Abstract
The purpose of this study was to investigate GPC3 gene expression in lung squamous cell carcinoma tissue and its correlation with clinical and tumor characteristics. Using RT–PCR, the presence of GPC3 gene expression was detected in cancer tissue and adjacent normal tissue in 66 cases of lung squamous cell carcinoma and positive rates were calculated. Using Western blot, changes in GPC3 protein expression were detected in lung squamous cell carcinoma and adjacent normal tissues. The percentage of tissue samples expressing GPC3 mRNA was significantly higher in lung squamous cell carcinoma than in adjacent normal tissue (P < 0.05). This percentage was also significantly higher for cases with lymph node metastasis than for those without lymph node metastasis (P < 0.05). Further, the percentage of samples expressing GPC3 mRNA was higher with lowering degrees of tumor differentiation (P < 0.05). Rates of GPC3 expression were, however, independent of patient gender, age, and tumor size (P > 0.05). The expression of GPC3 protein in lung squamous cell carcinoma was significantly higher than that in adjacent normal tissues (P < 0.05). The expression in cases with lymph node metastasis was significantly higher than in those without lymph node metastasis (P < 0.05), and GPC3 protein expression increased with lowering degrees of tumor differentiation (P < 0.05). Further investigation is warranted for the association of initiation, development, invasion, and metastasis of disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite great progress in the treatment of lung cancer, the survival rate remains poor. The main reason for this poor survival is the complex biological characteristics of lung cancer [1]. Currently, lung cancer research focuses on discovering tumor markers involved in the regulation of cell cycle, apoptosis, and tumor angiogenesis.
Phosphatidylinositol proteoglycan-3 (Glypican-3, GPC3) is a member of the heparan sulfate proteoglycan (HSPG) family. Currently, six members (GPC1, GPC2, GPC3, GPC4, GPC5, and GPC6) have been identified in this family. Among them, GPC3 is an extracellular glycoprotein that can regulate cell proliferation as a tumor suppressor gene [2]. Since its discovery in 1995, GPC3 has continued to gain attention and has been found to be closely related to the occurrence, development, and the prognosis of a variety of tumors, including ovarian cancer, gastric cancer, liver cancer, melanoma, and others [3–7]. Interestingly, the expression patterns of GPC3 vary in different tumors. Research has shown that the expression of GPC3 is upregulated in hepatocellular carcinoma, Wilms’ tumor, neuroblastoma, and melanoma, with low or no expression in the tissues surrounding the tumor. It may also function as the carcinoembryonic antigen (CEA) protein [8–10]. However, compared with normal tissue, the GPC3 expression is decreased in breast and ovarian cancer, where it may function as a potential tumor suppressor by induction of apoptosis [11, 12]. Although it is known that the expression patterns of GPC3 vary in different tumor tissues, it is unclear how GPC3 is expressed in lung squamous cell carcinoma, and, to date, few studies have been reported. Kim et al. [13, 14] found that GPC3 expression was decreased in lung adenocarcinoma. Patients with lung adenocarcinoma expressing GPC3 had a better prognosis than those patients with no tumor GPC3 expression. Daniel et al. [15] reported that the positive rate of GPC3 expression was 54% in lung squamous cell carcinoma, 10% in lung adenocarcinoma, and 10% in large cell lung cancer, suggesting that GPC3 expression in lung cancer tissue varies by carcinoma type.
Using RT–PCR to evaluate GPC3 mRNA levels and Western blot to evaluate GPC3 protein levels, we designed this study to detect changes in GPC3 expression in lung squamous cell carcinoma and in the adjacent normal tissue, as well as to explore the relationship between these expression changes and the biological characteristics of lung squamous cell carcinoma, such as degree of differentiation, metastasis, etc. We further discuss the association of GPC3 expression with the occurrence, development, invasion, and metastasis of lung squamous cell carcinoma; data such as this may one day provide the tools necessary for early diagnosis, more accurate estimation of prognosis, and targeted treatment for this disease.
Materials and methods
Specimens
Fresh resection tissue specimens were collected from 66 patients with squamous cell carcinoma in our hospital from March 2008 to March 2010. Both derived cancer tissue and its adjacent normal tissue were collected for each case. Samples were preserved in liquid nitrogen immediately and stored for later testing. Our sample population included 36 men and 30 women. The patients were aged 28–72 years old (mean = 58 years old); 38 patients were younger than 60 years old, with the remainder aged 60 years or older. Without any preoperative treatment, the 66 cases were all pathologically diagnosed as squamous cell carcinoma. The degree of differentiation for these cases was as follows: highly differentiated in 20 cases, moderately differentiated in 24 cases, and poorly differentiated in 22 cases. Forty-five cases presented with lymph node metastasis, while 21 cases presented without lymph node metastasis. The primary tumor was less than 3 cm in 28 cases and was greater than or equal to 3 cm in 38 cases.
Reagents
Main reagents include but were not limited to the following: Trizol kit (INVITROGEN); RT–PCR kit (GIBCO-BRL); RIPA lysate buffer [1% NP-40, 1% Deoxycholate, 0.1% SDS, 500 mM Tris, 150 mM NaCl, 1 mM PMSF, and 19 Protease Inhibitor Cocktail (Roche, New Jersey, USA)]; TBST (10 mM Tris–HCl, pH 7.5, 150 mM NaCl, and 0.1% Tween-20). The GPC3 (GI: 23271173) upstream primer was 5′-CAGCTCCTGAGAACCATGTCTATG-3′, and the downstream primer was 5′-CCCTTCCTCATCCAGGTTTTT-3′. The GAPDH (GI: 163954974) upstream primer was 5′-AATCCCATCACCATCTTCC-3′, and the downstream primer was 5’-CATCACGCCACAGTTTCC-3’. All primer sequences were provided by Shanghai Invitrogen Biotechnology Co. Ltd. The GPC3 primary antibody was rabbit anti-human polyclonal antibody (Abcam, UK); the secondary antibody was HRP-labeled goat anti-rabbit polyclonal antibody (Abbiotec Co., USA). The internal reference was goat anti-human GAPDH polyclonal antibody (Abcam, UK). The secondary antibody was HRP-labeled goat anti-rabbit polyclonal antibody (Abbiotec Co., USA). ECL chemiluminescence reagent was used for Western detection (Pierce Co., USA), and the BCA protein concentration assay kit was a product of the domestic Beyotime Institute of Biotechnology.
RT–PCR
A 100 mg sample of both cancer tissue and adjacent tissue was obtained. For each sample, 1 ml Trizol reagent was added and homogenized. After transferring the sample to a 1.5-ml centrifuge tube, 0.2 ml chloroform was added and the preparation was shaken for 20 s and then incubated for 3 min. After the sample was centrifuged at 12,000g for 10 min, the aqueous supernatant was taken; 0.5 ml of isopropanol was added and the preparation was mixed gently. After incubation at room temperature for 10 min, the preparation was centrifuged at 15,000 r/min for 10 min and the supernatant was discarded. Adding 1 ml 75% ethanol, the precipitate was gently washed and centrifuged and the supernatant was discarded; after dry out, the precipitate was dissolved with 20 μl of DEPC water (65°C dissolution for 10–15 min). After this, the RT reaction was performed. In the 20 μl of reaction volume, 1.0 μg of total RNA was added and incubated at 37°C for 1 h. The samples were inactivated at 95°C for 5 min and then immediately incubated in ice. They were stored at −20°C for later use. Primers were designed, followed by PCR. In a 25 μl PCR system, 5 μl cDNA was added. The amplification conditions were as follows: initial denaturing at 94°C for 3 min, denaturing at 94°C for 30 s, annealing at 56°C for 30 s, extension at 72°C for 30 s; after 30 cycles, final extension at 72°C for 10 min. The PCR products were detected by 1.5% agarose gel electrophoresis.
Western blot
Each tissue sample (100 mg, preserved in liquid nitrogen) was ground and then homogenized with 1 ml RIPA lysate buffer. The homogenate was transferred to a 1.5-ml centrifuge tube and centrifuged at 16,000g for 30 min. The supernatant was saved, and its protein concentration was determined by the BCA method. The protein sample of lung squamous cell carcinoma or adjacent tissue was obtained by mixing 10 μg of cancer or adjacent tissue, respectively, from each case. The lymph node metastasis-positive group was the mixture of 10 μg samples from all the individual patients with positive lymph node metastasis; the lymph node metastasis-negative group was the mixture of 10 μg samples from all the individual patients with negative lymph node metastasis. The high differentiation group was the mixture of 10 μg samples from all the individual patients with highly differentiated tumors; the moderate differentiation group was the mixture of 10 μg samples from all the individual patients with moderately differentiated tumors; the low differentiation group was the mixture of 10 μg samples from all the individual patients with poorly differentiated tumors. After the 6% stacking gel and the 12% separation gel were cast, 50 μg of total protein were applied to each lane and the electrophoresis separation was performed. The protein from the gel was then transferred to a PVDF membrane by semi-drying transfer (Amresco, USA). The PVDF membrane was blocked with TBST solution containing 5% skim milk at room temperature for 1 h. Then the rabbit anti-human polyclonal GPC3 antibody (1:1,000 dilution) and the goat anti-human GAPDH polyclonal antibody (1:1,000 dilution) were added in one by one, with overnight incubation at 4°C. The HRP-labeled goat anti-rabbit IgG (1:2,000 dilution) and the HRP-labeled goat anti-rabbit polyclonal antibody (1:2,000 dilution) were then added, with incubation at 37°C for 1 h. After TBST washing, ECL chemiluminescence reagent was used for autoradiography. The relative content of GPC3 was represented by the gray scale ratio of GPC3/GAPDH. The gray scale was analyzed with QuantityOne software (Bio-Rad, USA).
Statistical analysis
Stata 7.0 software was used for statistical analysis. χ2 tests and t tests were performed. Results were considered statistically significant at P < 0.05.
Results
By RT–PCR, the percentage of samples expressing GPC3 mRNA was 51.52% (34/66) in lung squamous cell carcinoma and 12.12% (8/66) in the adjacent tissues; this was a statistically significant higher rate of GPC3 mRNA expression in lung squamous cell carcinoma tissues compared with the adjacent tissues (P < 0.05). The percentage of samples expressing GPC3 mRNA was significantly higher in cases with lymph node metastasis than in those without lymph node metastasis (P < 0.05). With decreasing degree of tumor differentiation, there was a higher percentage of samples expressing GPC3 mRNA (P < 0.05). The percentage of samples expressing GPC3 mRNA was, however, independent of patient gender, age, and tumor size (P > 0.05). Data are shown in Table 1.
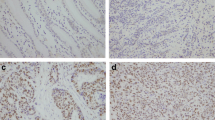
Western blot results showed that the GPC3 protein expression in lung squamous cell carcinoma was significantly higher than that in adjacent normal tissue (P < 0.05). The expression in samples from patients with lymph node metastasis was significantly higher than those from patients without lymph node metastasis (P < 0.05). With decreasing degree of tumor differentiation, there was higher expression of GPC3 protein (P < 0.05). The results are shown in Fig. 1.
The expression of GPC3 protein in lung squamous cell carcinoma and in the adjacent tissues (A: lung squamous cell carcinoma, B: cancer/adjacent tissue, C: negative lymph node metastasis, D: positive lymph node metastasis, E: low differentiation, F: moderate differentiation, G: high differentiation); II The changes in relative expression of GPC3 protein in lung squamous cell carcinoma and in the adjacent tissues (asterisks indicates P < 0.05, A: lung squamous cell carcinoma, B: cancer/adjacent tissue, C: negative lymph node metastasis, D: positive lymph node metastasis, E: low differentiation, F: moderate differentiation, G: high differentiation)
Discussion
All of the HSPG family members are composed of a glycosaminoglycan (GAG) connected to core proteins. The core proteins have a similar size (60 kDa). A repeat sequence containing Ser–Gly in the C terminus forms the HS binding site, which anchors to the cell membrane by covalently binding with glycosyl phosphatidylinositol (GPI). As the HS chain interacts with a number of large, functional bio-molecules (including growth factors and their receptors, extracellular matrix proteins and adhesion molecules), it is currently believed that HSPG is involved in regulating cell proliferation, differentiation, adhesion, and migration. It may also be involved in organ growth and suppressing or regulating most of the mesoderm tissue [16]. In the human embryo, GPC3 has high expression in the gastrointestinal tract and tissues of mesoderm origin. In adults, it has low expression only in a few tissues, such as the lung, kidney, heart, and ovary [17].
GPC3 plays an important regulatory role in cell growth and development. Its expression varies widely in different malignant tumors and is closely related to variety of tumors, including liver cancer, malignant melanoma, ovarian cancer, and breast cancer. It plays different roles in different tumors, where these may be the exact opposite, depending on the cancer [3–7]. Interestingly, the expression patterns of GPC3 vary in different tumors. Research has shown that the expression of GPC3 was upregulated in hepatocellular carcinoma, Wilms’ tumor, neuroblastoma, hepatoblastoma, melanoma, testicular germ cell tumors, and others [8–10, 18–20], with low or no expression in tissues surrounding the tumor. It may function as the carcinoembryonic antigen (CEA) protein. GPC3 can be used diagnostically as serum and immunohistochemical markers of hepatocellular carcinoma [3, 21]. Zhu et al. [22] found that mRNA of GPC3 had low or even no expression in normal liver tissue, liver focal nodular hyperplasia, and cirrhosis of the liver; however, in the 30 cases of hepatocellular carcinoma in the study, 20 cases showed significant expression and 5 cases had moderate expression. The average increase in the expression of GPC3 mRNA in hepatocellular carcinoma was significant compared with that in normal liver tissue (an increase of 21.7 times, P < 0.01). Compared with tissue from patients with hepatic focal nodular hyperplasia and cirrhosis, the expression of GPC3 mRNA in hepatocellular carcinoma is increased by 7.2-fold (P < 0.05) and 10.8-fold (P < 0.01), respectively. Therefore, in most cases, one can determine whether liver disease is benign or malignant based on the GPC3 expression. A study by Ho et al. [21] suggests that GPC3 can act as a new tumor immunotherapy target. IGF-2 is known to be an important growth factor in the early stages of tumor development. GPC3 can specifically bind to IGF-2, which plays a role in the signal transduction pathway mediated by IGF-2 [23]. However, the most recent study on this subject found that the GPC3 knockout hepatoma (HepG2) can significantly promote the growth of liver tumor cells; however, inhibition by GPC3 does not function by downregulating the signal transduction of IGF-2. Therefore, the relationship between GPC3 and IGF-2 in cancer occurrence and development still needs further study. Toretsky et al. found that the expression of GPC3 was upregulated in Wilms’ tumor and hepatoblastoma, whereas low or no expression was detected in the tissues surrounding the tumor. They proposed that the high expression of GPC3 in Wilms’ tumor and hepatoblastoma tissues may promote tumor growth [8]. Nakatsura et al. found that there was significant expression of GPC3 in more than 80% of melanoma tumor tissues. GPC3 expression was detected in the serum of 39.6% of patients, while no GPC3 was detected in the serum of healthy individuals (60 cases). In stage 0, I, and II melanoma, positive rates of GPC3 in the serum of melanoma patients (44.4, 40.0, and 47.6%, respectively) were significantly higher than those of 5-S-cysteinyldopa (0.0, 8.0, and 10.0%, respectively). After surgical treatment, GPC3 was no longer detectable in the serum of 11 patients. These data suggest that GPC3 can be a very effective diagnostic marker of melanoma, especially in the early stages [10].
However, compared with normal tissue, the GPC3 expression is decreased in breast cancer [11, 17], ovarian cancer [12, 24], and mesothelioma [9]. It may function as a potential tumor suppressor, by inducing apoptosis. The decreased GPC3 expression in breast cancer may be related to the hyper-methylation of the GPC3 promoter. In addition, ectopic expression of GPC3 inhibited the growth of 80% of breast cancer cell lines [25]. A study using a mouse breast cancer cell line transfected with the rat GPC3 gene found that there were very few local invasions in this cell line compared with the control group. Almost no experimental spontaneous growth and lung metastases were observed, with increased sensitivity to apoptosis. Further, the GPC3-transfected samples showed a high expression of cadherin and β-catenin proteins. The downregulation of these proteins was associated with the degree of malignancy of breast cancer. Meanwhile, the expression of GPC3 can also inhibit the activity of MMP2 [11]. Lin et al. found that the expression of GPC3 was silenced in ovarian carcinoma tissue, and the ectopic expression of GPC3 inhibited the growth of the ovarian cancer cell line. In addition, GPC3 mRNA expression in advanced ovarian cancer was lower than that in lower-stage cancer, suggesting that this gene may be involved in ovarian cancer disease progression [12]. Gonzalez et al. [15] confirmed that GPC3 can induce apoptosis of the mesothelioma cell line II 14 and the breast cancer cell line MCF-7, but such effects can be reversed by exogenous IGF-II peptide. The apoptosis function was lost after mutating the GPI anchor protein so that it could no longer be anchored to the surface of cell membrane, but the apoptotic function remained intact after mutating its GAG binding site in the protein. Therefore, these data suggest that the apoptosis function of the GPC3 gene may be related with GPI, and the GPC3-induced apoptosis is cell specific. For NIH3T3 and HT29 colon cancer cell lines, apoptosis cannot be induced by GPC3. Murthy et al. found that the decreased GPC3 expression in mesothelioma was related to the hyper-methylation of its promoter. After a 2-h treatment with DAC (2-Deoxy-5-azacytidine) to the mesothelioma cell line, the expression of GPC3 returned to normal. Further, the ectopic expression of GPC3 can inhibit the formation of colony in mesothelioma cells [9]. Therefore, GPC3 may serve as a tumor suppressor gene in the occurrence and development of breast cancer, ovarian cancer, and mesothelioma, functioning by inhibition of tumor occurrence [2]. Song et al. [26] found that the deletion of GPC3 in vivo can inhibit the nonclassical Wnt/JNK signaling pathway, as well as enhancing the activity of classic Wnt/β- catenin pathway. In vitro, GPC3 in mesothelioma cells can enhance the activity of JNK responding to Wnt5a. Therefore, in some cell types, GPC3 may be a selective mediator in the Wnt signaling pathway.
This study detected changes in GPC3 expression in lung squamous cell carcinoma, using mRNA levels determined by RT–PCR and protein levels determined by Western blot. In our study, we found that a greater percentage of lung squamous cell carcinoma samples expressed GPC3 mRNA, compared with adjacent normal tissues. A higher percentage of expression was seen in tissues from patients with lymph node metastasis, compared with patients with no lymph node metastasis, and in samples from patients with a tumor with a lower degree of differentiation, compared with more highly differentiated tumors. GPC3 protein expression showed a similar pattern.
Kim et al. found that GPC3 expression was decreased in lung adenocarcinoma [13, 14]. Patients with lung adenocarcinoma expressing GPC3 had a better prognosis than those patients with no GPC3 expression. Daniel et al. [15] reported that the positive rate of GPC3 expression was 54% in lung squamous cell carcinoma, 10% in lung adenocarcinoma, and 10% in large cell lung cancer. Our study found that the positive rate of GPC3 expression was 51.52% in lung squamous cell carcinoma, which is consistent with the results reported by Daniel et al. The expression of GPC3 in lung squamous cell carcinoma may be related to its function as CEA protein, which could promote tumor growth. The GPC3 expression positive rate was especially high in poorly differentiated lung squamous cell carcinoma. Therefore, GPC3 expression can serve as a prognosis indicator for lung squamous cell carcinoma. Lee et al. found that the classic Wnt pathway played an important role in the occurrence and development of lung squamous cell carcinoma [27]. This study also found high GPC3 expression in lung squamous cell carcinoma. This highly expressed GPC3 may be anchored in the cell membrane by GPI, so that it can adhere and bind to Wnt, resulting in the formation of a GPC3-Wnt complex. This increases the interaction between the ligand and the receptor in the Wnt pathway and promotes the high expression of Wnt. The increased Wnt protein binds to the cell surface receptor Fz, activating Wnt signaling. This causes the expression of targeted genes (lung cancer-related genes), leading to uncontrolled proliferation of lung epithelial cells and cancer. Further investigation is still required to determine the specific mechanisms. Zynger et al. studied differential GPC3 expression in germ cell tumors composed of different tissues, which suggested that GPC3 may play a role in tumor differentiation [18]. The results of our study showed that GPC3 might plays an important role in the occurrence and development of lung squamous cell carcinoma and is closely related to the degree of differentiation and the invasive ability of the tumor. Further investigation is warranted to describe the relation of whether GPC3 protein contributes to the mechanism of occurrence, development, differentiation, and invasiveness of the tumor.
In conclusion, if GPC3 is expressed in the normal tissue, little or no GPC3 is expressed in the tumor tissue; if GPC3 is not expressed in the normal tissue, GPC3 is normally expressed or even highly expressed in the tumor tissue [18]. Thus, the relationship between GPC3 and its function in normal and tumor tissues is complicated. Functionally, it may participate in and/or influence the signaling of various growth factors. Further study is required to determine the mechanism of silencing or upregulation of its expression in different tumor tissues. The relationship between GPC3 expression and cancer is complicated, with differing and sometimes opposite roles in different tumor types. Additional in-depth study of GPC3 would help to further elucidate the role of GPC3 expression on the occurrence and development of tumors.
References
Shao WL, Wang DY, He JX. The role of gene expression profiling in early-stage non-small cell lung cancer. J Thorac Dis. 2010;2:89–99.
Gonzalez AD, Kaya M, Shi W, Song H, Testa JR, Penn LZ, Filmus J. OCI-5/GPC3, a glypican encoded by a gene that is mutated in the Simpson-Golabi-Behmel overgrowth syndrome, induces apoptosis in a cell line-specific manner. J Cell Biol. 1998;141:1407–14.
Yamauchi N, Watanabe A, Hishinuma M, Ohashi K, Midorikawa Y, Morishita Y, Niki T, Shibahara J, Mori M, Makuuchi M, et al. The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol. 2005;18:1591–8.
Kandil D, Leiman G, Allegretta M, Evans M. Glypican-3 protein expression in primary and metastatic melanoma: a combined immunohistochemistry and immunocytochemistry study. Cancer Cytopathol. 2009;117:271–8.
Oishi C, Baba T, Kubota Y, Shimotsuma Y, Kitamura K, Honma T, Ikegami A, Inokuchi M, Umeda T, Yoshida H, et al. Two cases of gastric cancer expressing Glypican 3, but producing AFP with different lectin affinity. Nippon Shokakibyo Gakkai Zasshi. 2009;106:805–12.
Buchanan C, Stigliano I, Garay-Malpartida HM, Rodrigues Gomes L, Puricelli L, Sogayar MC, Bal de Kier Joffe E, Peters MG. Glypican-3 reexpression regulates apoptosis in murine adenocarcinoma mammary cells modulating PI3 K/Akt and p38MAPK signaling pathways. Breast Cancer Res Treat. 2010;119:559–74.
Maeda D, Ota S, Takazawa Y, Aburatani H, Nakagawa S, Yano T, Taketani Y, Kodama T, Fukayama M. Glypican-3 expression in clear cell adenocarcinoma of the ovary. Mod Pathol. 2009;22:824–32.
Toretsky JA, Zitomersky NL, Eskenazi AE, Voigt RW, Strauch ED, Sun CC, Huber R, Meltzer SJ, Schlessinger D. Glypican-3 expression in Wilms tumor and hepatoblastoma. J Pediatr Hematol Oncol. 2001;23:496–9.
Murthy SS, Shen T, De Rienzo A, Lee WC, Ferriola PC, Jhanwar SC, Mossman BT, Filmus J, Testa JR. Expression of GPC3, an X-linked recessive overgrowth gene, is silenced in malignant mesothelioma. Oncogene. 2000;19:410–6.
Nakatsura T, Kageshita T, Ito S, Wakamatsu K, Monji M, Ikuta Y, Senju S, Ono T, Nishimura Y. Identification of glypican-3 as a novel tumor marker for melanoma. Clin Cancer Res. 2004;10:6612–21.
Peters MG, Farias E, Colombo L, Filmus J, Puricelli L, Bal de Kier Joffe E. Inhibition of invasion and metastasis by glypican-3 in a syngeneic breast cancer model. Breast Cancer Res Treat. 2003;80:221–32.
Lin H, Huber R, Schlessinger D, Morin PJ. Frequent silencing of the GPC3 gene in ovarian cancer cell lines. Cancer Res. 1999;59:807–10.
Powell CA, Xu G, Filmus J, Busch S, Brody JS, Rothman PB. Oligonucleotide microarray analysis of lung adenocarcinoma in smokers and nonsmokers identifies GPC3 as a potential lung tumor suppressor. Chest. 2002;121(3 Suppl):6S–7S.
Kim H, Xu GL, Borczuk AC, Busch S, Filmus J, Capurro M, Brody JS, Lange J, D’Armiento JM, Rothman PB, et al. The heparan sulfate proteoglycan GPC3 is a potential lung tumor suppressor. Am J Respir Cell Mol Biol. 2003;29:694–701.
Baumhoer D, Tornillo L, Stadlmann S, Roncalli M, Diamantis EK, Terracciano LM. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4, 387 tissue samples. Am J Clin Pathol. 2008;129:899–906.
Filmus J, Capurro M, Rast J. Glypicans. Genome Biol. 2008;9:224.
Buchanan C, Stigliano I, Garay-Malpartida HM, Rodrigues Gomes L, Puricelli L, Sogayar MC, Bal de Kier Joffe E, Peters MG. Glypican-3 reexpression regulates apoptosis in murine adenocarcinoma mammary cells modulating PI3 K/Akt and p38MAPK signaling pathways. Breast Cancer Res Treat. 2010;119:559–74.
Zynger DL, Dimov ND, Luan C, Teh BT, Yang XJ. Glypican 3: a novel marker in testicular germ cell tumors. Am J Surg Pathol. 2006;30:1570–5.
Akutsu N, Yamamoto H, Sasaki S, Taniguchi H, Arimura Y, Imai K. Shinomura Y: Association of glypican-3 expression with growth signaling molecules in hepatocellular carcinoma. World J Gastroenterol. 2010;16:3521–8.
Suzuki M, Sugimoto K, Tanaka J, Tameda M, Inagaki Y, Kusagawa S, Nojiri K, Beppu T, Yoneda K, Yamamoto N, et al. Up-regulation of glypican-3 in human hepatocellular carcinoma. Anticancer Res. 2010;30:5055–61.
Ho M, Kim H. Glypican-3: a new target for cancer immunotherapy. Eur J Cancer. 2011;47:333–8.
Zhu ZW, Friess H, Wang L, Abou-Shady M, Zimmermann A, Lander AD, Korc M, Kleeff J, Buchler MW. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut. 2001;48:558–64.
Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, Neri G, Cao A, Forabosco A, Schlessinger D. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet. 1996;12:241–7.
Umezu T, Shibata K, Kajiyama H, Yamamoto E, Nawa A, Kikkawa F. Glypican-3 expression predicts poor clinical outcome of patients with early-stage clear cell carcinoma of the ovary. J Clin Pathol. 2010;63:962–6.
Xiang YY, Ladeda V, Filmus J. Glypican-3 expression is silenced in human breast cancer. Oncogene. 2001;20:7408–12.
Song HH, Shi W, Xiang YY, Filmus J. The loss of glypican-3 induces alterations in Wnt signaling. J Biol Chem. 2005;280:2116–25.
Lee EH, Chari R, Lam A, Ng RT, Yee J, English J, Evans KG, Macaulay C, Lam S, Lam WL. Disruption of the non-canonical WNT pathway in lung squamous cell carcinoma. Clin Med Oncol. 2008;2:169–79.
Conflict of interest
The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Qiang Lin and Li-wen Xiong contributed equally to this article.
Rights and permissions
About this article
Cite this article
Lin, Q., Xiong, Lw., Pan, Xf. et al. Expression of GPC3 protein and its significance in lung squamous cell carcinoma. Med Oncol 29, 663–669 (2012). https://doi.org/10.1007/s12032-011-9973-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-9973-1