received 1 g/m2 oral capecitabine (Roche) administered twice daily from day 1+ 60 mg oral pioglitazone (Takeda), 4.5 MU IFN-α sc. (Roche) 3 times per week, from day 1+, and 60 mg oral etoricoxib (MSD) daily starting with day 1+. Treatment was continued until disease progression was documented or for a maximum of 6 weeks after confirmation of complete remission.
Efficacy assessment
Response was evaluated in patients who had a follow-up duration of ≥3 weeks by the treating physicians and centrally (blinded) by the imaging unit of the University Hospital Regensburg. Response categories were assigned by means of the RECIST criteria [9]. All major responses were reconfirmed in 4- to 6-week intervals. Stable disease was suggested if no tumor progression occurred within 6 months of treatment. Clinical response was defined as stable disease (SD) >6 months, partial response (PR), and complete remission (CR). Data reported represent the best response obtained during treatment according to study protocol.
Dosage modification
Drug administration was paused for grade 2–3 toxicity and resumed at a reduced dosage on resolution to less than grade 2. In case of reoccurrence of dosage-limiting grade 3–4 toxicity, the corresponding drug was discontinued. Capecitabine therapy was continued with a 75% starting dosage for the first and 50% for the second occurrence. IFN-α administration was continued at a dose of 3 MU three times a week, COX-2 inhibitor administration at a dose of 30 mg etoricoxib every day, and pioglitazone at a reduced dose of 45 mg.
Statistical considerations
The current multicenter non-randomised phase II trial was designed to assess (1) objective response, (2) CRP response, and (3) qualitative and quantitative toxicity of the treatment schedules.
The Kaplan–Meier methodology served to analyse time to progression and overall survival (OS). Overall survival and progression-free survival (PFS) were calculated from the initiation of treatment until death or until November 2009 (date of final data analysis), which ever came first. Survival analyses were done on the intent-to-treat population. Patients who died as a result of unrelated causes during therapy or who were lost to follow-up were censored.
Results
Patients’ characteristics
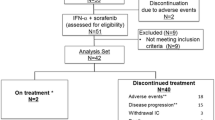
Detailed patient characteristics of the 45 patients with non-respectable metastatic RCC are listed in Table 1.
Treatment
All patients received at least three 3-week cycles of study medication. The median duration of study treatment was 10.5 months (95% CI, 7.2–14.7 months).
Treatment efficacy
All 45 patients were assessable for response. At present, 11 patients are alive (24%), 3 of 4 CR patients with histologically confirmed CR, 5 patients in PR (11%) are still on treatment for 22.0+ to 58.0+, and 2 patients with progressive disease are alive with alternative therapy approaches. Five patients achieving partial remissions with only residual measurable metastatic disease in CT scans had negative positron emission tomography results, probably indicating complete remissions.
Overall clinical response (SD, PR, and CR) was 76% as detailed in Table 2. Objective responses were diagnosed after a median time of 4.5 months (range 2.8–8.7 months). Responses were seen at all major tumor localisations (lung, pancreas, lymph nodes, liver, bone, and contralateral kidney). Metastases of patients with complete response were localised in the lung (n = 3), liver (n = 1), bone (n = 1), and in the lymph nodes (n = 4). All these patients had undergone prior tumor nephrectomy. The clinical response rate of patients who had or had not received previous systemic therapy (n = 19; n = 26) was 53 and 92%, respectively. Two responders received previously IFN-α.
After a median follow-up of 26.1 months, 12- to 24-month progression-free survival rates were 36–16%.
12-, 24-, and 36-month survival rates were 82, 62, and 36%, respectively. The median PFS and OS rate were 7.2 months (95% CI: 3.2–11.1 months) and 26.9 months (95% CI: 22.7–31.0 months) (Fig. 1). Objective response to treatment was observed in all Motzer risk categories.
Progression-free survival and Overall survival of patients with praetherapeutic elevated CRP levels (n = 32) versus patients with normal praetherapeutic CRP levels (n = 13). Progression-free survival and Overall survival of patients with C-reactive protein (CRP) elevation vs. patients without elevated CRP levels
CRP response
CRP levels were available for follow-up in all 45 patients, and 32 patients (67%) had elevated CRP levels. During therapy, CRP levels significantly decreased (>30%) in all patients with initially elevated CRP levels from mean 42.3 mg/L, range 9.1–236, to 11.1 mg/L, range 1.1–35.6 mg/L (P = 0.006). ECOG status improved in 45% of the patients with CRP response.
Evaluation of patients with praetherapeutic elevated CRP levels and patients without baseline CRP increase showed significantly improved PFS (P = 0.017) and a tendency to improved overall survival (P = 0.082) for the elevated CRP level group (Fig. 1).
Tolerability and safety
The treatment regimen aimed at facilitating long-term administration of the entire study medication by a scheduled early dosage reduction in case of toxicity > grade 1.
The main treatment-related toxicity was capecitabine-associated hand-foot-syndrome, which led to a dosage reduction. Secondly, interferon-α dosage had to be reduced. Mild fever reactions and depression were specifically related to the additional administration of low-dose IFN-α. Fatigue after the initiation of interferon-alpha was also observed, albeit less frequently.
Because of renal insufficiency (4 patients) and hypertension (1 patient), COX-2 inhibitors were discontinued after 3–5 treatment cycles. Dosage reduction in pioglitazone became necessary in only two patients due to edema.
Only 2 patients discontinued therapy because of drug-related toxicities after 2.5 months (depression grade 3) and 6 months (hand-foot syndrome grade 3).
Discussion
We can now provide the long-term data of an extended study population treated with a combined anti-inflammatory therapy approach for non-resectable, partially systemically pre-treated metastatic RCC.
Although cancer-related inflammation represents a potential target for innovative diagnostic and therapeutic strategies, clinical approaches to this are just at the beginning. A phase II clinical trial of the TNF-α antagonist infliximab in patients with advanced renal cell carcinoma cancer resulted in disease stabilisation and some partial responses [10], as well did a combination therapy with meloxicam, a COX-2 inhibitor, and natural interferon-alpha [11]. The addition of celecoxib to IFN-alpha in a patient cohort with metastatic RCC disclosed significant association between clinical outcome and maximal COX-2 expression: Objective response was found in a patient subgroup, demonstrating strong COX-2 immunostaining in their kidney tumors [12].
Besides etoricoxib, the transcriptional modulators interferon-alpha and pioglitazone act synergistic in the presented schedule: All drugs have––similar to low-dose capecitabine—only poor monoactivity at the respective dosage levels. Interferon-alpha decisively attenuates inflammation in normal volunteers [13], adding a certain clinical benefit in RCC patients. This benefit was missing in a historical control group that had not received interferon-alpha, although CRP response could be frequently observed in this regimen.
Pioglitazone, a selective ligand of peroxisome Proliferator-activated receptor gamma (PPAR-gamma), can mediate direct antitumoral effects and a broad spectrum of stroma-modulating activity including antiangiogenetic, antiinflammatory, and immunoaugmentative effects [14, 15]. Examples of superadditive complementation of PPAR-gamma agonists by COX2 inhibitors are well documented, experimentally and in clinical trials, respectively [16, 17]. The related targets for the drugs are ubiquitously available in the tumor compartment, and the activity profile of the administered drugs builds upon their ability to regulate systems functions both in tumor and adjacent stroma cells. Adding a low-dose chemotherapeutic drug like Capecetabine, given continuously on a daily basis, appears promising mainly due to the fact that its potential antiangiogenic and antitumorigenic effects are accompanied by low toxicity [6, 18, 19].
C-reactive protein, an acute-phase reactant, has emerged as a promising prognostic tool for RCC: Elevated CRP levels have a negative impact on the overall survival rate in patient populations receiving surgery for primary or metastatic RCC [20–24].
Correlating anti-inflammatory response with therapeutic outcome, the present study provides impressive proof that the resolution or even the attenuation of the tumor-associated inflammatory processes can be identified in time: Objective response to the treatment regime was paralleled by strong CRP decline not later than 4–6 weeks’ treatment. In contrast––and contradictory to current investigations—absence of elevated CRP at baseline was associated with poor treatment outcome.
The study results are confirmatory in every aspect: First, combined anti-inflammatory and angiostatic treatment has the capacity to induce durable, even pathologically confirmed complete remission in metastatic RCC, although 42% of the study population had been systemically pre-treated and 51% of the patients had an ECOG performance status >0. Second, 67% of the included patients with elevated CRP levels at base-line as a poor prognostic parameter [25, 26] demonstrated CRP response greater than 30% or normalisation thereby predicting clinical response and third, clinical response occurred in a range of comparably low toxicity rates.
Comparing PFS and OS of selected second-line TKI studies (Table 3) with the outcome of our systemically pre-treated patient cohort, the results of our study are noteworthy and support the further investigation of this multi-targeted approach as a second-line therapy option in patients with metastatic RCC, who failed to prior TKI therapy.
References
Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–44. doi:10.1038/nature07205.
Solinas G, Marchesi F, Garlanda C, et al. Inflammation-mediated promotion of invasion, metastasis. Cancer Metastasis Rev. 2010;29(2):243–8.
Motzer RJ, Molina AM. Targeting renal cell carcinoma. J Clin Oncol. 2009;10:3274–6. doi:10.1200/JCO.2009.21.8461.
Escudier B, Bellmunt J, Négrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28(13):2144–50. doi:10.1200/JCO.2009.26.7849.
Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol. 2010;28(13):2137–43. doi:10.1200/JCO.2009.26.5561.
Bellmunt J, Trigo JM, Calvo E. Activity of a multitargeted chemo-switch regimen (sorafenib, gemcitabine, and metronomic capecitabine) in metastatic renal-cell carcinoma: a phase 2 study (SOGUG-02–06). Lancet Oncol. 2010;11(4):350–7. doi:10.1016/S1470-2045(09)70383-3.
Reichle A, Vogt T. Systems biology: a therapeutic target for tumor therapy. Cancer Microenviron. 2008;1:159–70. doi:10.1007/s12307-008-0012-5.
Reichle A, Grassinger J, Bross K, et al. C-reactive protein in Patients with Metastatic Clear Cell Renal Carcinoma: An Important Biomarker for Tumor-associated Inflammation. Biomarker Insights. 2007;1:87–98.
Gehan EA, Tefft MC. Will there be resistance to the RECIST (response evaluation criteria in solid tumors) J. Natl Cancer Inst. 2000;92:179–81. doi:10.1093/jnci/92.3.179.
Harrison ML, Obermueller E, Maisey NR, et al. Tumor necrosis factor alpha as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose. J Clin Oncol. 2007;25(29):4542–9. doi:10.1200/JCO.2007.11.2136.
Shinohara N, Kumagai A, Kanagawa K, et al. Multicenter phase II trial of combination therapy with meloxicam, a cox-2 inhibitor, and natural interferon-alpha for metastatic renal cell carcinoma. Jpn J Clin Oncol. 2009;39(11):720–6. doi:10.1093/jjco/hyp089.
Rini BI, Weinberg V, Dunlap S, et al. Maximal COX-2 immunostaining and clinical response to celecoxib and interferon alpha therapy in metastatic renal cell carcinoma. Cancer. 2006;106(3):566–75. doi:10.1002/cncr.21661.
Tilg H, Vogel W, Dinarello CA. Interferon-alpha induces circulating tumor necrosis factor receptor p55 in humans. Blood. 1995;85:433–5.
Koeffler HP. Peroxisome proliferator-activated receptor gamma and cancers. Clin Cancer Res. 2003;9(1):1–9.
Panigrahy D, Huang S, Kieran MW, et al. PPAR-gamma as a therapeutic target for tumor angiogenesis and metastasis. Cancer Biol Ther. 2005;4:687–93.
Hafner C, Reichle A, Vogt T. New indications for established drugs: combined tumor-stroma-targeted cancer therapy with PPARγ agonists, COX-2 inhibitors, mTOR antagonists and metronomic chemotherapy. Current Cancer Drug Targets. 2005;5(6):393–419.
Hazra S, Peebles KA, Sharma S et al. The role of PPARγ in the cyclooxygenase pathway in lung cancer. PPAR Res 2008 doi:10.1155/2008/790568.
Gasparini G. Metronomic scheduling: the future of chemotherapy? Lancet Oncol. 2001;2(12):733–40. doi:10.1016/S1470-2045(01)00587-3.
Sunela KL, Koskinen S, Kellokumpu-Lehtinen P. A phase-II study of combination of pegylated interferon alfa-2a and capecitabine in locally advanced or metastatic renal cell cancer. Cancer Chemother Pharmacol. 2010;66(1):59–67. doi:10.1007/s00280-009-1134-2.
Ljungberg B, Grankvist K, Rasmuson T. Serum interleukin-6 in relation to acute-phase reactants and survival in patients with renal cell carcinoma. Eur J Cancer. 1997;33:1794–8. doi:10.1016/S0959-8049(97)00179-2.
Bromwich E, McMillan DC, Lamb GW, et al. The systemic inflammatory response, performance status and survival in patients undergoing alpha-interferon treatment for advanced renal cancer. Br J Cancer. 2004;91:1236–8. doi:10.1038/sj.bjc.6602152.
Saito K, Tatokoro M, Fujii Y, et al. Impact of C-reactive protein kinetics on survival of patients with metastatic renal cell carcinoma. Eur Urol. 2009;55:1145–53. doi:10.1016/j.eururo.2008.10.012.
Johnson TV, Abbas A, Owen-Smith A, et al. Absolute preoperative C-reactive protein predicts metastasis and mortality in the first year following potentially curative nephrectomy for clear cell carcinoma. J Urol. 2010;193:480–5.
Limura Y, Saito K, Fujii Y, et al. Development and external validation of a new outcome prediction model for patients with clear cell renal cell carcinoma treated with nephrectomy based on preoperative serum C-reactive protein and TNM classification: the TNM-C score. J Urol. 2009;181:1004–12.
Kedar I, Mermershtain W, Ivgi H, et al. Thalidomide reduces serum C-reactive protein and interleukin-6 and induces response to IL-2 in a fraction of metastatic renal cell cancer patients who failed IL-2- based therapy. Int J Cancer. 2004;110:260–5. doi:10.1002/ijc.20089.
Kerr C. Inflammatory response predicts survival in renal cancer. Lancet Oncol. 2006;7:284. doi:10.1016/S1470-2045(06)70632-5.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walter, B., Schrettenbrunner, I., Vogelhuber, M. et al. Pioglitazone, etoricoxib, interferon-α, and metronomic capecitabine for metastatic renal cell carcinoma: final results of a prospective phase II trial. Med Oncol 29, 799–805 (2012). https://doi.org/10.1007/s12032-011-9982-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-9982-0