Abstract
The HER2 gene, which is located on chromosomes 17, is a therapeutic target for cancer. Amplification of HER2 has been described in several tumor types. However, few studies of HER2 gene amplification and protein expression in esophageal carcinoma have been conducted. This study was to investigate the relationship between the expression of HER2/neu and the clinical characteristics, including survival rate, of esophageal squamous carcinoma. The clinical data of 145 patients admitted in Renmin Hospital of Wuhan University, from 2000 to 2005, were reviewed. The HER2 protein expression and gene status in 145 esophageal carcinomas were evaluated using immunohistochemistry and fluorescence in situ hybridization. The survival rate was calculated by the Kaplan–Meier method and the log-rank test using SPSS13.0 software. Compared to normal esophageal epithelium (23/95, 24.2%), HER2 protein was overexpressed in most esophageal squamous carcinoma tissues (60/145, 41.4%), of which 45 (31.0%) were 2+ and 15 (10.4%) were 3+, HER2 overexpression associated significantly with HER2 gene amplification. There is a correlation between the overexpression of HER2 and the differentiation of the carcinoma, the HER2 gene amplification and the differentiation of the carcinoma and the tumor stage. According to univariate analysis, there was a significant difference in survival rates when cases with and without HER-2/neu overexpression or amplification were compared. HER-2/neu amplification/overexpression may be used as an independent prognostic factor in patients with esophageal squamous cancer, and patients with HER-2/neu amplification/overexpression might be potential candidates for new adjuvant therapies that involve the use of humanized monoclonal antibodies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal carcinoma remains the 8th leading cause of malignancy-related deaths worldwide [1]. Squamous cell carcinoma is the most frequent histological type of cancer of the esophagus (ESCC) [2]. It is usually associated with a poor prognosis because it is often at an advanced stage when diagnosed and there is a high frequency of lymph node metastases. Treatment for esophageal carcinoma remains a challenge for physicians. Recently, targeted therapy has been applied to esophageal carcinoma, which may open new avenues for cancer treatment. Current targeted therapy depends on the evaluation of the status of target genes [3, 4].
A member of the epidermal growth factor receptor (EGFR) family, c-erbB-2 (HER2), has received much attention because it is the therapeutic target in several tumors. Overall, 15–25% of patients with breast cancer [5–7] have been found to be HER2 2+/3+, which is associated with an unfavorable prognosis, especially in patients with lymph node metastases. In addition, there is accruing evidence to indicate that HER2 is an important predictive factor of response to chemotherapy and hormonal therapy in breast cancer [8, 9].
A number of studies have analyzed HER2 overexpression in esophageal carcinoma. A recent study has reported 30.3% of patients with esophageal squamous cell carcinomas had overexpression of HER2 [10]. The clinical significance of HER2 gene amplification and protein overexpression is not yet fully understood. In some studies, HER2 appears to be an important prognostic factor in ESCC [11, 12]. However, the literature is conflicting in this respect, and not all studies have shown an association between HER2 overexpression and poor prognosis [13–15].
The objectives of this study were (1) to determine the frequency of HER-2/neu amplification and overexpression in ESCC, (2) to clarify whether the same mechanisms of gene amplification and protein overexpression function in ESCC as in breast cancer, and (3) to investigate the relationship between HER-2/neu amplification/overexpression and the clinicopathological characteristics of tumors, including survival rates. This study was conducted with a view toward the future introduction of Herceptin therapy for the treatment of patients with ESCC.
Methods
Tumor tissue collection and human subjects approval
The specimens were selected from archive paraffin embedded blocks in Renmin Hospital of Wuhan University by two pathologists. A total of 312 Chinese patients with esophageal carcinoma who underwent surgery at the Department of Surgery, Renmin Hospital of Wuhan University, during the period of 2000–2005, were eligible. Only those patients whose clinical data (including diagnosis, age, sex, address, and disease history) were intact were included; 145 Chinese patients with ESCC were finally selected in this study. None of the patients had undergone preoperative radiation or chemotherapy. Ninety-five cases of normal esophagal tissues were cut from the distal esophagus of the same patients.
For all patients, we reviewed age, gender, tumor size, histological grade, extent of infiltration, lymphatic invasion, and evolution of disease. The clinicopathological data are summarized in Table 1. The age of the 145 patients selected for this study ranged from 34 to 81 years, with a mean of 59.2 years.
The follow-up time ranged from 0 to 120 months with an average of 47.9 months. The causes of death were ascertained from medical records or autopsy, if performed. Patients who had died within 4 weeks of radical surgery were excluded from our analyses. Deaths due to other causes resulted in censored observations beginning at time of death. The institutional review board at the Renmin Hospital of Wuhan University approved this study, and informed consent was obtained from all patients. Each specimen was routinely fixed in 10% formalin and embedded in paraffin. Before inclusion in the study, each specimen was verified by a histopathologist.
Immunohistochemistry
All esophageal tumor and normal esophageal epithelium specimens were fixed in 10% buffered formalin and embedded in paraffin according to standard procedures. Serial sections (4 μm thickness) placed on positively charged slides (Menzel-Glasser, German) were used for hematoxylin and eosin staining, immunohistochemistry, and FISH detection of HER2.
Immunohistochemistry for HER2 was performed using the Hercep Test kit (DakoCytomation, Denmark), according to the manufacturer’s instructions. Antibody binding was visualized by the EnVison detection kit (DakoCytomation, Denmark).
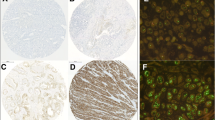
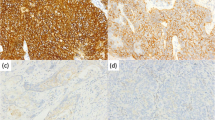
Immunohistochemical (IHC) staining was scored by two pathologists and evaluated following the criteria recommended by the manufacturer: no staining, or weak staining in fewer than 10% of the tumor cells (0); weak staining in part of the membrane in more than 10% of the tumor cells (1+); complete staining of the membrane with weak or moderate intensity in more than 10% of the neoplastic cells (2+); and strong staining in more than 10% (3+). HER-2/neu protein overexpression was defined as either negative (score 0 and 1+) or positive (score 2+ and 3+).This cutoff point was predicted on the results of previous breast cancer studies. Interpretations were made independently by two pathologists, who had been blinded to each other’s findings, and to the results of the other assays. We used paraffin slides of invasive breast carcinoma as a positive control.
Fluorescence in situ hybridization
HER-2/neu amplification was analyzed using FISH HER2 PharmDx (Dako, Denmark),which contains both fluorescently labeled HER-2/neu gene and chromosome 17 centromere probes. In brief, the sections were incubated at 56°C overnight and deparaffinized by washing in xylene, ethanol, and distilled water. After incubation in 0.2 M HCl at room temperature for 20 min, they were heat-treated in citrate buffer (2 × SSC, pH 6.0) at 80°C for 1–1.5 h. They were then digested with pepsin at room temperature for 8–14 min, rinsed in 2 × SSC at room temperature for 2 min and dehydrated in graded ethanol (75, 80, and 100%) for 2 min. After the HER2/CEN17 probe mix was applied to the dry slides, the tissue area was cover slipped and sealed with rubber cement. The slides were then incubated in hybridizer (Hybridizer Instrument for in situ hybridization, DAKO, Denmark) for denaturation at 82°C for 5 min and hybridization at 45°C for about 18 h. Post-hybridization washes were performed in urea/0.1 × SSX at 45°C for 30 min and in 2 × SSC at room temperature for 2 min. The slides were dehydrated in graded ethanol, and after application of 15 μL of mounting medium containing 4′,6′-diamidino-2-phenylindole (DAPI), the tissue area was cover slipped.
FISH analyses were performed according to the HER2 FISH PharmDx (Dako, Denmark) criteria. In each case, 100 non-overlapped, intact interphase tumor nuclei identified by DAPI staining were evaluated, and HER2 gene (red signal) and CEN17 (green signal) copy numbers in each nucleus were assessed. The cases were considered to be amplified when the average copy number ratio, HER2/CEN17, was ≥2.0 in all nuclei evaluated or when the HER2 signals formed a tight gene cluster. Among the cases in which HER2 gene was not amplified, samples showing more than four copies of the HER2 gene and more than four CEN17 in more than 10% of the tumor cells were considered to be polysomic for chromosome 17.
Statistical analyses
All statistical analyses were performed using SPSS for Windows 13.0, SPSS Inc. Categorical variables were compared by the Pearson Chi-square test or Fisher’s exact test, depending on the expected values found in the contingency table. The overall survival rates were calculated using the Kaplan–Meier method, and the curves were compared by the log-rank test. In all statistical tests, the alpha error was set at 5%. The survival period was calculated from the date of hospital admission to death or the date of last follow-up.
Results
HER-2/neu immunohistochemistry
The HER2 protein was overexpressed in most esophageal carcinoma tissues (60/145, 41.4%), of which 45 (31.0%) were 2+ and 15 (10.4%) were 3+ (Fig. 1), compared to normal esophageal epithelium (23/95, 24.2%) (Table 2). Statistical analysis revealed an association between the expression of HER2 and the differentiation of the carcinoma (Table 1).
HER-2/neu amplification
The same cases evaluated by immunohistochemistry were also examined using FISH. Gene amplification was found in 24 (16.6%) cases; 121 (83.4%) showed no amplification (Fig. 2). The HER2 amplification cases included all fifteen of the HER2 3+ cases, six of the HER2 2+ cases, two of the HER2 1+ case and one of the HER2 0 cases by immunohistochemistry. HER2 amplification was associated with the differentiation of the carcinoma and the tumor stage (Table 1). Statistical analysis revealed an association between HER2 overexpression and HER2 amplification (P < 0.0001) (Table 3). 13.1% (19/145)of the patients showed chromosome 17 polysomy. Two of the 19 patients with chromosome 17 polysomy showed HER2 amplification. There was no association between chromosome 17 polysomy and HER2 amplification. Four patients with HER2 overexpression showed polysomy of chromosome 17, two (50%) were 3+ and two (25%) were 2+. However, there was no significant association between chromosome 17 polysomy and HER2 overexpression (Table 4).
Survival analysis
Survival analysis was performed on 145 patients who had survived for more than 4 weeks after surgery. The survival curves, according to HER-2/neu amplification and overexpression, are shown in Figs. 3 and 4. Tumors associated with HER-2/neu amplification exhibited poor mean survival rates (28.0 vs. 50.7 months). Tumors associated with HER-2/neu overexpression also exhibited poor mean survival rates (38.3 vs. 52.8 months).
Discussion
HER2 is a transmembrane receptor with an intracellular domain with tyrosine kinase activity [16]. If HER2 is overexpressed in the malignant cell, there is a subsequent signaling from the receptor, resulting in increased cell proliferation and mitosis, ultimately causing tumor progression and metastasis [17].
In this study, we demonstrated that HER2 is overexpressed and amplified in ESCC; similar results were observed in other researches [1, 10–13, 18–22]. Therefore, it is reasonable to focus on HER2 overexpression and/or gene amplification when developing therapeutic strategies to target carcinomas.
Immunohistochemical analysis showed that 41.4% of the cases were positive for expression of HER2. The few studies reporting HER2 expression in ESCC show discrepant frequencies ranging from 0 to 64% [18–22]. This variability may have resulted from differences in immunohistochemical protocols, different antibody sources used by the different authors, or different criteria for evaluating expression.
Previous studies to determine whether HER2 expression was associated with the clinicopathologic characteristics of ESCC have been controversial. We found there is significant association between HER2 expression and the clinicopathological findings, such as histological grade and tumor stage.
HER2 overexpression is associated with lower rates of survival. These findings are consistent with previous reports [19, 23].
The reported frequencies of HER-2/neu amplification in ESCC vary from 2 to 19.1% in the patients [10–15]. This variability may have resulted from differences in tissue preparation, probes, and the methods used to evaluate the alterations. Our study demonstrated 16.6% of cases showed gene amplification.
Mimura et al. (2005) reported that all 3+ cases, 50% of 2+ cases showed gene amplification [11]. Another study showed a significant association between gene amplification and protein overexpression in 70% of 3+ and in 30% of 2+ amplified cases [12]. In contrast to these results, Sunpaweravong et al. (2005) found no significant association between gene amplification and immunohistochemical expression; the one case positive for amplification did not overexpress c-erbB-2 [13]. Our date agree that most c-erbB-2 overexpression is caused by gene amplification [24].
Chromosome 17 harbors a number of important oncogenes and tumor suppressor genes, including HER2, TOP2A, DARPP32, p53, and BRCA1 [25]. We found chromosome 17 polysomy did not correlate with HER2 amplification or with HER2 overexpression. Our results suggest that increased HER2 gene dosage resulting from gene amplification is the most important determinant for HER2 overexpression, whereas any influence resulting from chromosome 17 polysomy alone is unlikely to play a significant role in HER2 gene overexpression at the transcriptional level. A similar finding was described previously in breast cancer [26].
FISH and IHC are two methods which have been used widely in clinical laboratories. These methods have both proven sensitive and specific in the laboratory. Compared to FISH, IHC is less time-consuming, less expensive, much easier to perform and requires minimal instrumentation. However, IHC methods can potentially be affected by a host of variables, including tissue fixation, processing, choice of primary antibodies, detection systems, and methods of antigen retrieval [27]. Furthermore, the interpretation for IHC may vary among observers, since the suggested scoring system for IHC is subjective. These factors, in addition to small study sample sizes, may also account for the variable rates of HER-2/neu immunoreactivity, as well as the conflicting reports suggesting the association of HER-2/neu with adverse clinical outcomes. FISH is currently regarded as the “gold standard” for the detection of HER-2/neu amplification: it is associated with both high sensitivity (96.5%) and high specificity (100%) [28]. FISH can be conducted with small tumor samples. Both formalin-fixed and paraffin-embedded tissue samples can be used since tissue preparation having little or no effect on the testing. It also allows for the direct visualization of gene amplification in the nuclei and provides an objective count of genes and chromosomes on a cell-by-cell basis. The disadvantage for FISH is that it requires a fluorescence microscope and special training in order to interpret the results. It also may prove quite difficult to visualize the morphological features of the tumor cells and, also, to separate in situ from invasive carcinoma when evaluating the amplification products via fluorescence. In addition, fluorescence fades quickly and does not create a permanent record [29].
Therefore, we think that c-erbB-2 expression must be evaluated initially by immunohistochemistry and, if the results are not conclusive, FISH should be performed. Such a practice has been standard procedure to assist in making therapeutic decisions in patients with breast and lung cancer [30–32].
With respect to overall survival rate, our data are in agreement with the findings of Mimura et al. (2005), showing significant differences in survival rates in cases with gene amplification and HER2 overexpression[11]. The significant association between HER-2/neu amplification and lower survival rate indicates a role for analyzing the alteration analysis in ESCC prognosis. Further studies with more cases are necessary for a better understanding of the influence of this gene on ESCC progression.
Antibody-based therapy with trastuzumab (Herceptin) is used clinically in HER-2–positive breast cancer [33–35]. Trastuzumab is most effective in patients with HER-2–positive breast cancer when used as adjuvant therapy [36]. Similarly, patients with ESCC having HER-2/neu gene amplification might also benefit from treatment with Trastuzumab, since HER-2/neu amplification indicates a group of cases in which this type of treatment could improve the prognosis [11, 37]. A recent study indicated that HER2-targeted therapy with trastuzumab (Herceptin) shows a significant primary tumor growth reduction as well as a reduction of lymph node metastases in an orthotopic mouse model of metastatic esophageal carcinoma. These preclinical results suggest a role for HER2-targeted antibody-based treatment of HER-2–overexpressing esophageal carcinoma. The results suggest, in particular, trastuzumab treatment in the adjuvant setting to prevent lymph node metastasis after primary tumor resection [38].
Conclusion
Our results indicate that HER-2/neu amplification/overexpression may constitute an independent prognostic factor in patients with esophageal squamous cancer and that patients exhibiting HER-2/neu amplification/overexpression might be potential candidates for new adjuvant therapies that involve the use of humanized monoclonal antibodies. Further studies with more cases and including additional techniques are necessary to verify other molecular alterations involved in tumor progression, which will contribute to the development of new therapies for ESCC.
References
Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83(10):2049–53.
Mascarello JT, Brothman AR, Davison K, Dewald GW, Herrman M, McCandless D, Park JP, Persons DL, Rao KW, Schneider NR, Vance GH, Cooley LD. Proficiency testing for laboratories performing fluorescence in situ hybridization with chromosome-specific DNA probes. Arch Pathol Lab Med. 2002;126(12):1458.
Corley DA, Buffler PA. Oesophageal and gastric cardia adenocarcinoma: analysis of regional variation using the Cancer Incidence in FIVE Continents database. Int J Epidemiol. 2001;30(6):1415–25.
Takehana T, Kunitomo K, Kono K, Kitahara F, Iizuka H, Matsumoto Y, Fujino MA, Ooi A. Status of c-ERBB-2 in gastric adenocarcinoma:a comparative study of immunohistochemistry, fluorescence in situ hybridization and enzyme-linked immunosorbent assay. Int J Cancer. 2002;98(6):833–7.
Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, Wilson C, Rong HM, Bauerfeind I, Felber M, Wang HJ, Bery M, Seshadri R, Hepp H, Slamon DJ. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003;95(2):142–53.
Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5(1):63–9.
Penault-Llorca F, Vincent-Salomon A, Mathieu MC, Trillet-Lenoir V, Khayat D, Marty M. Incidence and implications of HER2 and hormonal receptor overexpression in newly diagnosed metastatic breast cancer (MBC). J Clin Oncol (Meeting Abstracts). 2005;23:69s. Abstract 764.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82.
Révillion F, Bonneterre J, Peyrat JP. ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer. 1998;34(6):791–808.
Sato-Kuwabara Y, Neves JI, Fregnani JHTG, Sallum RA, Soares FA. Evaluation of gene amplification and protein expression of HER-2/neu in esophageal squamous cell carcinoma using Fluorescence in situ Hybridization (FISH) and Immunohistochemistry. BMC Cancer. 2009;9(6):1207–471.
Mimura K, Kono K, Hanawa M, Mitsui F, Sugai H, Miyagawa N, Ooi A, Fujii H. Frequencies of HER-2/neu expression and gene amplification in patients with oesophageal squamous cell carcinoma. Br J Cancer. 2005;92(7):1253–60.
Reichelt U, Duesedau P, Tsourlakis MC, Quaas A, Link BC, Schurr PG, Kaifi JT, Gros SJ, Yekebas EF, Marx A, Simon R, Izbicki JR, Sauter G. Frequent homogeneous HER-2 amplification in primary and metastatic adenocarcinoma of the esophagus. Mod Pathol. 2007;20(1):120–9.
Sunpaweravong P, Sunpaweravong S, Puttawibul P, Mitarnun W, Zeng C, Baron AE, Franklin W, Said S, Varella-Garcia M. Epidermal growth factor receptor and cyclin D1 are independently amplified and overexpressed in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2005;131(2):111–9.
Friess H, Fukuda A, Tang WH, Eichenberger A, Furlan N, Zimmermann A, Korc M, Buchler MW. Concomitant analysis of the epidermal growth factor receptor family in esophageal cancer:overexpression of epidermal growth factor receptor mRNA but not of c-erbB-2 and c-erbB-3. World J Surg. 1999;23(10):1010–8.
Suzuki H, Abo S, Kitamura M, Hashimoto M, Izumi K, Terada K, Sugiyama T. Gene amplification of int-2 and erbB in human esophageal cancer: relationship to clinicopathological variables. Cancer Invest. 1997;15(5):411–15.
Wells A. EGF receptor. Int J Biochem Cell Biol. 1999;31(6):637–43.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37.
Hardwick RH, Barham CP, Ozua P, Newcomb PV, Savage P, Powell R, Rahamin J, Alderson D. Immunohistochemical detection of p53 and c-erbB-2 in oesophageal carcinoma; no correlation with prognosis. Eur J Surg Oncol. 1997;23(1):30–5.
Lam KY, Tin L, Ma L. C-erbB-2 protein expression in oesophageal squamous epithelium from oesophageal squamous cell carcinomas, with special reference to histological grade of carcinoma and pre-invasive lesions. Eur J Surg Oncol. 1998;24(5):431–5.
Dreilich M, Wanders A, Brattstrom D, Bergstrom S, Hesselius P, Wagenius G. Bergqvist M: HER-2 overexpression (3+) in patients with squamous cell esophageal carcinoma correlates with poorer survival. Dis Esophagus. 2006;19(4):224–31.
Suo Z, Su W, Holm R, Nesland JM. Lack of expression of c-erbB-2 oncoprotein in human esophageal squamous cell carcinomas. Anticancer Res. 1995;15(6B):2797–8.
Suwanagool P, Parichatikanond P. Maeda S: Expression of c-erbB-2 oncoprotein in primary human tumors: an immunohistochemistry study. Asian Pac J Allergy Immunol. 1993;11(2):119–22.
Dreilich M, Wanders A, Brattström D, Bergström S, Hesselius P, Wagenius G, Bergqvist M. HER-2 overexpression (3+) in patients with squamous cell esophageal carcinoma correlates with poorer survival. Dis Esophagus. 2006;19(4):224–31.
Hofmann M, Stoss O, Gaiser T, Kneitz H, Heinmoller P, Gutjahr T, Kaufmann M, Henkel T, Ruschoff J. Central HER2 IHC and FISH analysis in a trastuzumab [Herceptin (R)] Phase II monotherapy study: assessment of test sensitivity and impact of chromosome 17 polysomy. J Clin Pathol. 2008;61(1):89–94.
Varis A, Zaika A, Puolakkainen P, Nagy B, Madrigal I, Kollola A, Väyrynen A, Kärkkäinen P, Moskaluk C, El-Rifai W, Knuutila S. Coamplified and overexpressed genes at ERBB2 locus in gastric cancer. Int J Cancer. 2004;109(4):548–53.
Wang S, Hossein Saboorian MH, Frenkel EP, Haley BB, Siddiqui MT, Gokaslan S, Hynan L. Ashfaq R: Aneusomy 17 in breast cancer: Its role in Her2/neu protein expression and implication for clinical assessment of Her-2/neu status. Mod Pathol. 2002;15(2):137–45.
Seidal T, Balaton AJ, Battifora H. Interpretation and quantification of immunostains. Am J Surg Pathol. 2001;25(9):1204–7.
Pauletti G, Godolphin W, Press MF, Slamon DJ. Detection and quantitation of HER-2/neu gene amplification in human breast cancer archival material using fluorescence in situ hybridization. Oncogene. 1996;13(1):63–72.
Park DI, Yun JW, Park JH, Oh SJ, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Yoo CH, Son BH, Cho EY, Chae SW, Kim EJ, Sohn JH, Ryu SH, Sepulveda AR. HER-2/neu Amplification Is an Independent Prognostic Factor in Gastric Cancer. Dig Dis Sci. 2006;51(8):1371–9.
Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, Haney J, Witta S, Danenberg K, Domenichini I, Ludovini V, Magrini E, Gregorc V, Doglioni C, Sidoni A, Tonato M, Franklin WA, Crino L, Bunn PA Jr, Varella-Garcia M. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97(9):643–55.
Cappuzzo F, Varella-Garcia M, Shigematsu H, Domenichini I, Ceresoli GL, Bartolini S, Rossi E, Ludovini V, Gregorc V, Toschi L, Franklin WA, Crino L, Gazdar AF, Bunn PA Jr, Hirsch FR. Increased HER2 gene copy number is associated with response to gefitinib therapy in epidermal growth factor receptor-positive nonsmall-cell lung cancer patients. J Clin Oncol. 2005;23(22):5007–18.
Tsuda H. HER-2 (c-erbB-2) test update: present status and problems. Br Cancer. 2006;13(3):236–48.
Baselga J. The EGFR as a target for anticancer therapy-focus on cetuximab. Eur J Cancer. 2001;37(Suppl 4):S16–22.
Leyland-Jones B. Trastuzumab: hopes and realities. Lancet Oncol. 2002;3(3):137–44.
Tripathy D, Slamon DJ, Cobleigh M, et al. Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol. 2004;22(6):1063–70.
Tuma RS. Trastuzumab trials steal show at ASCO meeting. J Natl Cancer Inst. 2005;97(12):870–1.
Kawaguchi Y, Kono K, Mimura K, Mitsui F, Sugai H, Akaike H, Fujii H. Targeting EGFR and HER-2 with cetuximab- and trastuzumab-mediated immunotherapy in oesophageal squamous cell carcinoma. Br J Cancer. 2007;97(4):494–501.
Gros SJ, Kurschat N, Dohrmann T, Reichelt U, Dancau AM, Peldschus K, Adam G, Hoffman RM, Izbicki JR, Kaifi JT. Effective therapeutic targeting of the overexpressed HER-2 receptor in a highly metastatic orthotopic model of esophageal carcinoma. Mol Cancer Ther. 2010;9(7):2037–45.
Acknowledgments
The authors thank Mr. Guo-sheng Xiong and Mis. Hong Hu for providing technical assistance, Dr. Ya-bing Huang and Dr. Lin Liu for assistance in standardizing the FISH protocol, Dr. Xiao-fan Li for revising this article.
Conflict of interests
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhan, N., Dong, WG., Tang, YF. et al. Analysis of HER2 gene amplification and protein expression in esophageal squamous cell carcinoma. Med Oncol 29, 933–940 (2012). https://doi.org/10.1007/s12032-011-9850-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-9850-y