Abstract
The aim of this study is to analyze the prognostic value of androgen receptor (AR) expression for patients with triple-negative breast cancer (TNBC). Clinical data of these patients were collected and analyzed, and immunohistochemical staining for AR was performed on tissue microarrays of operable breast cancer from 287 patients with TNBC, who were treated at Sun Yat-sen University Cancer Center from January 1995 to December 2008. AR expression was found in 25.8% of the cases with TNBC. TNBC patients with AR negative have a higher proportion of positive lymph node. A significant correlation was found between AR expression and disease-free survival (DFS) and overall survival (OS). Univariated analysis indicated that AR expression had a significant prognostic value in TNBC patients, whereas multivariate analysis indicated that AR was a significant independent prognostic factor of DFS (P = 0.032) in all patients. Our results suggested that AR was a favorable prognostic factor of DFS and OS in patients with TNBC. Therefore, TNBC may be further divided into two subtypes according to AR status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple-negative breast cancers (TNBC) are characterized by the lack of expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2/neu). TNBC accounts for about 15–20% of all breast cancers [1–4]. It is associated with poor overall prognosis, a high probability of early relapse after diagnosis, and increased risk of death after relapse. These clinical characteristics represent a major challenge for physicians in optimizing patient management [5–8]. TNBC have not benefited from anti-HER2 drugs or the endocrine treatment. However, several pathways of interest are being studied. One of the areas is the role of the androgen receptor (AR).
AR, a member of the steroid hormone receptor family, is expressed in more than 70% of breast cancers and has been implicated in breast cancer pathogenesis [9, 10]. Emerging evidence demonstrates that women with high androgen levels have a increased risk of breast cancer [11], androgen can stimulate growth in AR(+)/ER(−) cell, and this proliferative effect was abrogated by the addition of the AR antagonist [12]. These findings suggest a connection between androgens and breast carcinogenesis, and AR may serve as a therapeutic target for triple-negative breast cancers. Also, recent retrospective studies suggested that AR status was a significant prognostic marker of breast cancer [13, 14].
Despite the prevalence of AR expression in breast cancer, the prognostic values of AR expression in TNBC are less well characterized. The purposes of this study were to evaluate the expression of AR in TNBC and to demonstrate the correlation with prognosis of patients with TNBC.
Materials and methods
Patients and tissues
This retrospective study comprised 287 female patients with TNBC diagnosed without any evidence of distant metastasis at the time of surgery between 1995 and 2008 at Sun Yat-sen University Cancer Center. Tissue samples were obtained from the patients through curative surgical resection. All specimens were formalin-fixed, paraffin-embedded. Clinical data, including patient age at diagnosis, menstrual status, tumor size, lymph node status, pathologic stage, treatment (surgery, chemotherapy, and radiotherapy), tumor recurrence, and follow-up status, were retrospectively obtained from hospital medical records. The data analysis was approved by our cancer center review board.
Tissue microarray
Tissue microarrays were constructed as described previously [15]. Briefly, 287 formalin-fixed, paraffin-embedded tissue blocks containing breast cancer specimens were retrieved from the archives of the Department of Pathology. Representative areas of each invasive carcinoma were identified on the corresponding slides stained with hematoxylin and eosin. Tissue cylinders with 1 mm diameter were punched from each donor tissue block and entered into a recipient paraffin block using a tissue microarrayer. The recipient paraffin block was subsequently cut, and the slices were transferred with adhesive tape onto coated slides. Then, the slides were dipped in paraffin to prevent oxidation. Each sample was arrayed in triplicate to minimize tissue loss and to overcome tumor heterogeneity.
Immunohistochemistry
Tissue microarray sections were immunohistochemically stained for AR. Briefly, tissue microarray slides generated from the paraffin-embedded tissue blocks were deparaffinized and rehydrated for 5 min. After microwave pretreatment in citrate buffer (pH 6.0) for antigen retrieval, the slides were immersed in 0.3% (vol/vol) hydrogen peroxide for 20 min to block endogenous peroxidase activity. The slides were then washed and incubated overnight at 4°C with primary antibodies against AR (1:500 dilution; DAKO). After a second incubation with biotinylated anti-goat antibodies, the slides were incubated with peroxidase-labeled streptavidin. The reaction products were visualized by immersing the slides in diaminobenzidine tetrachloride and counterstaining with Harris hematoxylin. Staining for AR was considered positive only if a minimum of 5% of definite tumor cells show positive reaction.
Statistical analysis
Statistical analysis was performed using SPSS16.0 software. Chi-square test was used to investigate the significance of the relationship between AR and the individual variables. The relationship between AR expression and the clinical outcomes was estimated through both univariate and multivariate analyses. The disease-free survival (DFS) and overall survival (OS) curves were estimated using the Kaplan–Meier method, and the differences in the survival curves were compared using the log-rank test. A multivariate analysis was performed using Cox’s regression model. The P values ≤0.05 denote statistical significance.
Results
Patient and tumor characteristics
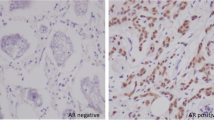
A total of 287 TNBC patients were grouped according to their AR status (positive or negative). Among the 287 tumor specimens, 74 (25.8%) were AR positive (Fig. 1). The association of AR expression with various clinicopathological parameters is listed in Table 1. When compared with TNBC patients with AR positive, patients with AR negative have a higher proportion of positive lymph node. The other clinicopathologic parameters, including tumor size, pathologic stage, histopathology, adjuvant chemotherapy, and adjuvant radiotherapy, were not significantly different between the two groups (Table 1).
Survival
As of December 2009, the median follow-up time was 72 months (range, 8–182 months). Of all 287 patients, 67 relapsed and 48 died. The five-year DFS and OS of all patients were 84.9 and 87.8%, respectively. When the patients were stratified in terms of AR status, the five-year DFS for AR-positive and AR-negative patients were 87.0 and 74.2%, respectively. The overall survival rates of AR-positive and AR-negative patients were 94.2 and 82.3%, respectively. AR-positive patients had a significant longer DFS (HR = 0.40, P = 0.008) and OS (HR = 0.34, P = 0.011, data not shown) than those of AR-negative patients (Fig. 2, Table 2).
Univariate analysis and multivariate analysis
Statistically significant predictors of DFS within the univariate analysis are listed in Table 2. Positive lymph nodes, higher stage, and positive AR were correlated with longer DFS. The patients with tumor cells positive for AR expression had significantly better outcomes in terms of DFS (P < 0.001) than patients with negative AR expression. In the multivariate analysis, positive AR remained a significant predictor of DFS when entered into a model containing all clinicopathologic variables (P = 0.032) (Table 2).
Discussion
AR and its ligand androgens may have some essential role in breast cancer, but the prognostic value of AR expression has not been well characterized. TNBC is characterized by an aggressive clinical history and overall poor prognosis, and there being no effective therapy represents one of the main reasons. However, several pathways of interest are being studied. One of the areas is the role of the androgen receptor (AR).
Our results demonstrated that the positive rate of AR was 25.8% in TNBC. The result confirmed previous studies indicating that AR expression in ER-negative breast cancers was lower than that in ER-positive breast cancers, in which AR expression exceeded 70% [9, 10].
When compared with TNBC patients with AR positive, patients with AR negative have a higher proportion of positive lymph node. This could become one of the reasons for poor prognosis in AR-negative patients. Our data demonstrated that AR expression was significantly associated with a longer DFS and OS. Other authors have also found that AR-negative TNBC patients have a trend toward a shorter DFS and OS than those patients with AR-positive tumors [14].
Our results showed that AR expression was a favorable prognostic factor of DFS and OS. Multivariate analysis indicated a significant relationship between AR expression and DFS or OS. TNBC patients with AR positive had a significantly decreased recurrence and death risk. These results could suggest that AR-positive tumor cells have a low biological aggressiveness.
In conclusion, our study indicated that TNBC may be further divided into two subtypes according to AR status. AR expression may be a favorable predictor of TNBC patient outcomes. AR-targeted therapy will become possible to improve survival for patients with TNBC.
References
Dent R, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34.
Carey LA, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–34.
Tischkowitz M, et al. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007;7:134.
Bauer KR, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer. 2007;109:1721–8.
Hines SL, et al. Clinical outcomes after a diagnosis of brain metastases in patients with estrogen- and/or human epidermal growth factor receptor 2-positive versus triple-negative breast cancer. Ann Oncol. 2008;19(9):1561–5.
Lin Nu, et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113(10):2638–45.
Nam BH, et al. Breast cancer subtypes and survival in patients with brain metastases. Br Cancer Res. 2008;10(1):R20.
Schneider BP, et al. Triple negative breast cancer: risk factors to potential targets. Clin Cancer Res. 2008;14(24):8010–8.
Brys M. Androgens and androgen receptor: do they play a role in breast cancer? Med Sci Monit. 2000;6:433–8.
Liao DJ, et al. Roles of androgens in the development, growth, and carcinogenesis of the mammary gland. J Steroid Biochem Mol Biol. 2002;20:175–89.
Hankinson SE, et al. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90:1292–9.
Doane AS, et al. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. 2006;25:3994–4008.
Agoff SN, et al. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol. 2003;120:725–31.
Gonzalez-Angulo AM, et al. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res. 2009;15:2472–8.
Kononen J, et al. Tissue microarrays for highthroughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–7.
Conflict of interest
All authors have no financial disclosures and conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Shusen Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
He, J., Peng, R., Yuan, Z. et al. Prognostic value of androgen receptor expression in operable triple-negative breast cancer: a retrospective analysis based on a tissue microarray. Med Oncol 29, 406–410 (2012). https://doi.org/10.1007/s12032-011-9832-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-9832-0