Abstract
The laryngeal squamous cell carcinoma (LSCC) is one of the most common cancers threatening people’s life. CXC-chemokine receptor type 2 (CXCR2) was reported to play critical roles in angiogenesis, tumorigenesis, and metastasis of several cancers such as colon cancer, melanoma, lung cancer, and so on. However, the expression of CXCR2 in LSCC and its association with clinical characters of LSCC remain unclear. Quantitative real-time reverse transcription-PCR and immunohistochemistry were used, respectively, to analyze the mRNA level and protein level of CXCR2 in 109 cases of LSCC tissues and 28 cases of tumor-adjacent normal tissues. The expression of CXCR2 in LSCC was significantly higher than that in tumor-adjacent tissues. Moreover, the expression level of CXCR2 protein in LSCC was significantly related to lymph node metastasis (P = .022), histopathological grade (P = .038), and 5 years’ survival (P = .007). Cox regression analysis revealed that CXCR2 expression (P = .031), as well as lymphatic metastasis (P = .026) and TNM classification (P = .042), is an independent prognostic marker of LSCC. High expression of CXCR2 is also associated with short survival of LSCC patients. Our data indicate that the expression of CXCR2 is associated with the development and progression of LSCC. CXCR2 expression may serve as an independent prognostic marker for LSCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Head and neck cancer accounts for about 6% of all human cancers. There are approximately 47,560 new cases in the USA and at least 500,000 cases worldwide each year [1, 2]. Approximately, one-fourth of all head and neck cancers are laryngeal squamous cell carcinoma (LSCC) [1, 3]. Despite many advanced methods for diagnosis and treatment, the mortality rate of LSCC has not changed too much (5 year–survival rate is 64%). Now, the incidence age of LSCC is getting younger (40–50 years old) [4, 5]. Therefore, it is urgent to improve the prognosis of LSCC patients.
Chemokines consist of a group of structurally related small molecular weight peptides (8–14 kD) that are soluble and mostly basic. The family of chemokines contains four members, designated as C, CC, CXC, and CX3C, on the basis of the position of their cysteine residues [6]. The CXC chemokines can be divided into ELR+ and ELR− subgroups depending on the presence or absence of the sequence motif (glutamic acid-leucine-arginine, ELR) in the N-terminus of the molecule [7]. The balance of the two groups may determine the tendency of angiogenesis and tumor growth [8]. CXCR2, also known as IL-8RB, has been shown to bind all of the ELR+ CXC chemokines, including IL-8; ENA-78; GRO-α, -β, and -γ; neutrophil-activating protein-2 (NAP-2); and GCP-2 with high affinity [9–13]. CXCR2 is a member of the seven-transmembrane domain rhodopsin-like G protein-coupled receptors, and it mediates functions of ELR(+) CXC chemokines such as angiogenesis [14]. CXCR2 is reported to participate in the pathogenesis of chronic inflammation, sepsis, and atherosclerosis [15]. In addition, a large body of evidence demonstrated that CXCR2 plays critical roles in angiogenesis, tumorigenesis, and metastasis of several human cancers such as colorectal carcinoma, melanoma, lung cancer, prostate carcinoma, pancreatic carcinoma, and so on [16–24]. However, the possible roles of CXCR2 expression in LSCC have not been determined.
In this study, we examined the expression levels of CXCR2 in LSCC tissues and tumor-adjacent normal tissues. Our data showed that compared to tumor-adjacent normal tissues, LSCC tissues have higher expression of CXCR2. In addition, our data suggested that CXCR2 expression in LSCC is associated with lymph node metastasis, tumor grade, and 5-year survival. We also revealed that CXCR2 expression might serve as the prognostic marker for LSCC.
Materials and methods
Patient specimens
One hundred and nine cases of LSCC were obtained from the Department of Pathology, the Affiliated Hospital of Nantong University between 2000 and 2009. Diagnosis of LSCC was performed according to the latest WHO criteria [25] and TNM stage classification (UICC 2002). The mean age of patients at the time of surgery was 60.8 years (ranging from 29 to 87 years old). Clinical data include gender, age, tobacco use, alcohol consumption, pTNM stage, lymph node metastasis, histopathological grade, and 5 years’ follow-up survival. Follow-up of all cases started from post-operation through May 2010. Before surgery, none of the patients had received radiation therapy, neoadjuvant chemotherapy, or immunotherapy. Nantong Tumour Hospital’s ethics committee approved the study protocol.
Tissue microarrays construction (TMA)
We use formalin-fixed, paraffin-embedded tissues from 109 LSCC and 28 tumor-adjacent normal tissues for our present study. TMA was produced by the Shanghai Xinchao Biotech Co., Ltd. Core tissue biopsies (2 mm in diameter) were taken from individual paraffin-embedded LSCC and arranged in the new recipient paraffin blocks. The tissue microarray was cut into 4-μm sections and placed on super frost charged glass microscope slides.
Immunohistochemistry
Paraffin tissue sections (4 μm) were deparaffinized in 100% xylene and re-hydrated in descending ethanol series and water according to standard protocols. Heat-induced antigen retrieval was performed in 10 mM citrate buffer for 2 min at 100°C. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide, and non-specific binding of immunoglobulin was blocked with serum for 1 h at room temperature. Tissue sections were incubated with monoclonal mouse anti-CXCR2 antibody (dilution or working concentration, ab24963, Abcam) overnight at 4°C. After washing, the sections were incubated with biotin-labeled goat anti-rabbit antibody for 30 min at room temperature and subsequently incubated with streptavidin-conjugated horseradish peroxidase (HRP). Sections were colorized with 3,3-diaminobenzidine (DAB) chromogen solution and counterstained with hematoxylin. Visualization and documentation were accomplished with XX microscope supporting XX camera. All sections were immunostained at the same time and under the same conditions. Results were examined by two investigators in blinded manner. In the case that the two investigators disagreed, related results were evaluated by the third party; if any two of the three investigators could not come to an agreement, related sections were excluded. Results were analyzed according to the method described as before [26]. Briefly, the percentage of CXCR2-positive cells was scored as follows: 0 for 0%, 1 for 1–33%, 2 for 34–66%, and 3 for 67–100%. The intensity of CXCR2 staining was also scored into four points: 0 for negative staining, 1 for yellow color staining, 2 for light brown color staining, and 3 for brown color staining. Samples with a sum score of 0–2 were regarded to have low expression of CXCR2, and those with a sum score of 3–6 were regarded to have high expression of CXCR2.
RNA extraction and quantitative real-time reverse transcription-PCR analysis
We collected 10 samples of fresh LSCC tissues and their adjacent tissues. Tissue samples were snap-frozen in liquid nitrogen and stored at −80°C before RNA extraction. Tissues were homogenized using tissue grinders. The quality of RNA was examined by gel electrophoresis. Total RNA from LSCC tissues or tumor-adjacent normal tissues was isolated using Trizol reagent (Invitrogen, Carlsbad, CA). Two microgram of total RNA was employed for cDNA synthesis using Moloney murine leukemia virus retrotranscriptase (Promega). Real-time PCR was performed using SYBR green dye in Bio-Rad iQ50 Real-time PCR system following the manufacturer’s instructions. Primers designed with Beacon Designer 7.0 software (Palo Alto, CA, USA) were as follows: CXCR2 forward 5′-TCTGGATGCCACCGAGATTCT-3′, CXCR2 reverse 5′-AGTCCATGGCGAAACTTC-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward 5′-TGCACCACCAACTGCTTAGC-3′, GAPDH reverse 5′-GGCATGGACTGTGGTCATGAG-3′. The transcription levels of GAPDH served as a loading control.
Statistical analysis
The following clinical data were evaluated: age, pTNM stage, Lymph node metastasis, histopathological grade, five years’ survival, and other clinicopathological information. The relationship between CXCR2 expression and clinicopathological factors was analyzed by chi-square test. Survival rate was estimated by Kaplan–Meier method. Univariate and multivariate analysis was carried out using Cox’s proportional hazards regression models. P < 0.05 was defined to be significant. The statistical analysis was performed using STATA V.9.0.
Result
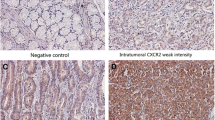
We examined the mRNA levels of CXCR2 in LSCC tissues and tumor-adjacent normal tissues by qPCR. Normalized to GAPDH, the means of CXCR2 mRNA level in laryngeal squamous cell cancer tissues (n = 10) and that in tumor-adjacent normal tissues (n = 10) were 4.66 ± 2.319 and 2.34 ± 1.51, respectively (t = 2.884, P < 0.05) (Fig. 1). Compared to tumor-adjacent normal tissues, the mRNA levels of CXCR2 in LSCC were significantly higher. Protein levels of CXCR2 were examined using immunohistochemistry. As shown in Fig. 2, compared to tumor-adjacent normal tissues, cancer tissues showed more positive staining cells and stronger staining. 73 Cases of 109 LSCC tissues (66.97%) showed high expression of CXCR2, however, only 9 cases of 28 tumor-adjacent normal tissues (32.14%) displayed high expression of CXCR2. The high expression rate of CXCR2 between cancer tissues and non-cancerous tissues showed significant difference (P = 0.001). The immunohistochemistry results are consistent with qPCR results, which suggest that the expression of CXCR2 might play an important role in the tumorigenesis of LSCC.
A1 Well-differentiated LSCC tissue (A2 and A3). The positive immunostaining of CXCR2. B1 Poorly differentiated LSCC tissue (B2 and B3). Strongly positive immunohistochemical reaction of CXCR2 in LSCC (C1). Tumor adjacent tissue (C2 and C3). Weakly positive expression of CXCR2. Original magnification ×40 in A1, A2, B1, B2, C1, and C2, ×400 in A3, B3, and C3
Correlations between various clinicopathological characteristics and CXCR2 expression in LSCC tissues were analyzed by χ2 test (Table 1). The analysis showed that high expression of CXCR2 in LSCC was significantly related to lymph node metastasis (P = 0.022), histopathological grade (P = 0.038), and 5 years’ survival (P = 0.007). However, no statistically significant correlation was found between CXCR2 expression and age, tobacco use, alcohol consumption, or pTNM stage.
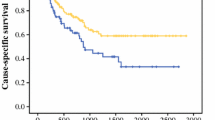
Univariate analysis showed that the life span of LSCC patients is correlated with lymph node metastasis (P < 0.001), tumor stage (P = 0.042), histopathological grade (P = 0.04), and expression level of CXCR2 as well (P = 0.001). Multivariate analysis using the Cox regression model adjusted to clinicopathological variables [tumor stage (I–II versus III–IV) and lymph node metastasis] showed that CXCR2 protein expression may serve as an independent prognostic factor for overall survival (Table 2). Moreover, we analyzed patients’ survival based on CXCR2 expression by stage separating stage II and stage III from the rest of the group. Kaplan–Meier survival curves showed that patients with high CXCR2 expression had a shorter survival time than patients with low CXCR2 expression (Fig. 3).
Discussion
Over the past two decades, even though patients with LSCC have benefited greatly from the latest advances in surgical techniques, radiation therapy and chemotherapy, the survival rate of LSCC has not improved significantly [4]. With the development of genomic and basic research, we have more knowledge about the molecular processes of tumorigenesis and progression. Cell biomarkers have been used as a variable for appraising the clinical malignancy of a cancer. Several molecular markers used for LSCC classification have been investigated, but there are few with great value in predicting patient prognosis. It is important to find some new molecular factors that may serve as prognostic markers for LSCC.
It was reported that CXCR2 and its ligands play important roles in inflammatory diseases, immune responses and wound healing [27–29]. Recently, some studies indicated that CXCR2 and its ligands participated in tumor growth and metastasis. CXCR2 was reported to bind all the ELR+ CXC chemokines with high affinity [14]. ELR+ CXC chemokines play an important role in tumor growth and progression in various tumors. All angiogenic ELR+ CXC chemokines mediate their angiogenic activity through CXCR2 [14]. Seema Singh et al. [30] found that CXCL-8-mediated angiogenesis in the regulation of melanoma growth and metastasis is dependent on CXCR2. Elena Loukinova et al. [31] reported that GRO-α can promote the growth of murine squamous cell carcinoma by a host CXCR2-dependent pathway. Aihua Li et al. [32] demonstrated that BM-derived EPC (endothelial progenitor cells) was correlated with pancreatic cancer growth and provided a cellular mechanism for CXCR2-mediated tumor neovascularization. These previous studies suggested that CXCR2 might play critical roles in tumor progression.
In the present study, we used quantitative real-time PCR and immunohistochemistry to examine the mRNA and protein levels of CXCR2 in 109 cases of LSCC and 28 cases of tumor-adjacent normal tissues. qRT-PCR result showed that LSCC tissues displayed higher level of CXCR2 mRNA compared with non-cancerous adjacent tissues (P < 0.05). This result is in accordance with previous reports in other tumors [20, 22]. Immunohistochemical analysis showed that CXCR2 protein was localized in the cell nucleus and cytoplasm of cancer cells. Some other reports showed that both the cell membrane and cytoplasm of cancer cells are positively stained with CXCR2 [22, 24]. The reason for the difference in subcellular localization of CXCR2 might be because of dissimilar antibodies and LSCC samples from various areas. Our results demonstrated that the expression of CXCR2 protein in LSCC was higher than that in adjacent normal tissues, which indicates that the expression of CXCR2 might be involved in the malignant transformation of LSCC [33]. It was reported that overexpression of CXCR2 is related to poor prognosis of various cancers including ovarian [24], breast [34], gastric cancer [35], and melanoma [36]. In our study, we found that high CXCR2 expression in LSCC was correlated with clinical pathologic characteristics including poor survival, lymph node metastasis, and stage grouping with TNM classification. Univariate analysis showed that not only tumor differentiation, stage grouping with TNM classification, and lymph node metastasis but also the expression level of CXCR2 is correlated to life span of LSCC patients. CXCR2 expression was found to be significantly related with prognosis in univariate survival analysis, and it still kept its prognosis value in multivariate survival analysis. The multivariate analysis revealed that strong expression of CXCR2 is an independent factor for poor prognosis of LSCC patients. Furthermore, we analyzed survival of LSCC patients based on CXCR2 expression by stage separating stage II and stage III from the rest of the group. The overall 5-year survival of high CXCR2 expression group was significantly shorter than that of low CXCR2 expression group. These findings suggest that CXCR2 expression may be associated with the development and progression of LSCC and that CXCR2 expression may act as an independent prognostic marker for LSCC.
Our study is the first report about the relationship between CXCR2 expression and the prognosis of LSCC patients; it will help to develop some new therapeutic strategies to improve the clinical treatments for LSCC and prolong the patients’ survival. Recently, it was reported that small molecules like SCH-479833 or SCH-527123 (antagonists targeting CXCR2) could reduce melanoma growth and angiogenesis [37]. SB225002, another antagonist of CXCR2, could block the proliferation of esophageal cancer cells [22]. In the future, more research needs to be done to confirm the therapeutic value of the regulation of CXCR2.
Conclusion
To the best of our knowledge, this is the first report that demonstrates the involvement of CXCR2 in the carcinogenesis of LSCC. In this study, we found that high expression of CXCR2 was correlated with poor survival, lymph node metastasis, and pTNM stage in LSCC patients. The overall survival of LSCC patients with high CXCR2 expression was significantly shorter than that with low CXCR2 expression. CXCR2 expression may serve as a prognostic marker in LSCC patients. Our study is helpful in understanding the roles of CXCR2 in the development and progression of LSCC. It also suggested the new therapeutic strategy by regulating CXCR2 expression to slow down the malignancy progression of LSCC and to improve the prognosis of LSCC patients.
References
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49.
Dirix P, Lambrecht M, Nuyts S. Radiotherapy for laryngeal squamous cell carcinoma: current standards. Exp Rev Anticancer Ther. 2010;10(9):1461–9.
Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116:1–13.
American Cancer Society: Cancer facts and figures. 2008
Boyle P, Ferlay J. Cancer incidence and mortality in Europe. Ann Oncol. 2005;16:481–8.
Zlotnik, A., Yoshie, O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–7.
Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95(10):3032–43
Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev. 2002;13:143–54.
Yang W, Schraw WP, Mueller SG, Richmond A. Interruption of G protein-coupling in CXCR2 does not alter ligand binding, but eliminates ligand-activation of GTPγ35S binding, calcium mobilization, and chemotaxis. Biochemistry. 1997;36:15193–200.
Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633.
Lee J, Horuk R, Rice GC, Bennett GL, Camerato T, Wood WI. Characterization of two high affinity human interleukin-8 receptors. J Biol Chem. 1992;267(23):16283–7.
Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253(5025):1280–3.
Ahuja SK, Murphy PM. The CXC chemokines growth-regulated oncogene (GRO) a, GROb, GROg, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem. 1996;271(34):20545–50.
Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR + CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269–77.
Gabellini C, Trisciuoglio D, Desideri M, Candiloro A, Ragazzoni Y, Orlandi A, Zupi G, Del Bufalo D. Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on human malignant melanoma progression. Eur J Cancer. 2009;45(14):2618–27.
Baier PK, Eggstein S, Wolff-Vorbeck G, et al. Chemokines in human colorectal carcinoma. Anticancer Res 2005;25:3581.
Singh S, Singh A, Sharma B, Singh R. CXCL8 and its cognate receptors in melanoma progression and metastasis. Future Oncol. 2010;6(1):111–6.
Ohri C, Shikotra A, Green R, Bradding P. Chemokine receptor expression in tumour islets and stroma in non-small cell lung cancer. BMC Cancer. 2010;10:172.
Reiland J, Furcht LT, McCarthy JB. CXC-chemokines stimulate invasion and chemotaxis in prostate carcinoma cells through the CXCR2 receptor. Prostate. 1999;41:78–88.
Liu Z, Yang L, Xu J, Zhang X, Wang B. Enhanced expression and clinical significance of chemokine receptor CXCR2 in hepatocellular carcinoma. J Surg Res. 2011;166(2):241–6.
Mestas J, Burdick MD, Reckamp K, Pantuck A, Figlin RA, Strieter RM. The role of CXCR2/CXCR2 ligand biological axis in renal cell carcinoma. J Immunol. 2005;175(8):5351–7.
Wang B, Hendricks DT, Wamunyokoli F, Parker MI. A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res. 2006;66(6):3071–7.
Matsuo Y, Raimondo M, Woodward TA, et al. CXC-chemokine/CXCR2 biological axis promotes angiogenesis in vitro and in vivo in pancreatic cancer. Int J Cancer. 2009;125:1027–37.
Yang G, Rosen DG, Liu G, Yang F, Guo X, Xiao X, Xue F, Mercado-Uribe I, Huang J, Lin SH, Mills GB, Liu J. CXCR2 promotes ovarian cancer growth through dysregulated cell cycle, diminished apoptosis, and enhanced angiogenesis. Clin Cancer Res. 2010;16(15):3875–86.
Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006;85:74.
Huang J, Zhang X, Tang Q, Zhang F, Li Y, Feng Z, Zhu J. Prognostic significance and potential therapeutic target of VEGFR2 in hepatocellular carcinoma. J Clin Pathol. 2011;64:343–8.
Nagarkar DR, Wang Q, Shim J, et al. CXCR2 is required for neutrophilic airway inflammation and hyperresponsiveness in a mouse model of human rhinovirus infection. J Immunol. 2009;183:6698–707.
Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21.
Devalaraja RM, Nanney LB, Du J, Qian Q, Yu Y, et al. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol. 2000;115:234–44.
Singh S, Varney M, Singh RK. Host CXCR2-dependent regulation of melanoma growth, angiogenesis, and experimental lung metastasis. Cancer Res. 2009;69(2):411–5.
Loukinova E, Dong G, Enamorado-Ayalya I, Thomas GR, Chen Z, Schreiber H, Van Waes C. Growth regulated oncogene-alpha expression by murine squamous cell carcinoma promotes tumor growth, metastasis, leukocyte infiltration and angiogenesis by a host CXC receptor-2 dependent mechanism. Oncogene. 2000;19(31):3477–86.
Li A, Cheng XJ, Moro A, Singh RK, Hines OJ, Eibl G. CXCR2-dependent endothelial progenitor cell mobilization in pancreatic cancer growth. Transl Oncol. 2011;4(1):20–8.
Richards BL, Eisma RJ, Spiro JD, Lindquist RL, Kreutzer DL. Coexpression of interleukin-8 receptors in head and neck squamous cell carcinoma. Am J Surg. 1997;174:507.
Snoussi K, Mahfoudh W, Bouaouina N, Fekih M, Khairi H, Helal AN, Chouchane L. Combined effects of IL-8 and CXCR2 gene polymorphisms on breast cancer susceptibility and aggressiveness. BMC Cancer. 2010;10:283.
Cheng WL, Wang CS, Huang YH, Tsai MM, Liang Y, Lin KH. Overexpression of CXCL1 and its receptor CXCR2 promote tumor invasion in gastric cancer. Ann Oncol. 2011;22(10):2267–76.
Sharma B, Singh S, Varney ML, Singh RK. Targeting CXCR1/CXCR2 receptor antagonism in malignant melanoma. Exp Opin Ther Targets. 2010;14:435–42.
Singh S, Sadanandam A, Nannuru KC, et al. Small-molecule antagonists for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival, and angiogenesis. Clin Cancer Res. 2009;15:2380–6.
Acknowledgments
This investigation was supported by grants from Science and Technique Development Fund (20107165) of Nantong, Jiangsu, China, and Academy level fund of Nantong Tomour Hospital, Nantong, Jiangsu, China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Han, L., Jiang, B., Wu, H. et al. High expression of CXCR2 is associated with tumorigenesis, progression, and prognosis of laryngeal squamous cell carcinoma. Med Oncol 29, 2466–2472 (2012). https://doi.org/10.1007/s12032-011-0152-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-0152-1