Abstract
As a transcription factor belonging to the basic helix-loop-helix leucine-zipper subgroup, AP-4 can control target gene expression by altering cell signal transduction, and regulate growth, development, and cell apoptosis. Under pathological circumstances, it is involved in tumorigenesis. Herein, immunohistochemistry and real-time PCR were used to detect the transcription factor AP-4 expression in gastric cancer, and these data were examined for correlation with histology, pTNM stage, and prognosis. The AP-4 expression rate was 83.67% in a total of 98 gastric cancer tissues, which was significantly higher than 40.91% in non-neoplastic tissues; AP-4 mRNA relative expression shows a significant difference between gastric cancer and normal tissues, and AP-4 expression has a significantly positive correlation with the depth of tumor invasion (P < 0.0001), degree of tumor differentiation (P = 0.0058), lymph node metastasis (P = 0.0255), and pTNM stage (P = 0.001). Survival analysis showed that AP-4-positive patients’ median survival time (12.60 months) was significantly shorter than that (41.40 months) of AP-4-negative patients. AP-4 expression in gastric cancer is associated with clinicopathological parameters of gastric cancer, such as differentiation, lymph node metastasis, depth of invasion (P = 0.0010), and pTNM stage. What’s more, AP-4 overexpression indicated a worse prognosis for patients. So AP-4 may be a molecular marker to predict the progression and prognosis of the tumor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite a decrease in incidence in recent decades, gastric cancer is one of the most common malignant neoplasms in the world [1]. More than one million new cases are diagnosed each year, especially in East Asia, like Japan, Korea, and China. In these countries gastric cancer remains the most common cause of cancer related deaths [2]. Until now, A number of studies have be carried out, but the precise nosogenesis remains unknown [3].
It is known that transcription factors belong to the helix-loop-helix family, which are important regulatory components [4, 5]; they play an important role in cell proliferation and differentiation, expression of intracellular genetic information, and other essential processes [6, 7]. As a member of the basic helix-loop-helix leucine-zipper (bHLH-LZ) subgroup of bHLH proteins [8], activating enhancer binding protein 4(AP-4) is an ubiquitously expressed transcription factor initially identified as a cellular protein that binds to the simian virus 40 enhancer and activates viral late gene transcription [9], and may control transcriptional networks during cellular differentiation by homodimer formation and binding to the symmetrical DNA sequence CAGCTG [9–12]. It was reported that AP-4 could regulate expression of some genes, such as angiotensinogen [13], APH-1A [14], Caspase-9 [15], PAHX-AP1 [16], human proenkephalin [17], and E7 oncoprotein [18], and it may play a role in the expression of the pancreatic exocrine gene family [19]. Recently, the overexpression of AP-4 in colorectal cancer, breast cancer, and prostate cancer was reported [11, 20, 21], but there has been no research carried out concerning AP-4 in gastric cancer. In this study, we will examine the AP-4 conditions of expression in gastric cancer and its relationship with prognosis.
Patients and methods
Patients and tissue samples
Gastric carcinoma specimens and normal tissue were obtained from 98 patients who underwent surgical gastrectomy with lymphadenectomy between 2005 and 2007. All the patients received a definite diagnosis by a clinical pathologist before operation. There were 68 male and 30 female patients with a mean age of 55.07, ranging from 23 to 80 years of age. Pathological types include adenocarcinoma, signet ring cell cancer, and mucinous carcinoma, with those that were signet ring cell and mucinous being considered poorly differentiated [22]. Among the 98 cases, 23, 7, 12, and 56 were classified as pTNM stage I, II, III, and IV, respectively (UICC, 2009). Folfox4 chemotherapy regimen was applied to the patients according to the NCCN guideline.
Immunohistochemistry
Fresh tissues were fixed with 4% paraformaldehyde, dehydrated in graded alcohols, embedded in paraffin, and cut into 4-μm sections. The sections were deparaffinized in xylene, rehydrated, and then washed in phosphate buffered saline (PBS). Tissue sections were subjected to antigen retrieval by boiling in 0.01 mol/L sodium citrate through microwave processing. After being washed with PBS, nonspecific background reactions were blocked by incubation with 3% H2O2 in methanol for 20 min at room temperature, and then blocked with 10% normal goat serum for 30 min. The sections were incubated with mouse anti-human TFAP4 monoclonal antibody (dilution 1:100, Sigma–Aldrich, Shanghai) overnight at 4°C, followed by three washes with PBS after which the sections were incubated with secondary antibody (goat–anti-mouse IgG, dilution 1:100, Boster, China) for 1 h at 37°C. After another three washes, color developing was performed by incubation with 3-3′-diaminobenzene tetrahydrochloride (DAB). Finally, the sections were counterstained with hematoxylin and examined microscopically. The primary antibody was replaced by PBS for negative control staining.
Evaluation of Immunostaining
AP-4 expression was evaluated by selecting ten fields randomly and counting a total of 200 tumor cells every field on high-power field (×400), then the percentage of stained cells was calculated. The results were graded as: negative (−), 0 ~ 10% of tumor cells stained; weakly positive (+), 11 ~ 25% of tumor cells stained; moderately positive (++), 26 ~ 50% of tumor cells stained; and strongly positive (+++), more than 50% of cancer cells positive.
Quantitative real-time PCR
For the detection of the differential expression of AP-4, quantitative real-time PCR was carried out for 50 samples selected from 98 samples. Total RNA extraction was performed using RNAiso Plus (Takara, Japan) according to the manufacturer’s protocol. The cDNAs from total RNA were synthesized using with PrimeScript® RT reagent Kit (Takara, Japan). The following primers were designed by Premier Primer 5.0 software: for human AP-4 forward primer 5′-GAGCCAGCCTGGGATTGTC-3′ and reverse primer 5′-GTGCTTAAAGGAGAAAGAAGAAAACC-3′; for human GAPDH forward primer 5′-TGTTGCCATCAATGACCCCTT-3′ and reverse primer 5′-CTCCACGACGTACTCAGCG-3′. Real-time PCR was performed using ABI StepOne Plus (Applied Biosystems, Singapore). The concentrations of the reagents were adjusted to reach a final volume of 20 μL, containing 2 μL reverse-transcribed product, 10 μl of Fast SYBR® Green Master Mix (Applied Biosystems, Foster City, CA), and 0.5 μl of 10 μM forward and reverse primers of AP-4 with GAPDH as an internal control. The reaction was carried out by 45 amplification cycles of 95°C for 3 s and 60°C for 30 s. The fold change for gene expression between the cancer and normal samples was calculated by using 2−ΔΔCt method.
Statistical analysis
The χ2 test was used to compare the relationship of over expression with pathological features and pTNM stage. The mRNA expression difference was analyzed with paired t-test. We used the Kaplan–Meier method to construct a survival curve, and the log-rank test analyzed cumulative survival durations. Cox’s proportional hazards model with forced entry of variables was employed for multivariate survival analysis. SPSS 12.0 for Windows software was used. P < 0.05 was considered significant and all tests are two-sided.
Results
Expression of AP-4
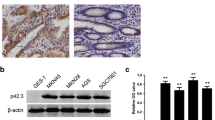
The expression of AP-4 in human gastric malignant tissues and normal tissues was analyzed by immunohistochemistry. AP-4 was predominantly expressed in the nucleus of epithelial cells. AP-4 was absent or weakly expressed in normal tissue, Positive cells tended to locate at the base of the crypt. In 83.67% (82 of 98 cases) of gastric cancer tissues AP-4 was overexpressed, significantly higher than 40.91% in nonneoplastic tissues, with a significant difference in the expression rates of AP-4 between the cancer and the normal tissues (χ2 = 28.7116 P < 0.0001)(Table 1, Fig. 1). The relationship between AP-4 expression and clinical pathological features of the patients was further analyzed. The relationship is shown in Table 2. The results revealed a positive association of AP-4 expression with degree of tumor differentiation (P = 0.0058); in poorly differentiated tumors, AP-4 showed high expression (Fig. 2). The intensity of immunostaining in AP-4 positive gastric cancer was intense in 33 of 98 (33.67%), moderate in 24 of 98 (24.49%), and mild in 25 of 98 (25.51%) cases.
Expression patterns of AP-4 in normal gastric mucosa and gastric cancer tissues. Positive cells widely distribute in gastric cancer tissues, strongly staining. In normal tissues, positive cells tended to locate at the base of the crypt. a–d tumor tissues, e–f normal tissues. Original magnification, a, b, e, f ×400, c, d, g, h ×200
In addition, AP-4 expression demonstrated a significantly positive correlation with the depth of tumor invasion (P < 0.0001), and AP-4 expression in patients with lymph node metastasis was significantly higher than that in patients without metastasis (P = 0.0255). According to TNM classification, stage I and II tumors showed significantly lower rates compared to stages III and IV (P = 0.0455). However, there was no significant difference in AP-4 expression and distant metastasis (P = 0.1559). There was no significant relationship found between AP-4 expression and gender or age (P = 0.3009, P = 0.4042) (Table 2).
We further detected the relative mRNA expression in a subtotal of 45 patients making use of quantitative real-time PCR and found that the mRNA level in the tumor tissue was 4.82-fold higher than in the normal tissue, with significant differences between them (P < 0.0001)(Fig. 3).
Survival analysis
Follow-up information was available on 98 gastric carcinoma patients for periods ranging from 6 months to 5 years. The median survival time of AP-4-positive patients (12.60 months) was significantly shorter than that of AP-4-negative patients (41.40 months). Univariate analysis using log-rank test and Kaplan–Meier test indicated the cumulative survival rate of patients with positive AP-4 expression to be obviously higher than without its expression (P = 0.0011; Fig. 4a and Table 3). This indicates that AP-4 expression decreased patients’ survival durations. In addition, the depth of tumor invasion (P < 0.0001) (Fig. 4b), TNM stage (P < 0.0001) (Fig. 4e), differentiation degree (P = 0.0075) (Fig. 4f) but not sex (P = 0.5410), age (P = 0.3894), lymph node metastasis (P = 0.0654) (Fig. 4c), or distant metastasis (P = 0.6201) (Fig. 4d) significantly influenced patient survival time (Table 3).
Kaplan–Meier survival curves of gastric cancer patients with respect to AP-4 expression, depth of invasion, lymph node metastasis, distant metastasis, TNM stage, and pathological types. A significant difference in survival was observed between the AP-4-positive and AP-4-negative groups (a); depth of tumor invasion (b), lymph node metastasis (c), and TNM stage (e) significantly influenced patient survival time; differentiation degree (f), and distant metastasis (d) had no affect on the extension of survival time
Multivariate analysis using Cox’s proportional hazard model indicated that depth of invasion (P = 0.0077), lymph node metastasis (P < 0.0001), differentiation degree (P = 0.0159), TNM stage (P = 0.0005), and AP-4 expression (P < 0.0001), but not patient sex (P = 0.3009), age (P = 0.4042), or distant metastasis (P = 0.7279), were independent prognostic factors of gastric carcinomas (Table 3).
Discussion
It was the pathologic stage and histologic grade that influenced the prognosis of patients with gastric cancer in this study, but these indicators do not always predict the progression of the tumor. There are a few studies which have indicated that some molecular markers may play important roles in the progression of gastric carcinomas [23, 24].
AP-4, belonging to the rapidly growing group of HLH proteins [10] which include enhancer binding proteins and transcription factors involved in differentiation and cellular proliferation [25–29], affects cell cycle events and apoptosis [12] and is located at chromosome 16p13.3 [30]. Recently, it has been reported that AP-4 was upregulated in colon carcinoma, breast cancer, and prostate cancer [11, 20, 21]. In addition, AP-4 positive expression indicated bad prognosis, with significance over grade, node status, size in ER + breast cancer, and a possible association with chemo-sensitivity [31].
In the present study, the AP-4 expression pattern was observed in gastric normal mucous and carcinomas, and it was significantly higher in carcinoma than in normal tissue, which indicated that the expression of AP-4 was upregulated in gastric cancer. Previous studies have suggested that AP-4 may repress apoptosis of gastric cancer cells to play an important role in the development and progression of stomach malignant tumors [11, 12]. It was reported that loss of p21 expression correlated with promotion of neoplasia, tumorigenesis, and progression in gastric cancer [32, 33]. Overexpression of c-myc may also play a role in the development and metastasis of stomach carcinomas [34, 35], and negative expression of p21 and positive expression of c-myc has been reported to be associated with an unfavorable prognosis [36, 37]. AP-4 could encode a c-MYC-inducible repressor to inhibit p21 expression [11], so AP-4 may be involved in carcinogenesis and tumor progression by inhibition of p21.
Analyzing the relationship between AP-4 expression and clinicopathological parameters of gastric cancer, we found that expression of AP-4 was significantly correlated with the progression of stomach carcinoma, the differentiation, lymph node metastasis, depth of invasion, and pTNM stage. Additionally, survival time in the AP-4 positive cases was much shorter than the AP-4-negative cases. These results indicate a relationship between AP-4 overexpression and gastric cancer development and metastasis. However, there was no statistical significance between AP-4 expression and distant metastasis, or survival time and distant metastasis although the reason may be that there were few patients with distant metastasis in this study.
In summary, AP-4 expression in gastric cancer is associated with worse prognosis; therefore it may be a useful prognostic factor for predicting progression of carcinoma and the outcome of gastric cancer patients. We believe that the control of AP-4 expression may be of far reaching importance for the regulation of carcinogenesis and progression, although further investigation is still needed to explore the exact function of AP-4 in gastric carcinogenesis.
References
Correia M, Machado JC, Ristimaki A. Basic aspects of gastric cancer. Helicobacter. 2009;14(Suppl 1):36–40.
Wu K, Nie Y, Guo C, Chen Y, Ding J, Fan D. Molecular basis of therapeutic approaches to gastric cancer. J Gastroenterol Hepatol. 2009;24(1):37–41.
Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71(2):127–64.
Jones S. An overview of the basic helix-loop-helix proteins. Genome Biol. 2004;5(6):226.
Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20(2):429–40.
Grau R, Punzon C, Fresno M, Iniguez MA. Peroxisome-proliferator-activated receptor alpha agonists inhibit cyclo-oxygenase 2 and vascular endothelial growth factor transcriptional activation in human colorectal carcinoma cells via inhibition of activator protein-1. Biochem J. 2006;395(1):81–8.
Atchley WR, Fitch WM. A natural classification of the basic helix-loop-helix class of transcription factors. Proc Natl Acad Sci USA. 1997;94(10):5172–6.
Lee SU, Song HO, Lee W, Singaravelu G, Yu JR, Park WY. Identification and characterization of a putative basic helix-loop-helix (bHLH) transcription factor interacting with calcineurin in C. elegans. Mol Cells. 2009;28(5):455–61.
Mermod N, Williams TJ, Tjian R. Enhancer binding factors AP-4 and AP-1 act in concert to activate SV40 late transcription in vitro. Nature. 1988;332(6164):557–61.
Hu YF, Luscher B, Admon A, Mermod N, Tjian R. Transcription factor AP-4 contains multiple dimerization domains that regulate dimer specificity. Genes Dev. 1990;4(10):1741–52.
Jung P, Menssen A, Mayr D, Hermeking H. AP4 encodes a c-MYC-inducible repressor of p21. Proc Natl Acad Sci USA. 2008;105(39):15046–51.
Jung P, Hermeking H. The c-MYC-AP4–p21 cascade. Cell Cycle. 2009;8(7):982–9.
Cui Y, Narayanan CS, Zhou J, Kumar A. Exon-I is involved in positive as well as negative regulation of human angiotensinogen gene expression. Gene. 1998;224(1–2):97–107.
Wang R, Zhang YW, Zhang X, Liu R, Hong S, Xia K, et al. Transcriptional regulation of APH-1A and increased gamma-secretase cleavage of APP and Notch by HIF-1 and hypoxia. FASEB J. 2006;20(8):1275–7.
Tsujimoto K, Ono T, Sato M, Nishida T, Oguma T, Tadakuma T. Regulation of the expression of caspase-9 by the transcription factor activator protein-4 in glucocorticoid-induced apoptosis. J Biol Chem. 2005;280(30):27638–44.
Kim MY, Jeong BC, Lee JH, Kee HJ, Kook H, Kim NS, et al. A repressor complex, AP4 transcription factor and geminin, negatively regulates expression of target genes in nonneuronal cells. Proc Natl Acad Sci USA. 2006;103(35):13074–9.
Unk I, Kiss-Toth E, Boros I. Transcription factor AP-4 participates in activation of bovine leukemia virus long terminal repeat by p34 tax. Nucleic Acids Res. 1994;22(23):4872–5.
Glahder JA, Hansen CN, Vinther J, Madsen BS, Norrild B. A promoter within the E6 ORF of human papillomavirus type 16 contributes to the expression of the E7 oncoprotein from a monocistronic mRNA. J Gen Virol. 2003;84(Pt 12):3429–41.
Fodor E, Weinrich SL, Meister A, Mermod N, Rutter WJ. A pancreatic exocrine cell factor and AP4 bind overlapping sites in the amylase 2A enhancer. Biochemistry. 1991;30(33):8102–8.
Cao J, Tang M, Li WL, Xie J, Du H, Tang WB, et al. Upregulation of activator protein-4 in human colorectal cancer with metastasis. Int J Surg Pathol. 2009;17(1):16–21.
Lin TX, Huang J, Huang H, Cai QQ, Xu KW, Yin XB, et al. Identification of response element gene sequence for non-steroid hormone transcription factors for the activation and up-regulation of L-plastin expression in prostate cancer. Zhonghua Nan Ke Xue. 2005;11(10):731–4.
Zheng L, Weng M, He J, Yang X, Jiang G, Tong Q. Expression of resistin-like molecule beta in gastric cancer: its relationship with clinicopathological parameters and prognosis. Virchows Arch. 2010;456(1):53–63.
Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48(13):3554–60.
Akyurek N, Akyol G, Dursun A, Yamac D, Gunel N. Expression of MUC1 and MUC2 mucins in gastric carcinomas: their relationship with clinicopathologic parameters and prognosis. Pathol Res Pract. 2002;198(10):665–74.
Edmondson DG, Olson EN. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989;3(5):628–40.
Lassar AB, Buskin JN, Lockshon D, Davis RL, Apone S, Hauschka SD, et al. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989;58(5):823–31.
Ludwig SR, Habera LF, Dellaporta SL, Wessler SR. Lc, a member of the maize R gene family responsible for tissue-specific anthocyanin production, encodes a protein similar to transcriptional activators and contains the myc-homology region. Proc Natl Acad Sci USA. 1989;86(18):7092–6.
Murre C, McCaw PS, Vaessin H, Caudy M, Jan LY, Jan YN, et al. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989;58(3):537–44.
Murre C, McCaw PS, Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989;56(5):777–83.
Bae Y, Kim H, Namgoong H, Baek M, Lee J, Hwang D, et al. Characterization of microsatellite markers adjacent to AP-4 on chromosome 16p13.3. Mol Cell Probes. 2001;15(5):313–5.
Buechler S. Low expression of a few genes indicates good prognosis in estrogen receptor positive breast cancer. BMC Cancer. 2009;9:243.
Xi YG, Ding KY, Su XL, Chen DF, You WC, Shen Y, et al. p53 polymorphism and p21WAF1/CIP1 haplotype in the intestinal gastric cancer and the precancerous lesions. Carcinogenesis. 2004;25(11):2201–6.
Seo YH, Joo YE, Choi SK, Rew JS, Park CS, Kim SJ. Prognostic significance of p21 and p53 expression in gastric cancer. Korean J Intern Med. 2003;18(2):98–103.
Hajdu J, Kozma L, Kiss I, Szentkereszty Z, Szakall S, Ember I. Is the presence of distant metastasis associated with c-myc amplification in gastric cancer? Acta Chir Hung. 1997;36(1–4):119–21.
Onoda N, Maeda K, Chung YS, Yano Y, Matsui-Yuasa I, Otani S, et al. Overexpression of c-myc messenger RNA in primary and metastatic lesions of carcinoma of the stomach. J Am Coll Surg. 1996;182(1):55–9.
Kouraklis G, Katsoulis IE, Theocharis S, Tsourouflis G, Xipolitas N, Glinavou A, et al. Does the expression of cyclin E, pRb, and p21 correlate with prognosis in gastric adenocarcinoma? Dig Dis Sci. 2009;54(5):1015–20.
Han S, Kim HY, Park K, Cho HJ, Lee MS, Kim HJ, et al. c-Myc expression is related with cell proliferation and associated with poor clinical outcome in human gastric cancer. J Korean Med Sci. 1999;14(5):526–30.
Acknowledgments
We thank Dr. Ie-Ming Shih and Dr. Chen Xu for critical reading of the manuscript and helpful suggestions, and thank Lin Xueke, Li Wei, Li Hang, Ma jingwei, Peng Zhao, Wang Xuesong, Piao Long, Liu Ke, Li Jiang, Xu Fei, Lv Qing, and Zhang Guanchao for their expert technical assistance. This work was supported by the National Natural Science Foundation of China (No.30400432).
Conflict of interest
All authors declare no conflict or competing interest for this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Liu Xinghua and Zhang Bo contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xinghua, L., Bo, Z., Yan, G. et al. The overexpression of AP-4 as a prognostic indicator for gastric carcinoma. Med Oncol 29, 871–877 (2012). https://doi.org/10.1007/s12032-011-9845-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-9845-8